Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11300
Peer-review started: April 24, 2021
First decision: May 12, 2021
Revised: May 13, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: December 26, 2021
Processing time: 243 Days and 6.1 Hours
The bidirectional link between periodontitis and diabetes mellitus (DM) has been established. Periodontitis causes systemic inflammatory burden through inflammatory mediators. The currently utilized tools [clinical attachment loss (CAL) and probing pocket depth (PPD)] are linear measurements, that do not exactly quantify the inflammatory burden of periodontitis. Periodontal inflamed surface area (PISA) quantifies the surface area of bleeding pocket epithelium and estimates the inflammatory burden. Studies relating to the periodontal status of diabetic patients with and without microvascular complications are scarce. This study assessed the proportion of periodontitis and correlation of PISA with glycemic status in controlled, uncontrolled type 2 DM (T2DM) with and without microvascular complications.
To assess the proportion of periodontitis and correlation of PISA with glycemic status in controlled, and uncontrolled T2DM with and without microvascular complications.
This study comprised 180 T2DM patients. Based on glycated hemoglobin (HbA1c) levels, they were grouped into: (1) Controlled T2DMgroup: (HbA1c ≤ 7%); (2) Uncontrolled T2DM group: (HbA1c > 7%) without microvascular complications; and (3) Uncontrolled T2DM group: (HbA1c > 7%) with microvascular complications. Each group comprised 60 patients. All patients were assessed for periodontal parameters (Bleeding on Probing, PPD, CAL, Oral hygiene index simplified and PISA), and systemic parameters (HbA1c, fasting plasma glucose and post prandial plasma glucose).
The proportion of periodontitis among controlled T2DM group, uncontrolled T2DM group without microvascular complications, uncontrolled T2DM group with micro
High proportion and severity of periodontitis, and increased inflamed surface area in uncontrolled T2DM may have contributed to the poor glycemic control and microvascular complications.
Core Tip: Poor glycemic control and diabetic complications result in severe periodontal destruction. Periodontitis causes systemic inflammatory burden through inflammatory mediators and affects glycemic control. Periodontal inflamed surface area (PISA) estimates periodontal inflammatory burden. This cross-sectional study assessed the proportion and severity of periodontitis and evaluated the correlation between PISA and glycated hemoglobin (HbA1c) in controlled, and uncontrolled type 2 diabetes mellitus (T2DM) with and without microvascular complications. There was a significant positive correlation between PISA and HbA1c. High proportion and severity of periodontitis, and increased inflamed surface area in uncontrolled T2DM may have contributed to poor glycemic control and microvascular complications.
- Citation: Anil K, Vadakkekuttical RJ, Radhakrishnan C, Parambath FC. Correlation of periodontal inflamed surface area with glycemic status in controlled and uncontrolled type 2 diabetes mellitus. World J Clin Cases 2021; 9(36): 11300-11310
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11300.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11300
Diabetes mellitus (DM) is a chronic disease of metabolic dysregulation characterized by hyperglycemia due to defects in insulin secretion, insulin action, or both. Type 2 DM (T2DM) constitutes about 90% to 95% of all DM cases[1]. It results from insulin resistance rather than from the total absence of insulin production. Periodontitis is an immunoinflammatory disease that affects the supporting tissues of the teeth. It is caused by a complex interplay between specific Gram-negative microorganisms, their byproducts, and the host-tissue response[2]. This results in progressive destruction of the periodontal ligament, alveolar bone and cementum[3]. Although it is initiated and maintained by a specific anaerobic or facultative Gram-negative bacterial infection, the onset and progression of the disease is a result of the inflammatory host response[4,5]. The inflammatory response to the presence of subgingival biofilm is characterized by the local production of various inflammatory mediators such as cytokines, prostanoids and enzymes like matrix metalloproteinases (MMPs). Dysregulated immune response results in an imbalance between the proportions of pro- and anti-inflammatory cytokines and results in periodontal tissue destruction.
The relationship between periodontitis and DM is bidirectional[5-7]. DM increases the risk and severity of periodontitis. Hyperglycemia and advanced glycation end-products (AGEs) affect collagen stability, vascular integrity, and cell functions (leukocytes, fibroblasts, and osteoclasts). AGEs aggregate macrophage and monocyte receptors and stimulate the release of proinflammatory cytokines and alteration in the RANKL/OPG ratio. This provokes an increase in the susceptibility to periodontal diseases. The biological model for the plausibility of periodontitis as a risk factor for diabetes showed that periodontitis causes a systemic inflammatory burden. It results from the entry of periodontopathogens and other coaggregating microorganisms and their virulence factors into the systemic circulation. The increased production of inflammatory cytokines in periodontitis aggravates insulin resistance, thereby affecting glycemic control and diabetic complications[6,7]. The more significant the amount of inflamed periodontal tissue, the greater the chance of periodontitis eliciting bacteremia and inflammatory response. The currently utilized tools [clinical attachment loss (CAL) and probing pocket depth (PPD)] are linear measurements that do not exactly quantify the inflammatory burden of periodontitis. Periodontal inflamed surface area (PISA) quantifies the surface area of the bleeding pocket epithelium and estimates the inflammatory burden. Studies relating to the periodontal status of diabetic patients with and without microvascular complications are scarce. This study assessed the proportion of periodontitis and correlation of PISA with glycemic status in controlled, uncontrolled T2DM with and without microvascular complications.
This cross-sectional study was conducted by the Department of Periodontics, Government Dental College, Calicut, in collaboration with the Department of Internal Medicine & Department of Microbiology, Government Medical College, Calicut, Kerala, India. The Institutional Ethics Committee Government Dental College Calicut (IEC No. 83/2016/DCC dated 29-11-16) approved this study, and it was registered under the Clinical Trial Registry of India (CTRI/2017/10/010217). Informed consent was obtained from the patients, and the study was conducted following the Helsinki Declaration of 1975, as revised in 2013. The duration of the study was 12 mo. T2DM patients in the age group between 30 and 60 years and with a minimum of 20 teeth were included in this study. Patients with known systemic diseases and conditions, pregnant and lactating mothers, patients with an acute condition that contraindicates a periodontal examination, patients who received systemic antibiotic therapy within the past 6 mo, patients who received periodontal therapy (scaling and root planing or surgery) within the past year were excluded from the study. In this study, 180 T2DM patients were selected from the Diabetic Clinic of the Department of Internal Medicine and divided into three groups (60 in each group) based on their glycated hemoglobin (HbA1c) levels: (1) Group I: controlled T2DM group: (HbA1c ≤ 7%); (2) Group II: uncontrolled T2DM group: (HbA1c > 7%) without microvascular complications; and (3) Group III: uncontrolled T2DM group: (HbA1c > 7%) with microvascular complications.
Patients were evaluated using a detailed questionnaire about their sociodemographic characteristics, medical history, oral hygiene practice, history of DM and drug allergy. HbA1c, fasting plasma glucose (FPG) and postprandial plasma glucose (PPPG) levels were also assessed. The oral and periodontal examination included PPD, CAL, bleeding on probing (BOP), Oral hygiene index-simplified (OHI-S) and periodontal inflamed surface area (PISA). All periodontal examinations were done by a single trained examiner. There were no sources of bias in this study.
The periodontal status was measured by probing pocket depth, gingival recession, and CAL in millimeters at six sites on each tooth using a William‘s graduated periodontal probe. The periodontal status was then recorded as no/mild periodontitis, moderate periodontitis, and severe periodontitis based on the criteria proposed by the CDC working group for use in population-based surveillance of periodontitis (CDC 2012 update)[8].
PISA was calculated with a Microsoft Excel spreadsheet available on the website: www.parsprototo.info. After filling CAL, gingival recession (GR) and BOP on six sites per tooth in this spreadsheet, mean CAL and GR for each tooth was calculated. Linear mean CAL and GR were translated into the periodontal epithelial surface area (PESA) for each specific tooth. The PESA measured in mm2 for a particular tooth consists of the root surface area of that tooth, which is covered with pocket epithelium. PISA for a particular tooth was estimated by multiplying PESA for a specific tooth with the proportion of sites with BOP. The Full-mouth PISA value (mm2) of each participant was calculated by using the sum of all individual PISAs around the individual tooth.
mean ± SD was calculated for quantitative variables, and frequency was calculated for qualitative variables. An independent t-test was used to compare the quantitative variables between controlled T2DM and uncontrolled T2DM groups. Quantitative data (age, BOP, PPD, CAL, OHI-S, HbA1c, FPG and PPPG) between groups were analyzed by analysis of variance. The χ2 test analyzed qualitative data such as gender, socioe
Mean age, gender distribution, and socioeconomic status showed no significant difference among the groups (Table 1). A significant difference was observed among these groups in terms of duration of diabetes, HbA1c, FPG and PPPG. The difference in BOP score, DI-S score, CI-S score, OHI-S score, mean PPD, mean CAL, and PISA showed significant differences among the groups (P < 0.001). The uncontrolled T2DM group with microvascular complications attained the highest values for all these parameters (Table 2). Bonferroni post hoc adjustment showed no significant difference between the uncontrolled T2DM without microvascular complications and uncontrolled type 2 DM with microvascular complications groups for mean PPD, CAL and PISA (P = 1.00) (Table 3).
| Sociodemographic characteristics | Group I | Group II | Group III | P value | |
| Age, yr (mean ± SD) | 48.83 ± 7.01 | 49.75 ± 5.99 | 50.87 ± 5.98 | 0.22 | |
| Gender (%) | Male | 23 (38.33) | 21 (35.00) | 13 (21.67) | 0.12 |
| Female | 37 (61.67) | 39 (65.00) | 47 (78.33) | ||
| Socio-economic Status (%) | APL | 18 (30.00) | 25 (41.67) | 13 (21.67) | 0.06 |
| BPL | 42 (70.00) | 35 (58.33) | 47 (78.33) | ||
| Variables | Group I | Group II | Group III | P valuea |
| Duration of DM | 8.95 ± 6.86 | 9.49 ± 6.873 | 13.67 ± 5.70 | < 0.001 |
| HbA1c | 6.73 ± 0.25 | 8.87 ± 1.203 | 9.40 ± 1.54 | < 0.001 |
| FPG | 117.25 ± 26.05 | 170.12 ± 57.86 | 173.67 ± 66.11 | < 0.001 |
| PPPG | 176.55 ± 59.63 | 234.62 ± 79.34 | 242.17 ± 73.16 | < 0.001 |
| BOP (% of site) | 55.31 ± 26.22 | 75.85 ± 22.72 | 79.14 ± 19.47 | < 0.001 |
| DI-S | 1.08 ± 0.73 | 1.55 ± 0.78 | 1.6 ± 0.66 | < 0.001 |
| CI-S | 1.31 ± 0.67 | 1.84 ± 0.71 | 1.94 ± 0.65 | < 0.001 |
| OHI-S | 2.37 ± 1.35 | 3.39 ± 1.41 | 3.5 ± 1.14 | < 0.001 |
| PPD | 2.59 ± 0.67 | 3.36 ± 0.77 | 3.43 ± 0.75 | < 0.001 |
| CAL | 2.88 ± 0.77 | 3.81 ± 0.96 | 3.96 ± 0.92 | < 0.001 |
| PISA | 852.22 ± 586.77 | 1506.5 ± 805.76 | 1530.05 ± 690.24 | < 0.001 |
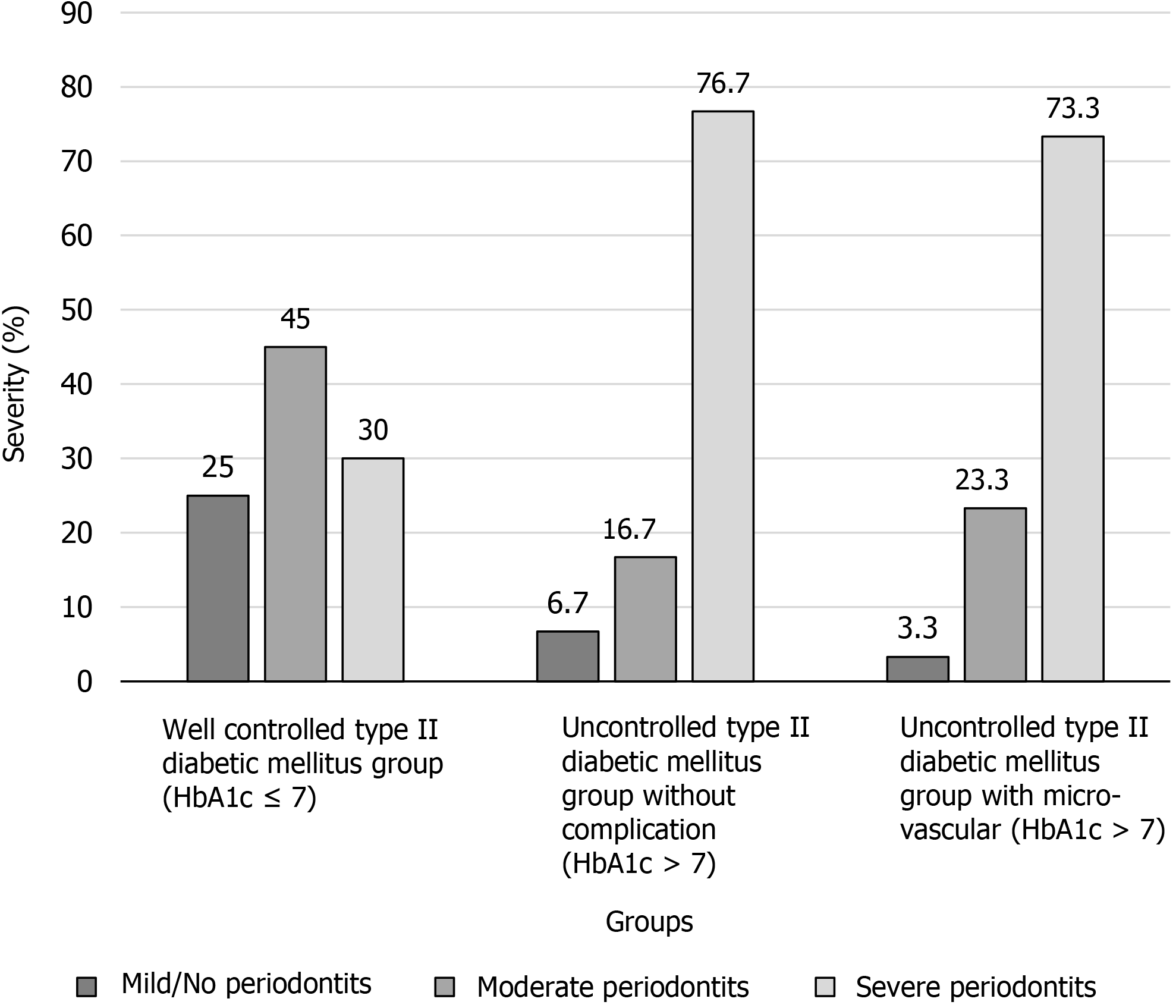
| Periodontitis (%) | 75 | 93.4 | 96.6 | < 0.001 |
| Dependent variable | Group | Group | Mean difference | SE | Significant | 95% confidence interval | |
| Upper bound | Lower bound | ||||||
| Mean PPD | I | II | 0.76778 | 0.1330 | 0.000 | 1.089 | 0.4463 |
| III | 0.83550 | 0.1330 | 0.000 | 1.157 | 0.5140 | ||
| II | III | 0.06772 | 0.1330 | 1.00 | 0.3892 | 0.2538 | |
| Mean CAL | I | II | 0.93488 | 0.1618 | 0.000 | 1.326 | 0.5438 |
| III | 1.08697 | 0.1618 | 0.000 | 1.478 | 0.6959 | ||
| II | III | 0.15208 | 0.1618 | 1.00 | 0.5432 | 0.2390 | |
| Mean PISA | I | II | 654.278 | 127.80 | 0.000 | 963.166 | 345.391 |
| III | 677.836 | 127.80 | 0.000 | 986.723 | 368.948 | ||
| II | III | 23.557 | 127.80 | 1.00 | 332.444 | 285.330 | |
The proportion of periodontitis among the study patients was 88.6%. The proportion of periodontitis among the controlled T2DM group, uncontrolled T2DM group without microvascular complications, and uncontrolled T2DM group with microvascular complications was 75%, 93.4%, and 96.6%, respectively. The difference in the proportion of periodontitis among these groups was significant (P < 0.001) (Table 2).
There was a significant difference in the severity of periodontal diseases among these groups (P < 0.001). The proportion of severe periodontitis among Group I, II and III was 30%, 76.7% and 73.3%, respectively (Figure 1). The uncontrolled T2DM group with microvascular complications showed the highest percentage of sites with CAL ≥ 6 mm. The highest percentage of sites with CAL ≤ 3 mm was observed in the controlled T2DM group. Percentage of sites with CAL ≤ 3 mm, 4–5 mm and ≥ 6 mm showed a significant difference among these groups (P < 0.001) (Table 4).
| Variable, CAL | Group I (% of site) | Group II (% of site) | Group III (% of site) | P valuea |
| CAL ≤ 3 mm | 75.07 | 47.64 | 43.67 | < 0.001 |
| CAL 4-5 mm | 22.16 | 37.53 | 38.43 | < 0.001 |
| CAL ≥ 6 mm | 2.77 | 14.83 | 17.90 | < 0.001 |
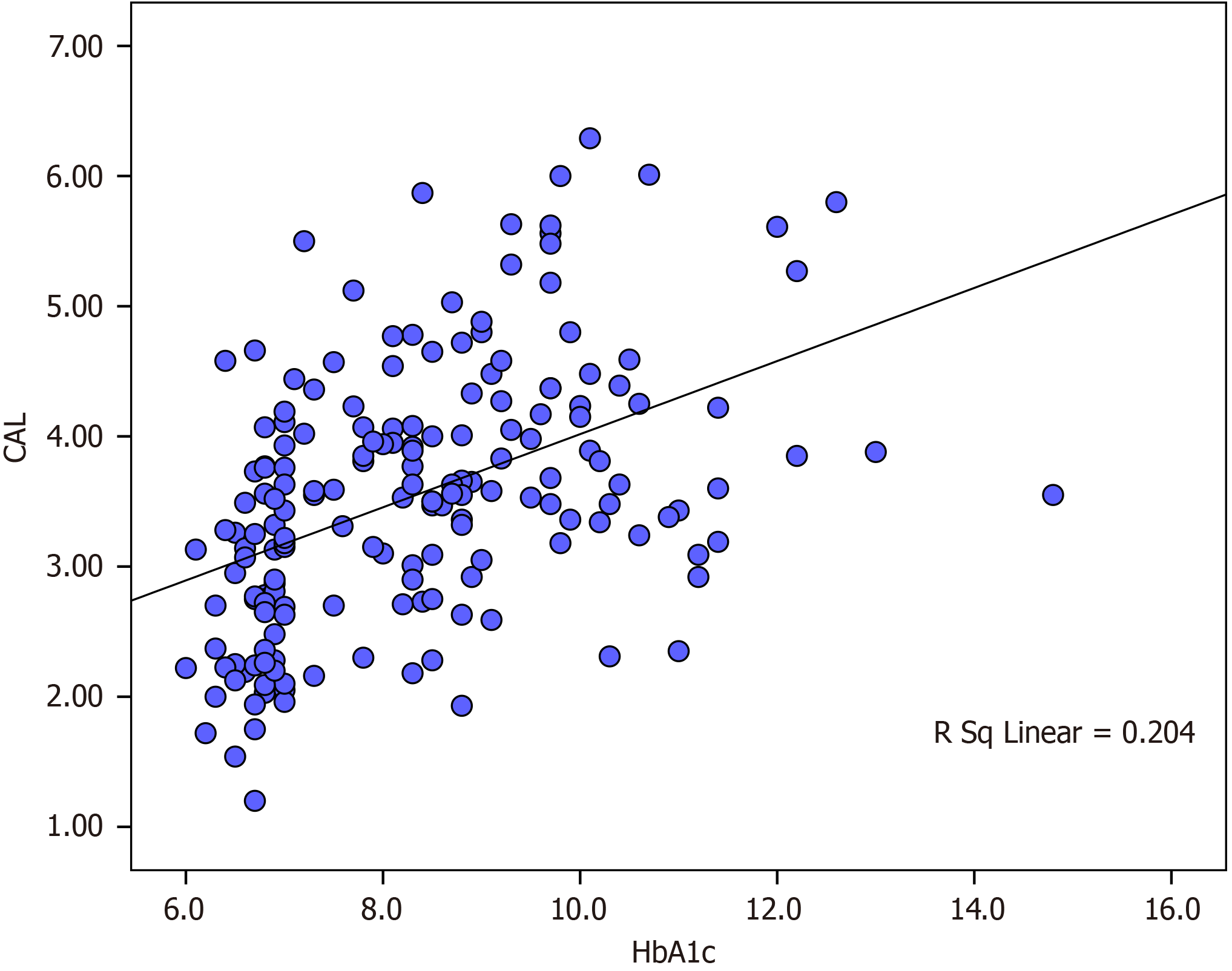
The multivariate linear regression model with the dependent variable PISA showed that age, duration of diabetes and HbA1C were significantly associated with PISA. A dose–response relationship between PISA and HbA1c was observed. An increase of PISA with 168 mm2 was associated with a 1.0% increase of HbA1c (Table 5). A significant positive correlation existed between the mean CAL and HbA1c in all patients (r = 0.451, P < 0.001) (Figure 2). A positive correlation was observed between PISA and HbA1c in all patients (r = 0.393, P < 0.001) (Figure 3).
| Model 1 | Unstandardized coefficients | Standardized coefficients | t | P value | |
| B | SE | β | |||
| Constant | 1558.557 | 1067.486 | 1.460 | 0.146 | |
| Age, yr | 18.249 | 8.546 | 0.152 | 2.135 | 0.034a |
| Gender | 89.509 | 141.724 | 0.055 | 0.632 | 0.529 |
| Occupation | 13.393 | 25.231 | 0.042 | 0.531 | 0.596 |
| Socio-economic status | 101.568 | 113.792 | 0.062 | 0.893 | 0.373 |
| Smoking | 173.619 | 145.772 | 0.094 | 1.191 | 0.235 |
| Chew tobacco | 171.018 | 272.268 | 0.044 | 0.628 | 0.531 |
| Duration of diabetes | 23.708 | 8.867 | 0.211 | 2.674 | 0.008a |
| Total dose of insulin | 0.399 | 1.942 | 0.016 | 0.205 | 0.838 |
| HbA1C | 167.690 | 35.502 | 0.355 | 4.723 | 0.000a |
A high proportion of periodontitis (88.6%) was observed in T2DM patients. The proportion of periodontitis among the uncontrolled T2DM group without microvascular complications and the uncontrolled T2DM group with microvascular complications was high compared to the controlled T2DM group. Several studies[9-13] have reported a higher prevalence of periodontitis in a poorly controlled group than in controlled T2DM. In this study, the mean overall OHI-S score of the controlled T2DM group came under the fair category, whereas the uncontrolled T2DM group with and without microvascular complications came under the poor category. Metabolic control of diabetes may be an important variable in the progression and aggravation of periodontal diseases. Conflicting reports are also available in the literature regarding glycemic control of diabetes and periodontitis. Sandberg et al[14]and Chuang et al[15] have opined that there is no relationship between metabolic control of diabetes and periodontitis.
In this study, a significant difference was obtained in mean PPD and mean CAL between these groups. The uncontrolled T2DM group with microvascular complications had the highest score. Hyperglycemia may have contributed to the increased PPD and CAL in this study. The PPD and CAL obtained in this study are in accordance with the studies of Taylor et al[11], Campus et al[13] and Tervonen et al[16]. In contrast to this, Bridges et al[17]in 1996 did not find any association between glycemic control of diabetes and periodontal parameters.
A significant difference was observed in the percentage of sites with CAL ≤ 3, CAL 4-5 mm and CAL ≥ 6 mm among these groups. The highest percentage of sites with CAL ≤ 3 mm was observed in Group I. Controlled glycemic status may have contributed to the maintenance of periodontal health in this group. The extent and severity of periodontitis were more in the uncontrolled T2DM group as compared to the controlled group. Uncontrolled T2DM group with microvascular complications showed the highest percentage of sites with CAL ≥ 6 mm. This observation is in accordance with Ternoven et al[16], who have reported that the percentage of sites with CAL ≥ 5 mm was significantly higher in poorly controlled T2DM than in a moderately controlled and controlled group. There are several studies that have reported a higher proportion of severe periodontitis in poorly controlled diabetic patients[16,18,19]. Although plaque is the main etiological agent for periodontitis, hyperglycemia and host immune responses to bacterial challenge also play an active role in the progression of periodontitis in patients with DM. Since a bidirectional relationship exists between periodontitis and diabetes, the systemic inflammation associated with periodontal diseases may contribute to the worsening glycemic control in patients with DM. Nonsurgical and surgical periodontal therapy improves glycemic status in DM and prediabetes[20,21]. Mammen et al[22] 2016 found a reduction in insulin resistance and an improvement in insulin sensitivity in patients with DM with chronic periodontitis after nonsurgical periodontal therapy.
Clinically meaningful description of periodontitis should include the proportion of sites with bleeding on probing along with CAL. The percentage of sites with bleeding on probing showed a significant difference among the study groups. The uncontrolled T2DM group with microvascular complications had a higher percentage of BOP. This is in accordance with the study by Emrich et al[9] in 1991 and Campus et al[13] in 2005. Ervasti et al[23] in 1985 found that patients with poorly controlled diabetes had higher gingival bleeding scores than those with good or moderate glycemic control but failed to find any correlation between diabetic complications and gingival bleeding. Hyperglycemia either directly or through AGE formation causes functional and structural modifications of cells. This will affect tissue hemostasis leading to a reduction in host resistance, which is reflected in gingiva as increased bleeding even with mild provocation[24].
Periodontitis may cause an inflammatory burden by the production of local inflammatory mediators entering the systemic circulation. A major disadvantage of the studies published on the relationship between periodontitis and systemic disease is the lack of a tool that adequately assesses the inflammatory burden of periodontitis. Currently, used tools such as CAL and PPD for grading periodontitis are linear measurements that do not quantify the inflammatory burden caused by periodontitis. So, in this study, PISA was used to assess the inflammatory burden of periodontitis. PISA reflects the surface area of the bleeding pocket epithelium. In this study, the uncontrolled T2DM group with and without microvascular complications had a higher percentage of severe periodontitis and a higher mean PISA than the controlled T2DM group. This is in accordance with the study by Leira et al[25] in 2017, which reported a higher PISA in severe periodontitis. From this study, it is evident that a greater estimate of PISA observed in this study could have contributed to the inflammatory link between periodontitis and diabetes. In this study, a positive correlation was obtained between PISA and HbA1c. A dose–response relationship between PISA and HbA1c was observed, and an increase of PISA with 168 mm2 was associated with a 1% increase of HbA1c. Previously, a dose–response association between PISA and HbA1c levels was reported by Nesse et al[26] in 2009. They reported that a 333 mm2 increase of PISA was associated with a 1% increase of HbA1c.
One of the limitations of this study was its small sample size. Moreover, inflammatory markers like interleukin (IL)-1, IL-6, tumor necrosis factor-α, C-reactive protein, MMPs and adipokines were not assessed in this study. Even though our patients had a fair–good OHI-S score, significant positive correlation was found between PISA, CAL and HbA1c. Metabolic control of diabetes may be an important variable in the progression and aggravation of periodontal diseases. Since a bidirectional relationship between diabetes and periodontal disease is well established, high proportion and severity of periodontitis, and increased inflamed surface area in uncontrolled T2DM patients may have contributed to the poor glycemic control and microvascular complications. As it was a cross-sectional study, it is impossible to confirm the direction of the relationship between periodontitis and T2DM with and without microvascular complications. It is necessary to conduct longitudinal studies to show the direction of the relationship between periodontitis and T2DM.
The high proportion and severity of periodontitis and increased inflamed surface area in uncontrolled T2DM patients may have contributed to poor glycemic control and microvascular complications. Since a bidirectional relationship exists between periodontitis and diabetes, the periodontal examination is mandatory for patients with diabetes. Proper periodontal therapy can help improve glycemic control and prevent microvascular complications associated with diabetes.
We are grateful to Dr. Thulaseedharan NK, Professor and Head, Department of Internal Medicine, Govt. Medical College, Calicut, for his support to conduct this study.
The bidirectional link between periodontitis and diabetes mellitus (DM) is well established. Periodontitis causes systemic inflammatory burden through inflammatory mediators. The currently utilized tools [clinical attachment loss (CAL) and probing pocket depth (PPD)] are linear measurements that do not exactly quantify the inflammatory burden of periodontitis. Periodontal inflamed surface area (PISA) quantifies the surface area of bleeding pocket epithelium and estimates the inflammatory burden.
Studies relating to the periodontal status of patients with diabetes with and without microvascular complications are scarce. This study assessed the proportion of periodontitis and correlation of PISA with glycemic status in controlled, uncontrolled type 2 DM (T2DM) with and without microvascular complications.
Firstly, to assess the prevalence and severity of periodontitis in T2DM patients (well-controlled T2DM group: [glycated hemoglobin (HbA1c) levels ≤ 7%], uncontrolled type T2DM group: (HbA1c > 7%) without microvascular complications, uncontrolled T2DM group: (HbA1c > 7%) with microvascular complications. Secondly, to assess the correlation between CAL and HbA1c. Finally, to assess the correlation between PISA and HbA1c.
This cross-sectional study was conducted by the Department of Periodontics, Government Dental College Calicut, in collaboration with the Department of Internal Medicine & Department of Microbiology, Government Medical College, Calicut, Kerala, India. The duration of the study was 12 mo. In this study, 180 T2DM patients were selected from the Diabetic Clinic of the Department of Internal Medicine and divided into three groups based on their HbA1c as follows: (1) Group I: controlled T2DM group: (HbA1c ≤ 7%); (2) Group II: uncontrolled T2DM group: (HbA1c > 7%) without microvascular complications; and (3) Group III: uncontrolled T2DM group: (HbA1c > 7%) with microvascular complications. Patients were evaluated using a detailed questionnaire about their sociodemographic characteristics, medical history, oral hygiene practice, history of diabetes and drug allergy. HbA1c, fasting plasma glucose and postprandial plasma glucose, PPD, CAL, bleeding on probing, oral hygiene index-simplified and PISA were assessed.
The proportion of periodontitis among the controlled T2DM group, uncontrolled T2DM group without microvascular complications, uncontrolled T2DM group with microvascular complications was 75%, 93.4% and 96.6%, respectively. The extent and severity of periodontitis were high in the uncontrolled T2DM group. A significant positive correlation was found between PISA and HbA1c among all patients (r = 0.393, P < 0.001).
The high proportion and severity of periodontitis and increased inflamed surface area in uncontrolled T2DM patients may have contributed to poor glycemic control and microvascular complications.
Since a bidirectional relationship exists between periodontitis and diabetes, the periodontal examination is mandatory for patients with diabetes. Proper periodontal therapy can help improve glycemic control and prevent microvascular complications associated with diabetes to some extent.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nong X S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Li JH
| 1. | Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 414] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1334] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 3. | Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31 Suppl 1:3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1430] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 4. | Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334. [PubMed] |
| 5. | Chapple IL, Genco R; Working group 2 of joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40 Suppl 14:S106-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Stanko P, Izakovicova Holla L. Bidirectional association between diabetes mellitus and inflammatory periodontal disease. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (2)] |
| 7. | Janket SJ, Jones JA, Meurman JH, Baird AE, Van Dyke TE. Oral infection, hyperglycemia, and endothelial dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:173-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 956] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 9. | Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 420] [Article Influence: 12.4] [Reference Citation Analysis (2)] |
| 10. | Novaes AB Jr, Gutierrez FG, Novaes AB. Periodontal disease progression in type II non-insulin-dependent diabetes mellitus patients (NIDDM). Part I--Probing pocket depth and clinical attachment. Braz Dent J. 1996;7:65-73. [PubMed] |
| 11. | Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998;69:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Collin HL, Uusitupa M, Niskanen L, Kontturi-Närhi V, Markkanen H, Koivisto AM, Meurman JH. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol. 1998;69:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and periodontal disease: a case-control study. J Periodontol. 2005;76:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF. Type 2 diabetes and oral health: a comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract. 2000;50:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Chuang SF, Sung JM, Kuo SC, Huang JJ, Lee SY. Oral and dental manifestations in diabetic and nondiabetic uremic patients receiving hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol. 1993;20:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR. Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors. J Periodontol. 1996;67:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (2)] |
| 19. | Lu HK, Yang PC. Cross-sectional analysis of different variables of patients with non-insulin dependent diabetes and their periodontal status. Int J Periodontics Restorative Dent. 2004;24:71-79. [PubMed] |
| 20. | Joseph R, Sasikumar M, Mammen J, Joseraj MG, Radhakrishnan C. Nonsurgical periodontal-therapy improves glycosylated hemoglobin levels in pre-diabetic patients with chronic periodontitis. World J Diabetes. 2017;8:213-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Correa FO, Gonçalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol. 2010;37:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Mammen J, Vadakkekuttical RJ, George JM, Kaziyarakath JA, Radhakrishnan C. Effect of non-surgical periodontal therapy on insulin resistance in patients with type II diabetes mellitus and chronic periodontitis, as assessed by C-peptide and the Homeostasis Assessment Index. J Investig Clin Dent. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ervasti T, Knuuttila M, Pohjamo L, Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol. 1985;56:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 373] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 25. | Leira Y, Martín-Lancharro P, Blanco J. Periodontal inflamed surface area and periodontal case definition classification. Acta Odontol Scand. 2018;76:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Nesse W, Linde A, Abbas F, Spijkervet FK, Dijkstra PU, de Brabander EC, Gerstenbluth I, Vissink A. Dose-response relationship between periodontal inflamed surface area and HbA1c in type 2 diabetics. J Clin Periodontol. 2009;36:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |