Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11406
Peer-review started: June 22, 2021
First decision: September 1, 2021
Revised: September 29, 2021
Accepted: November 15, 2021
Article in press: November 15, 2021
Published online: December 26, 2021
Processing time: 184 Days and 3.4 Hours
To describe the characteristics, diagnosis and surgical treatment of inguinal endometriosis (IEM).
We retrospectively analyzed 10 patients diagnosed with IEM at Beijing Chao-Yang Hospital from 2011 to 2019. Relevant features, symptoms, images, surgical treatment, hormonal therapy and follow-up were collected and discussed. A total of 10 cases of IEM diagnosed by surgery and pathology were characterized by a lesion on the right side (9/11); five patients had symptoms related to the menstrual cycle, and only 3 patients were clearly diagnosed before surgery. Ultrasonography was of little assistance in confirming the diagnosis, but magnetic resonance imaging showed specific, high-intensity patterns. Anatomically, most of the IEM lesions were located in the extraperitoneal ligament (10/11); nine patients had inguinal hernias (IH), five had concurrent or prior pelvic endometriosis, and four had infertility. The clinical results from extensive resection were satisfactory.
IEM is an extremely rare condition that can easily be misdiagnosed prior to surgery. A right IH may contribute to the formation of right-sided IEM, and extensive resection involving the round ligament and hernia sac is essential to prevent recurrence.
Core Tip: An inguinal hernia on the right side may be one of the causes of the formation of right inguinal endometriosis. This condition may present clinically as a painful mass that can vary in size, possibly according to menstrual cyclicity. Preoperative imaging using ultrasound and/or magnetic resonance imaging may be useful for preoperative diagnosis. Extensive resection involving the round ligament and hernia sac is necessary to prevent recurrence.
- Citation: Li SH, Sun HZ, Li WH, Wang SZ. Inguinal endometriosis: Ten case reports and review of literature. World J Clin Cases 2021; 9(36): 11406-11418
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11406.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11406
Endometriosis (EM) is a disease characterized by the presence of tissue resembling endometrium outside the uterus, causing a chronic inflammatory reaction[1]. The disease is mainly found in women of childbearing age, mostly in the pelvis, with a prevalence in healthy women of 6%–10%[2]. Inguinal EM (IEM) occurs in 0.3% to 0.6% of patients affected by EM, a rare form of extraperitoneal partial round ligament involvement. It is often confused with other groin pathologies and is mostly diagnosed during histological examination[3,4]. IEM was first described by in 1986[5], and to date, fewer than 150 cases have been reported in the literature[6]. In addition, most of the cases related to IEM have been reported as individual cases.
IEM mainly occurs in the right groin area and is characterized by a groin mass with pain and periodic exacerbations or no pain but with menstrual symptoms. The size of the mass may change with the menstrual cycle or have a bleeding tendency[7]. Catamenial symptoms are present in 50% of patients[8,9]. Niitsu et al[10] classified IEM into three types according to the location of the endometrial tissue: Type I, hernial sac or hydrocele in the Nuck canal; type II, round ligament; and type III, under the skin. The etiology of IEM is still unclear, although several theories have been proposed, including but not limited to metaplasia of the mesothelium, retrograde menstruation and seeding into a hernia, oxidative stress, inflammation, autoimmune dysfunction, apoptosis suppression and regeneration of stem cells[11].
IEM is extremely rare, and its occurrence is usually associated with pelvic EM. It can also be easily misdiagnosed before surgery due to its coexistence with inguinal hernia (IH) and other inguinal lesions, which increases the difficulty of diagnosis. Therefore, it is essential to explore the pathogenesis, clinical features, diagnosis and differential diagnosis of IEM. This article retrospectively analyzed the clinical presentation, diagnosis, and treatment of 10 patients with IEM at our hospital and reviewed the relevant literature findings.
The medical records of all patients who underwent IEM surgery in Beijing Chao-Yang Hospital between 2011 and 2019 were retrospectively reviewed. The initial inclusion criteria were as follows: (1) Complete surgical resection; and (2) The presence of both endometrial glands and stromal cells in all lesions excised from the inguinal region. When IEM was diagnosed before or during the operation due to thickening of the hernia sac wall or round ligament, the following inclusion criteria were applied: (1) Complete removal of the hernia sac; (2) Relatively wide surgical margins (approximately 1 cm) for broad ligament resection; and (3) Adequate hernia repair, if necessary. Furthermore, we identified the number of female patients treated for pelvic EM or IH during the same period to estimate the incidence of IEM.
This research project was approved by the constituted Ethics Committee of our hospital (2016-Science-166) and conformed to the Declaration of Helsinki. Informed consent was given by all subjects, and patient anonymity was preserved.
Ten IEM patients were included in this case report; all diagnosed through surgery and pathology, and 4 were nulliparous. Table 1 summarizes the characteristics of all 10 patients, including age, surgical history, and lesion characteristics. The median age at diagnosis was 38 years (range 32–53 years). Of the 10 IEM patients, four had a history of pelvic EM surgery, 8 in the right area of the groin, 1 on the left, and 1 on both sides.
| Index | Cases, n (%) | Percentage, n (%) |
| Age at diagnosis (yr) | 38 (32-53) | |
| Age distribution | Total: 10 | |
| 30–39 yr | 5 | 50 |
| 40–49 yr | 3 | 30 |
| 50 yr and older | 2 | 20 |
| Parity | ||
| Nulliparous | 4 | 40 |
| Dysmenorrhea | ||
| Yes | 7 | 70 |
| No | 3 | 30 |
| Surgical history | 6 | 60 |
| Cesarean section | 1 | 10 |
| Pelvic endometriosis | 4 | 40 |
| Inguinal hernia | 1 | 10 |
| No surgical history | 4 | 40 |
| Department of admission | ||
| Gynecology | 2 | 20 |
| General surgery | 8 | 80 |
| Laterality of inguinal endometriosis | ||
| Right | 8 | 80 |
| Left | 1 | 10 |
| Right and left | 1 | 10 |
| Lesion size (cm) | 3.2 ± 1.2 | |
| Symptoms | ||
| Presenting symptoms | 10 | 100 |
| Symptoms related to menstruation | 5 | 50 |
| Symptoms not related to menstruation | 5 | 50 |
| No symptoms (incidental findings) | 0 | 0 |
| Swelling | ||
| Yes | 10 | 100 |
| No | 0 | 0 |
| Block size varies with body position | ||
| Yes | 9 | 90 |
| No | 1 | 10 |
| Pain | ||
| Yes | 10 | 100 |
| No | 0 | 0 |
All patients had pain and swelling in the groin. Among them, 9 had an increase in groin mass when standing and a slight decrease in groin mass when lying supine. Five patients had catamenial symptoms, including bloating and pain in the groin during menstruation, but the symptoms disappeared between menstruation periods. The duration of complaints prior to surgery ranged from 3 to 108 mo.
The mean size of the lesions was 3.2 ± 1.2 cm. Eight patients were admitted to the hernia department, and only two patients were treated by a gynecologist. A total of 2478 female patients underwent surgery for a hernia or swelling in the groin, and 1958 female patients underwent surgery for EM during the same period in our hospital. The prevalence of IEM in the two groups was 0.4% (10/2478) and 0.5% (10/1958), respectively.
The 10 cases were diagnosed of inguinal EM by surgery and pathology.
Five had prior pelvic EM, and four had infertility.
All patients had no family history.
No special findings except for masses in the groin area were found under physical examinations.
The blood ca125 of case 9 was slightly elevated to 78.5 U/mL, the blood ca125 of case 10 was normal, and the remaining 8 patients did not receive laboratory examinations.
Ultrasonography (US) was performed in all 10 patients (Table 2); 8 patients showed inconclusive heterogeneous lesions, and one patient with bilateral IEM had a heterogeneous, hypoechoic lesion on the left side and a cystic lesion on the right side. Only one patient was diagnosed with IEM by US, in which the mass appeared as a hypoechoic lesion with a cluster of small cysts in the right groin.
| Case | Age (yr) | EM history | Parity | Surgery year | Laterality | ICS | Preoperative diagnosis | US/MRI | Site of EM | Surgery | Postoperative diagnosis | Follow-up (mo) |
| 1 | 47 | - | P | 2018 | R | - | IH/cyst of Nuck’s canal? | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 24 |
| 2 | 52 | + | P | 2016 | R | - | IH/IEM? | US: Low echo with cluster; Cysts (IEM); MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 41 |
| 3 | 33 | - | P | 2017 | R | + | IH/cyst of Nuck’s canal? | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 24 |
| 4 | 53 | - | P | 2018 | R | + | IH | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 12 |
| 5 | 35 | + | N | 2018 | R | - | IH | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 19 |
| 6 | 36 | + | N | 2019 | R | - | IH | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 13 |
| 7 | 42 | - | P | 2015 | R | + | IH | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (R); IH (R) | 60 |
| 8 | 40 | + | N | 2012 | L | - | IH | US: Hetero-geneous echo; MRI:/ | Extraperitoneal round ligament | Wide excision of the lesion; Hernia repair | IEM (L); IH (L) | 70 |
| 9 | 32 | - | N | 2014 | R | + | IEM; Pelvic EM | US: Hetero-geneous echo; MRI: IEM (R) | Extraperitoneal round ligament | Wide excision of the lesion | IEM (R); Pelvic EM | 88 |
| 10 | 36 | - | P | 2011 | B | + | Bilateral IEM; IH (R) | US: Hetero-geneous echo (L) + cyst (R); MRI: IEM (bi) | Extraperitoneal round ligament (L); Hernial sac (R) | Wide excision of the lesion (L + R); Hernia repair (R) | IEM + IH (R); IEM (L) | 97 |
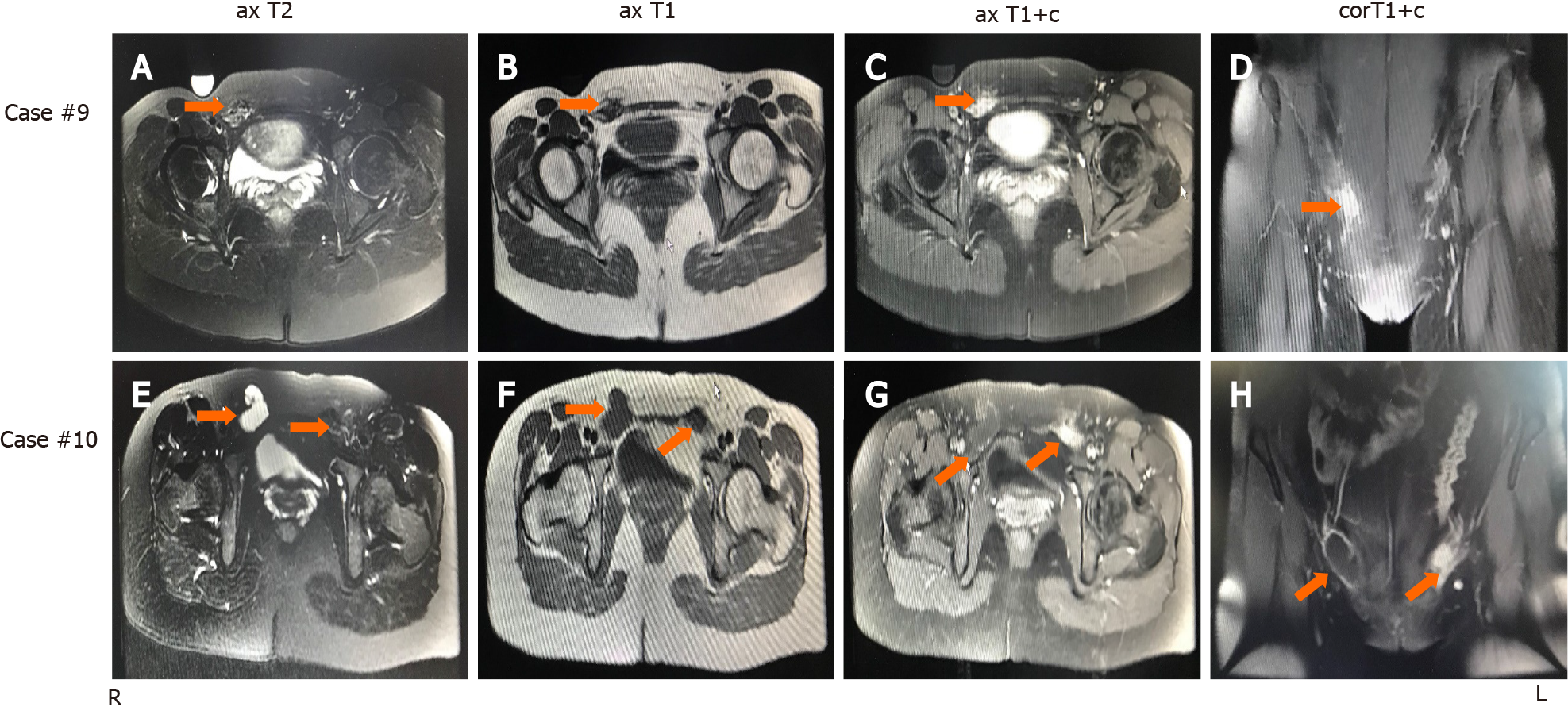
Magnetic resonance imaging (MRI) was performed on 2 patients who were treated by a gynecologist. As shown in Figure 1, case 10 (cystic lesion) showed a hyperintense nodule in the wall of a cystic lesion on T1-weighted axial imaging. Furthermore, T1-weighted contrast-enhanced imaging showed an enhanced hyperintense lesion in the wall of the cystic lesion. Case 9 (solid mass) showed mixed hypointense and hyperintense patterns on T1-weighted and T2-weighted imaging, but T1-weighted contrast-enhanced images showed an enhanced hyperintense pattern at the same lesion. Therefore, the IEM lesion showed specific performance on MRI, in which a hyperintense pattern was found on T1-weighted images or T1-weighted contrast-enhanced images, particularly the latter.
A total of 10 cases of IEM diagnosed by surgery and pathology were characterized by a lesion on the right side.
As shown in Table 1, all patients received surgical intervention through a skin incision in the groin, and the involved extraperitoneal round ligaments were resected extensively. In addition, 9 patients underwent complete excision of the IH sac and hernia repair. Simultaneous laparoscopic exploration of the pelvis was performed only for case 9, and the EM lesion in her right sacral ligament was completely removed.
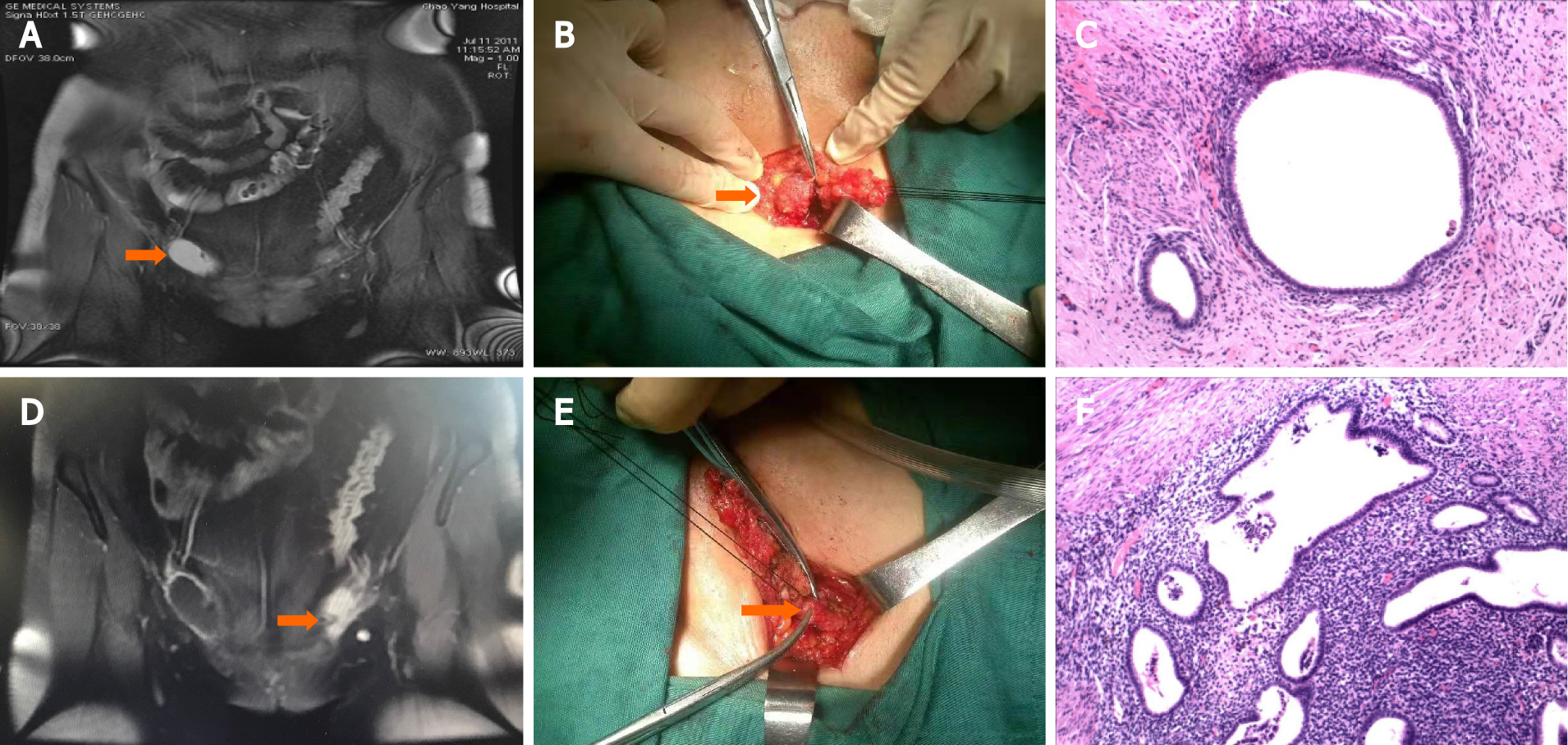
Case 10 (Figure 2) had EM lesions in both inguinal areas. The inner wall of the right hernia sac was locally thickened with dark red fluid and adhesions, and the left extraperitoneal round ligament was significantly thickened with a purplish-blue lesion on the cut surface. Extensive excision of the left extraperitoneal round ligament and the right hernia sac was performed. In addition, the hernia on the right side was repaired. All specimens underwent routine pathological examination, and characteristic endometrial glands with stromal cells were found in all cases.
Only one patient (Case 9), who was surgically confirmed to have pelvic EM, was treated with gonadotropin-releasing hormone agonist (GnRH-a) for six months postoperatively. The remaining nine patients did not receive any drugs.
The median follow-up time was 36 mo (range 12–108 mo), and no complications or recurrence of IEM were observed. In the follow-up of 4 patients, 2 conceived spontaneously and delivered at full term within 2 years. One patient underwent assisted reproduction and full-term delivery 18 mo after surgery, and one patient remained infertile with adenomyosis 2 years after surgery.
Table 3 summarizes the characteristics, symptoms, imaging examinations, surgical treatments, medications and follow-up results of our cases and 14 other retrospective case reports related to IEM.
| Ref. | Case number | Age (yr) | Side | Size (cm) | EM history, n (%) | Reproductive history | Presenting symptoms | Imaging | Preoperative diagnosis | Site of lesion | Operation | Recurrence |
| Kapan et al[14], 2005 | 3 | 39-51 | R: 2; L: 1 | 2-4 | / | / | Groin lump (100%) | / | 0 | Type I (1); Type II (2) | Wide excision of lesion (3); Hernia repair (3) | / |
| Gaeta et al[22], 2010 | 8 | 30 (22-46) | R: 8 | 1.5-4.5 | / | / | Groin lump (100%); Pain (50%); Catamenial symptom (25%) | MRI: Type I: Prevalently cystic (2/8); type II: Prevalently solid with small scattered cysts (6/8) | 100% | Type I (8) | Wide excision of lesion (8) | / |
| Sun et al[9], 2010 | 9 | R: 8; L: 1 | / | / | / | Catamenial symptom (66%) | / | 33% | Excision of inguinal lesion (8/9); Extra round ligament (1/9); Laparo-scopy (4 pelvic EMs) | 0% | ||
| Wong et al[39], 2011 | 1 | 48 | R | 4 × 5 | No | Gravida 3, para 3 | Period pain at the groin during menses | US: A slightly bulky uterus | Fineneedle aspiration biopsy of the mass revealed EM | Proliferative endometrium | Solid, fibroid-like tumor was removed from the right groin | Remained asymptomatic and underwent a second exploratio: Revealed a multinodular subinguinal endometriotic lesion |
| Rajendran et al[15], 2012 | 1 | 36 | L | 2 × 2 | Crampy lower abdominal pain and a lump in her left groin. The lump present for 3 yr | CT: Mass adjacent to the rectus femoris muscle. US: A 2 × 2 cm solid mass with evidence of blood flow at the posterior aspect | Biopsy of the lesion revealed endometrial tissue | |||||
| Albutt et al[44], 2014 | 1 | 23 | L | 2.1 | No | No | A new-onset tender bulge with subjective fevers and chills | US: Avascular complex cystic lesion measuring 2.1 cm in the left groin. CT: A tubular cystic structure along left inguinal canal, round ligament | Inguinal hernia | Fpathology: A hydrocele with concordant EM | The cystic structure was dissected away from the round ligament | no |
| Mourra et al[17], 2015 | 42 | 35 (20-53) | R: 29; L: 11; Unk: 2 | 3.36 (1-5) | 5 | / | Groin lump (100%) | / | 31% | / | Wide excision of lesion (42); Hernia repair (8); Laparo-scopy (4) | 1 (2.38%) |
| Çayır et al[37], 2018 | 1 | 35 | R | 2.5 × 1.5 | US: Hypoechoic solid mass of 2.5 cm × 1.5 cm | |||||||
| Wolfhagen et al[13], 2018 | 9 | 32.5 (27-43) | R: 7; L: 2 | / | 0 | P: 4; N: 5; S: 2 | Groin lump (100%); Catamenial symptom (44%) | US: 1/7 suggestive for IEM; MRI: 0/4 suggestive for IEM; Image: 3/7 suggestive for hernia; Fin: 1/2 suggestive for IEM; 1/2 inconclusive | 33% | Type I (7); No mention (2) | Wide excision of lesion (9); Laparo-scopy (1 pelvic EM) | 0% |
| Niitsu et al[10], 2019 | 28 | 20-50 | R: 25; L: 3 | 1-3.3 | 4 | / | Groin lump (100%); Catamenial symptom (57.1%) | US: Low or with cyst (28/28); CT: Soft tissue density (18/18); MRI: T1 low/T2 low (3/3) | 71.4% | Type I (15); Type II (10); Type Ⅲ (3) | Wide excision of lesion (28) | 2 (7.1%) |
| Arakawa et al[18], 2019 | 20 | 37.2 ± 6.7 | R: 13; L: 5; R and L: 2 | 2.4 ± 1.1 | 11 | P: 3; N: 17 | Groin lump (100%); Pain (100%); Swelling (70%); Catamenial symptom (80%) | US: Solid mass (15/20), cystic mass (2/20), mixed (1/20), no record (2/20); CT: Inguinal mass (13/13); MRI: Solid (9/18), cystic + solid (8/18), cystic (1/18) | 5/6 | No mention | Operation: Radical excision of lesion (5/6), Wide excision of lesion (1/6); Hormone: OC (4/8), DNG (4/8); Chinese medicine (1); No treatment (5) | 1 (5%) |
| Jena et al[4], 2020 | 1 | 25 | r | 3 × 2 | No | 2-yr history of painful persistent mass in the right groin and her symptoms fluctuated with the menstrual cycle | MRI: 2.7 cm × 1.7 cm × 1.6 cm heterogeneous nodular lesion in the right inguinal subcutaneous plane superficial to the adductor muscles and at the lower edge of the rectus abdominis muscle | Inguinal hernia | Mass showed the possibility of intramuscular endometriosis | Excision of the lesion and the | Patient was symptom free on subsequent follow-up | |
| Zihni İ et al[25], 2020 | 1 | 31 | r | 2.1 × 1.2 | The patient had given birth by caesarean section 2 yr previously | Pain and swelling in the right inguinal area. The complaints had been ongoing for approximately 1 yr, and the pain and swelling increased undertaking strenuous labour | US: A cystic structure, 21 mm × 12 mm in size, was seen within the hernia pouch in the right inguinal canal | |||||
| Basnayake et al[23], 2020 | 1 | 27 | r | 4 cm × 4 cm | No | No | Enlarging, painless, right inguinal swelling of 4 mo duration | US: Multiloculated, thin septated, anechoic cystic swelling without increased internal vascularity at the right inguinal region | There was no demonstrable hernia | The histology: Type I endometriosis | A complete excision of the cyst was performed | Follow-up after 1 yr showed no evidence of recurrence |
| This study | 10 | 38 (32-53) | R: 8; L: 1; B: 1 | 3.2 ± 1.2 | 4 | P: 6; N: 4 | Groin lump (100%); Pain (100%); Swelling (100%); Catamenial symptom (50%) | US: Low echo with cluster cysts (1/10); heterogeneous mass (8/10); heterogeneous low echo lesion (L) + cyst echo (R) (1/10); MRI: T1 +c high (2/2) | 30% | Type II (9); Type I + II (1) | Wide excision of lesion (10); Hernia repair + mesh (9) | 0% |
It is challenging to recognize IEM, which is therefore often confused with more common diseases, such as hernias, soft tissue tumors, cysts, lymphadenopathy, granulomas, and hydrocele. Catamenial symptoms, such as changes in size and tenderness, appear in 50% of patients, indicating that the suspicion index for IEM should be increased[12]. IEM is prone to misdiagnosis before surgery[11,13], possibly due to its uncommon location, the noncyclic pain pattern in a number of patients[14-16] or the inconclusive results from US[17,18]. Symptomatic patients often visit a surgeon rather than a gynecologist[3,6,10,19], so IEM is often misdiagnosed as a common groin disease such as IH[17,20]. More explicitly, IEM can be discovered in the extraperitoneal part of the round ligament, subcutaneous fat tissue, inguinal lymph nodes, and even in the sac wall of the inguinal or femoral hernia[6,21].
In our study, 0.5% of patients affected by EM developed IEM, which is consistent with the literature[3]. According to reports, 72%–90%[3,10,17,19] of patients experience right IEM. Similarly, our series of results showed that 9 out of 11 lesions were on the right. Hypotheses for the predominance of the right side in IEM are as follows[3,8,9,19]. First, due to its clockwise circulation, peritoneal fluid persists on the right side for an extended period of time in patients with EM. Second, the sigmoid colon prevents peritoneal fluid from flowing into the left inguinal ring. Third, pre-existing EM of the pelvis makes the round ligament a route for the groin. Pathological diagnosis is still the key for identifying IEM. The characteristic finding is the presence of endometrial glands with stromal cells in the connective tissue.
In our study, 8 patients had a right IH, which thus may be one of the causes for the formation of right IEM. The exact pathogenesis of IEM has not been well explained[15,22]. In the present study, most of the patients with IEM had IHs on the same side, and approximately half of the patients also had pelvic EM. Therefore, regional anatomical defects in the groin and pre-existing pelvic EM may be the main causes of IEM. Although there is an association between abdominal wall EM (e.g., scar EM) and cesarean delivery, a possible association of IEM with cesarean delivery has not been reported. Only one of the 10 patients in this study had a history of cesarean delivery, and this patient also had ipsilateral IH, which did not suggest an association with IEM. The preoperative diagnosis of IEM remains a clinical challenge. Although catamenial symptoms should increase the index of suspicion for IEM[3,8,9,19], typical catamenial symptoms are present in only 50% of IEM cases. Due to the uncommon location of IEM lesions, symptomatic patients usually see a general surgeon rather than a gynecologist[3,6,15,17,19,20], and the diagnosis is frequently incidental or is made upon histologic examination.
A groin lump or subcutaneous mass of the inguinal area can be difficult to distinguish from a wide range of entities, such as hernia, lymph node enlargement, cancer, EM, Nuck hydrocele, lipomas, and abscess[23]. Cutaneous EM with a hernia sac can occur in the absence of normal EM symptoms such as dysmenorrhea. The differential diagnosis for inguinal painless nodules might include IEM[24]. If EM in the inguinal area is detected, it might be within the IH sac or EM of the Nuck canal (NC), round ligament, and subcutaneous tissue[25]. The most frequent manifestation of IEM is a painful lump with cyclical discomfort and growth[26]. Preoperative imaging using US and/or MRI may be useful in this regard, although surgical exploration and histology may clearly establish the genesis and ultimate diagnosis of the disease[13]. Additionally, features of the discomfort, such as frequency, duration, relationship with menstruation, and location, should be extensively questioned. Likewise, appropriate preoperative imaging is important. Ultrasound has been widely used in the examination of the pathology of IEM[27,28].
Computed tomography (CT), MRI, or even a positron emission tomography-CT (PET-CT) has been used to augment the US findings in some situations. High-resolution ultrasound is a low-cost and dependable technique for establishing the suspicion of an IEM diagnosis[29]. Nonetheless, the final diagnosis is based on histology and immunohistochemistry.
In our study, eight patients were treated by a non-gynecologist, and only three patients were diagnosed preoperatively, which is consistent with less than 50% of most reports reported in the literature[3,13,17,19]. In addition, IEM is often associated with IH. In 2 large case studies reported by Niitsu et al[10] and Mourra et al[17], the prevalence of IEM with IH was 54% and 19%, respectively. In our study, the incidence was 90% (9/10). General surgeons tend to focus on the IHs and ignore the presence of IEM, leading to preoperative misdiagnosis. Compared with gynecologists, general surgeons encounter IEM more often. Since this diseases happens rarely, diagnosis is very challenging. It has been stated in many series that general surgeons have performed operations on patients diagnosed with IEM[30]. In these rare cases, it may be helpful if the patient has a history of swelling in the groin area, especially during menstruation or a history of uterine surgery. However, a definite diagnosis can only be made after pathological examination[31]. Although most patients experience pelvic EM, there are also a considerable number of patients with extrapelvic manifestations[32]. Association of the anterior abdominal wall or inguinal groin area is usually correlated with previous surgery, secondary to implantation, and it is rare for IEM lesions to appear in these areas in patients who had not undergone prior surgery[33].
Ultrasound is usually used as the first noninvasive imaging approach for inguinal lesions, but it has limited diagnostic value for IEM. US and CT usually cannot diagnose IEM[3]. Since IEM simulates common surgical conditions in the area, including hydrocele of the canal of Nuckand IH, imaging methods such as US are helpful before surgery. These diseases can yield imaging signs such as cystic components or hypoechoic solid masses[34]. US is usually unable to provide a definitive diagnosis because IEM masses frequently show indeterminate hypoechoic, heterogeneous, or cystic lesions[17,35,36]. We also confirmed that only one patient with a history of pelvic EM was suggested to have IEM on ultrasound. Therefore, the preoperative diagnosis rate based on US alone is very low. If general surgeons focus on the periodic pain pattern of the inguinal mass and the patient’s previous history of EM, the preoperative diagnosis rate might be greatly improved (71.4%)[10].
It has been stated that MRI is a valuable diagnostic method for pelvic EM, with a specificity of 98% and a sensitivity of 90%[22], as MRI can recognize iron in hemosiderin deposits in endometrioma, so pure fibrous lesions are hypointense on T1- and T2-weighted scans, whereas hemorrhages are hyperintense on fat-suppressed T1-weighted or T1-weighted scans. Gaeta et al[22] indicated that there are two main MRI patterns of IEM on T1-weighted images: A solid hypointense mass with a hyperintense hemorrhagic cyst and clusters of hemorrhagic cysts. The role of MRI in the diagnosis of thoracic/pelvic EM has been investigated, and it was discovered that MRI has significantly greater accuracy than other tools, but the role of MRI in abdominal wall EM is undefined[36]. It has been reported that IEM was accidentally found in systemic iodine 131 (I-131) scans following total thyroidectomy[37].
In our study, the IEM lesion located in the round ligament presented with mixed hyper- and hypointensity with an irregular edge in the shape of a cluster of dots and foci on T1- or T2-weighted images, but the lesion in the hernia sac presented as a cystic lesion with hyperintense nodules in part of the cyst wall on T1-weighted images. All lesions presented an enhanced, hyperintense pattern on T1-weighted contrast-enhanced images. T1-weighed contrast-enhanced imaging clearly showed a slight lesion in the hernia sac of case 10. Therefore, extensive use of MRI can improve the accuracy of the preoperative diagnosis of IEM.
Drug treatment for IEM has been poorly reported and is controversial. For isolated pelvic ectopic EM, postoperative suppressive hormone therapy is not necessary[3]. Hormone suppression therapy should only be used for patients with pelvic EM treated laparoscopically[38].
Surgical treatment has been reported in most cases[10,14,39]; primary treatment of IEM relies on extensive resection of the lesion to avoid subsequent recurrence[40]. Niitsu et al[10] reported three cases of recurrence separately from the subcutaneous area, the remnant hernia sac and the distal end of the round ligament. In our study, removal of the affected round ligament with a 1 cm surgical margin was carried out in all patients. Additionally, all-inclusive resection of the hernia sac and hernia repair were performed on nine patients. It is critical to diagnose IEM prior to surgery. Based on the preoperative diagnosis, surgeons should select suitable surgical techniques depending on the anatomical location of the IEM lesion[41]. When the general surgeon performs IH surgery and finds significant thickening of the hernia sac wall, adhesions, bloody fluid inside the sac, or significant thickening of the extraperitoneal round ligament and purple–blue lesions on the cut surface, the possibility of IEM should be considered, and a gynecologist should be asked to consult on the staging, remove the hernia sac and perform complete excision of the IEM lesion as well as evaluate the presence of pelvic EM and, if necessary, perform laparoscopic exploration, especially in patients with infertility.
Finally, to prevent intraoperative cell implantation, proper normal saline irrigation should be performed prior to wound closure. To minimize the risk of artificially inducing EM, it is recommended that gloves, suture materials and sponges be changed in the final stage of uterine surgery. In cesarean section surgery, it is beneficial to clean the operative field with a high-flow saline solution before closing the incision line[42]. Due to the possibility of pelvic EM, laparoscopic diagnosis or postoperative referral to a gynecologist should be performed at the same time, especially for infertile women[3,18-20]. Individualized clinical treatment should be provided in collaboration with general practitioners and obstetrics and gynecology specialists[43].
In this study, there was only one patient for whom the coexistence of pelvic EM was surgically confirmed and who received GnRH-a treatment six months after surgery. The remaining nine patients did not receive any medication. In the short-term and long-term follow-ups, none of the patients experienced postoperative groin discomfort. Therefore, for IEMs without pelvic EM, extensive surgical resection is sufficient. For larger lesions that are highly suspected of IEM, preoperative hormone therapy may sometimes be considered to help achieve radical resection. IEM has been shown to be associated with pelvic EM, and some authors recommended simultaneous laparoscopy to evaluate concomitant pelvic EM[3,10,18,20,44].
Follow-up showed that none of the patients had recurrence of IEM or IH. Therefore, extensive resection of the involved round ligament and hernia sac is the key to preventing recurrence, but this needs to be confirmed by more clinical IEM cases. Because IH occasionally coexists in patients with IEM, peritoneal pseudomyxoma, perivascular epithelioid cell tumors, and especially malignancy have been infrequently found in the hernia sac, some of which may not show serious abnormalities; hence, even if the surgeon is not aware of the coexistence of IEM, the hernia sac should be removed as widely as possible, and pathological examination should be performed. If malignant tumors are suspected, cryosectioning should be performed, as this may help in the decision to perform more aggressive surgical procedures. No developmental abnormalities or malignant changes were encountered in our series. It is very rare for EM lesions in the lateral wall of the pelvis to undergo malignant transformation. However, oncologists should pay greater attention to patients with a history of EM or pelvic surgery[45-48]. In our study, 5 patients had pelvic EM, including 4 patients with a surgical history of pelvic EM and 1 patient who underwent simultaneous pelvic EM surgery. Four out of these 5 patients were infertile. Therefore, it is necessary for a gynecologist to evaluate concomitant pelvic EM, especially for infertile patients. Treatments include complete surgical excision with minimal spillage to avoid recurrence, hormonal therapy and anticipatory management[3]. Gynecology referral is recommended because pelvic EM often occurs at the same time as IEM. IEM rarely recurs after appropriate extensive surgical resection[10,17,19], and postoperative hormonal therapy is not recommended for patients with isolated pelvic EM who have undergone appropriate surgical treatment[38].
IEM is extremely rare and can easily be misdiagnosed before surgery. MRI helps in making a preoperative diagnosis. Right IH may be one of the reasons for the formation of right IEM, and extensive resection is the key to preventing IEM recurrence. Since IEM is usually associated with pelvic EM, consultation with a gynecologist is recommended. In this article, the clinical characteristics, pathogenesis, diagnosis and differential diagnosis of IEM were further enriched by the clinical management experience of the 10 patients we treated and by a systematic literature review. In women with inguinal masses associated with menstrual cycle changes (especially on the right side), gynecological disease-IEM cannot be excluded.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Palmeri M, Parra RS S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1231] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 2. | Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 769] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 3. | Licheri S, Pisano G, Erdas E, Ledda S, Casu B, Cherchi MV, Pomata M, Daniele GM. Endometriosis of the round ligament: description of a clinical case and review of the literature. Hernia. 2005;9:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Jena SK, Begum J, Kumari S, Kar C. The Groin Endometriosis: A Great Mimicker of Common Groin Conditions. J Gynecol Sur. 2020;36:30-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Blanco RG, Parithivel VS, Shah AK, Gumbs MA, Schein M, Gerst PH. Abdominal wall endometriomas. Am J Surg. 2003;185:596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Andres MP, Arcoverde FVL, Souza CCC, Fernandes LFC, Abrão MS, Kho RM. Extrapelvic Endometriosis: A Systematic Review. J Minim Invasive Gynecol. 2020;27:373-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 7. | Savelli L, Manuzzi L, Di Donato N, Salfi N, Trivella G, Ceccaroni M, Seracchioli R. Endometriosis of the abdominal wall: ultrasonographic and Doppler characteristics. Ultrasound Obstet Gynecol. 2012;39:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Sun ZJ, Zhu L, Lang JH. A rare extrapelvic endometriosis: inguinal endometriosis. J Reprod Med. 2010;55:62-66. [PubMed] |
| 10. | Niitsu H, Tsumura H, Kanehiro T, Yamaoka H, Taogoshi H, Murao N. Clinical Characteristics and Surgical Treatment for Inguinal Endometriosis in Young Women of Reproductive Age. Dig Surg. 2019;36:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 12. | Miranda L, Settembre A, Capasso P, Piccolboni D, De Rosa N, Corcione F. Inguinal endometriosis or irreducible hernia? Hernia. 2001;5:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Wolfhagen N, Simons NE, de Jong KH, van Kesteren PJM, Simons MP. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Kapan M, Kapan S, Durgun AV, Goksoy E. Inguinal endometriosis. Arch Gynecol Obstet. 2005;271:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Rajendran S, Khan A, O'Hanlon D, Murphy M. Endometriosis: unusual cause of groin swelling. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Mourra N, Cortez A, Bennis M, Guettier C, Zaatari G, Duvillard P, Validire P, Balaton A. The groin: an unusual location of endometriosis-a multi-institutional clinicopathological study. J Clin Pathol. 2015;68:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Arakawa T, Hirata T, Koga K, Neriishi K, Fukuda S, Ma S, Sun H, Nagashima N, Harada M, Hirota Y, Wada-Hiraike O, Fujii T, Osuga Y. Clinical aspects and management of inguinal endometriosis: A case series of 20 patients. J Obstet Gynaecol Res. 2019;45:2029-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Hagiwara Y, Hatori M, Moriya T, Terada Y, Yaegashi N, Ehara S, Kokubun S. Inguinal endometriosis attaching to the round ligament. Australas Radiol. 2007;51:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Fujikawa H, Uehara Y. Inguinal Endometriosis: An Unusual Cause of Groin Pain. Balkan Med J. 2020;37:291-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Majeski J. Scar endometriosis manifested as a recurrent inguinal hernia. South Med J. 2001;94:247-249. [PubMed] |
| 22. | Gaeta M, Minutoli F, Mileto A, Racchiusa S, Donato R, Bottari A, Blandino A. Nuck canal endometriosis: MR imaging findings and clinical features. Abdom Imaging. 2010;35:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Basnayake O, Jayarajah U, Seneviratne SA. Endometriosis of the Inguinal Canal Mimicking a Hydrocele of the Canal of Nuck. Case Rep Surg. 2020;2020:8849317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Chen PC, Cheng CH, Ding DC. Primary inguinal subcutaneous endometriosis accompanied with an inguinal hernia: A case report. Medicine (Baltimore). 2021;100:e25460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Zihni İ, Karaköse O, Özçelik KÇ, Pülat H, Eroğlu HE, Bozkurt KK. Endometriosis within the inguinal hernia sac. Turk J Surg. 2020;36:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Kiyak G, Ergul E, Sarikaya SM, Yazgan A. Endometriosis of the groin hernia sac: report of a case and review of the literature. Hernia. 2010;14:215-217. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Guerriero S, Conway F, Pascual MA, Graupera B, Ajossa S, Neri M, Musa E, Pedrassani M, Alcazar JL. Ultrasonography and Atypical Sites of Endometriosis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Rees MA, Squires JE, Tadros S, Squires JH. Canal of Nuck hernia: a multimodality imaging review. Pediatr Radiol. 2017;47:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Prodromidou A, Paspala A, Schizas D, Spartalis E, Nastos C, Machairas N. Cyst of the Canal of Nuck in adult females: A case report and systematic review. Biomed Rep. 2020;12:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Wasfie T, Gomez E, Seon S, Zado B. Abdominal wall endometrioma after cesarean section: a preventable complication. Int Surg. 2002;87:175-177. [PubMed] |
| 31. | Kara C, Derici H, Bozdag A, Ermete M. Endometriosis of the abdominal wall: report of three cases. Ulus Cerrahi Derg. 2005;21:201-203. |
| 32. | Audebert A, Petousis S, Margioula-Siarkou C, Ravanos K, Prapas N, Prapas Y. Anatomic distribution of endometriosis: A reappraisal based on series of 1101 patients. Eur J Obstet Gynecol Reprod Biol. 2018;230:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Horton JD, Dezee KJ, Ahnfeldt EP, Wagner M. Abdominal wall endometriosis: a surgeon's perspective and review of 445 cases. Am J Surg. 2008;196:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 34. | Hensen JH, Van Breda Vriesman AC, Puylaert JB. Abdominal wall endometriosis: clinical presentation and imaging features with emphasis on sonography. AJR Am J Roentgenol. 2006;186:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Yang DM, Kim HC, Ryu JK, Lim JW, Kim GY. Sonographic findings of inguinal endometriosis. J Ultrasound Med. 2010;29:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Medeiros LR, Rosa MI, Silva BR, Reis ME, Simon CS, Dondossola ER, da Cunha Filho JS. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | Çayır D, Araz M, Apaydın M, Çakal E. Inguinal Endometriosis Visualized on I-131 Whole Body Scan. Mol Imaging Radionucl Ther. 2018;27:52-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Fong KNY, Lau TWS, Mak CCC, Lui KW. Inguinal endometriosis: a differential diagnosis of right groin swelling in women of reproductive age. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Wong WS, Lim CE, Luo X. Inguinal endometriosis: an uncommon differential diagnosis as an inguinal tumour. ISRN Obstet Gynecol. 2011;2011:272159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A, Prentice A, Saridogan E, Soriano D, Nelen W; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 1342] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 41. | Wang L, Maejima T, Fukahori S, Shun K, Yoshikawa D, Kono T. Laparoscopic surgical treatment for hydrocele of canal of Nuck: A case report and literature review. Surg Case Rep. 2021;7:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Nominato NS, Prates LF, Lauar I, Morais J, Maia L, Geber S. Caesarean section greatly increases risk of scar endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;152:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Mu B, Zhang Z, Liu C, Zhang K, Li S, Leng J, Li M. Long term follow-up of inguinal endometriosis. BMC Womens Health. 2021;21:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Albutt K, Glass C, Odom S, Gupta A. Endometriosis within a left-sided inguinal hernia sac. J Surg Case Rep. 2014;2014. [PubMed] |
| 45. | Wang T, Vajpeyi R. Hernia sacs: is histological examination necessary? J Clin Pathol. 2013;66:1084-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Milam MR, Atkinson JB, Currie JL. Adenosarcoma arising in inguinal endometriosis. Obstet Gynecol. 2006;108:753-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Slomovitz BM, Soslow RA, Chang RC, Golub R, Kuo DY. Serous adenocarcinoma of the inguinal region arising from endometriosis followed by a successful pregnancy. Gynecol Oncol. 2002;87:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Abaid LN, Cupp JS, Chang M, Beanes SR, Goldstein BH. Clear cell carcinoma of the pelvic side wall arising from endometriosis. Gynecol Oncol Rep. 2018;25:24-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |