Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11285
Peer-review started: April 22, 2021
First decision: May 24, 2021
Revised: June 4, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: December 26, 2021
Processing time: 244 Days and 17.3 Hours
In recent years, biological therapies have revolutionized the management of inflammatory bowel disease (IBD); however, they are expensive. The devel
To analyze clinical response rates to CT-P13 and adverse events in IBD patients treated in real-life practice.
An observational, prospective, multicenter study of IBD patients treated with CT-P13 in clinical practice who were naïve to biological treatments or failed to respond to other anti-tumor necrosis factor drugs or had switched from infliximab originator was carried out. No diagnostic or follow-up interventions were conducted on patients outside usual clinical practice. The primary endpoints were clinical response rates and number of adverse events. The primary efficacy variable was the proportion of patients who were in clinical remission and/or had a clinical response at 3, 6, 9, and 12 mo.
A total of 220 IBD patients treated with CT-P13 (Remsima®) were included in the study: 87 (40%) with ulcerative colitis and 133 (60%) with Crohn’s disease. Mean age of the patients was 41.47 (SD 15.74) years, and 58% were female. Nineteen (9%) patients started treatment with CT-P13 after switching from infliximab. Of the remaining 201 patients, 142 (65%) were naïve to biologic agents. At baseline, 68.6% (n = 138/201) of patients presented with active disease. After 12 mo of treatment, 14.8% (n = 12/81) presented with active disease, and 64.2% (n = 52/81) were in clinical remission without corticosteroids. After 3 mo, 75.5% (n = 115/152) had a clinical response or achieved clinical remission, which was sustained for 12 mo (85.2%; n = 69/81). There was a decrease in specific IBD indices at 3, 6, 9, and 12 mo (P < 0.001). A total of 34 adverse events were reported by 27 (12.3%) patients, 9 (26.5%) of which were serious.
CT-P13 is an effective and safe infliximab biosimilar for the treatment of IBD in real-life practice and may be a valid and attractive alternative for the treatment of IBD.
Core Tip: In this study, the effectiveness and safety of the infliximab biosimilar CT-P13 in the treatment of inflammatory bowel disease (IBD) in real-life practice were analyzed. Our results show that CT-P13 is an effective and safe biosimilar of infliximab, and is a valid and attractive alternative for the treatment of IBD as it allows for a reduction of healthcare costs and facilitates access to biological treatments for more patients.
- Citation: Huguet JM, Cortés X, Bosca-Watts MM, Aguas M, Maroto N, Martí L, Amorós C, Paredes JM. Real-world data on the infliximab biosimilar CT-P13 (Remsima®) in inflammatory bowel disease. World J Clin Cases 2021; 9(36): 11285-11299
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11285.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11285
Crohn’s disease (CD) and ulcerative colitis (UC) are the two main conditions of inflammatory bowel disease (IBD). CD is characterized by chronic inflammation of any part of the gastrointestinal tract, has a progressive and destructive course, and is increasing in incidence worldwide. UC is a chronic IBD of unknown etiology affecting the colon and rectum. Multiple factors, such as genetic background, environmental and luminal factors, and mucosal immune dysregulation, have been suggested to contribute to its pathogenesis[1,2]. Both chronic diseases are associated with high morbidity and strongly affect quality of life[3-8]. In the last two decades, biological therapies have revolutionized the management of these diseases. Seven biologic agents are currently approved by the United States Food and Drug Administration (FDA) for IBD treatment, although only five are approved by the European Medicines Agency (EMA): three anti-tumor necrosis factor (TNF) agents (infliximab, adalimumab, and golimumab), one anti-IL12/23 (ustekinumab), and one anti-integrin agent (vedolizumab)[9]. These biological treatments induce mucosal healing, prolong periods of remission, and improve patients’ quality of life, leading to a reduction in hospitalizations and surgical procedures[5,9-15]. However, they are expensive; cons
The expiry of patents for some biologics has led to the development of biosimilar products with two main goals: reducing healthcare costs and improving patients’ access to these treatments[10,16,19]. According to the World Health Organization (WHO), a biosimilar is a biotherapeutic product similar in terms of quality, safety, and efficacy to an already licensed reference biotherapeutic product[10,20]. Although a biosimilar and its reference drug are mostly the same product, there may be dif
Although various studies support the similarity between CT-P13 and infliximab in terms of efficacy and safety, there are unmet needs regarding research on these agents in the context of IBD. Due to this fact and limited evidence in real-life practice, many physicians are still reluctant to prescribe biosimilars[10]. Most observational trials that have evaluated CT-P13 in patients who were naïve to biological treatment or had switched from infliximab had a retrospective design and limited sample size[23,24], with few exceptions[25,26]. Furthermore, prospective trials had follow-up periods of less than one year and/or study samples of less than 100 patients[27-32], with the exception of the studies by Kolar et al[33], Jung et al[26], and Bergqvist et al[34]. Up to now, only one randomized controlled trial has compared infliximab with CT-P13 in IBD patients[35]. Furthermore, most studies included patients who were either naïve to biologic agents or had switched from infliximab to CT-P13, although little is known regarding patients switching from other biologic agents, such as adalimumab[10,36].
In this study, we aimed to analyze clinical response rates to CT-P13, marketed under the trade name of Remsima®, and adverse events in patients with IBD treated in real-life practice.
This observational, prospective, multicenter study was conducted at eight Spanish hospitals: Hospital General Universitario de Valencia, Hospital de Sagunto, Hospital Universitario Dr. Peset, Hospital Arnau de Vilanova de Valencia, Hospital Universitari i Politecnic la Fe, Hospital Clínico Universitario de Valencia, Hospital Francesc de Borja de Gandia, and Hospital de Manises, Valencia.
Patients’ assignment to a specific therapeutic strategy was determined as in daily clinical practice. Thus, the decision to prescribe a drug was clearly dissociated from the decision to include a patient in the study. No diagnostic or follow-up interventions were conducted on patients outside usual clinical practice, and all data were collected in a case report form (CRF) for the epidemiological analysis by the attending physician.
The duration of the study was at least one year. Study variables were recorded in patients’ clinical histories and in an electronic database (Excel).
The study was conducted following the principles outlined in the current revised version of the Declaration of Helsinki and in compliance with Good Clinical Practice (GCP) and all applicable laws and regulatory requirements in Spain. The study was approved by the Hospital General Universitario de Valencia Ethics Committee, and all patients signed an informed consent.
Based on the ECCO guidelines, patients start biological treatment for CD or UC with moderate to severe activity when they have presented an inadequate response to conventional treatment, which includes corticosteroids and immunomodulators (IMM), such as 6-mercaptopurine (6-MP) or azathioprine (AZA), or when they are intolerant or have medical contraindications to such treatments. Biological treatment is also started in active, fistulizing CD that has not responded despite a complete and adequate course of conventional treatment (including antibiotics, drainage, and immunosuppressive therapy)[37,38].
The study included IBD patients treated with Remsima® (infliximab biosimilar, CT-P13, Kern Pharma, Spain) in clinical practice who were naïve to biological treatments, or had failed to respond to other anti-TNF drugs, or had switched from infliximab originator (Remicade®). Patients who were unresponsive to infliximab or allergic or intolerant to infliximab or its excipients or had a contraindication to infliximab were excluded. CT-P13 (Remsima®) was administered at a standard dose of 5 mg/kg via a 2-h intravenous infusion. Patients were invited to take part and were included in the study if they agreed to participate and their eligibility was confirmed.
The primary endpoints were clinical response rate and number of adverse events in IBD patients treated with CT-P13 (Remsima®) in real-life practice. Variables were collected at baseline and every three months during the 12 mo follow-up period (Table 1). CT-P13 (Remsima®) trough levels and anti-drug antibodies were determined according to clinical practice before each infusion. The primary efficacy variable was the proportion of patients who were in clinical remission and/or had a clinical response at each visit.
| Variable | Baseline | Follow-up |
| Demographic data | ||
| Date of birth | X | - |
| Gender | X | - |
| Smoking habit | X | - |
| Disease data | ||
| Date of diagnosis | X | - |
| IBD type (Crohn’s disease, ulcerative colitis) | X | - |
| Montreal classification | X | - |
| Type of fistula (perianal, entero-enteral, enterovesical, enterovaginal, enterocutaneous, other) | X | - |
| Intra-intestinal complications (yes or no) | X | - |
| Extraintestinal complications (dermatological, osseous, optical, hepatic) | X | - |
| Harvey-Bradshaw index | X | X |
| Crohn’s disease activity index | X | X |
| Partial Mayo Score | X | X |
| Truelove-Witts severity index | X | X |
| Laboratory analysis | ||
| C-reactive protein | X | X |
| Erythrocyte sedimentation rate | X | X |
| Hemoglobin | X | X |
| Calprotectin | X | X |
| CT-P13 trough levels and antibodiesx | - | X |
| Imaging tests | ||
| Disease severity and lesion location according to endoscopy result (mild, moderate, severe) | X | X |
| Disease severity and lesion location according to magnetic resonance enterography result (mild, moderate, severe, fibrotic stenosis) | X | X |
| Treatment data | ||
| Current treatments (mesalazine, cortisone, AZA/6-MP, MTX) | X | X |
| Previous treatments (mesalazine, corticosteroids, AZA/6-MP, MTX, infliximab, adalimumab) | X | - |
| Reasons to start treatment with CT-P13 (corticosteroid dependence, corticosteroid resistance, failure of AZA/6-MP, perianal disease/fistula, start of severe illness) | X | - |
| Clinical situation | ||
| Active clinical disease | X | X |
| Clinical remission | X | X |
| Clinical response | - | X |
Clinical disease was considered active when the HBI (Harvey-Bradshaw Index) was > 4 (CD) or PMS (Partial Mayo Score) was > 2 (UC) or the patient had an active perianal fistula in CD. A fistula was considered active if it presented suppuration spontaneously or with digital pressure, and inactive if there was no suppuration. Clinical remission was defined as HBI ≤ 4 (CD) or PMS ≤ 2 (UC), without corticosteroids, or an inactive perianal fistula. A clinical response was defined as a decrease of at least 2 points in the HBI or PMS or improvement of the perianal fistula. The disease was considered stable when HBI was ≤ 4 (CD) or PMS was ≤ 2 (UC) and C-reactive protein (CRP) was < 5 mg/L for at least 6 mo. Relapse or exacerbation was defined as the need to scale the dosage of CT-P13 (Remsima®) (shorten interval and/or increase doses), the addition of corticosteroids, or switching to another biologic agent based on the physician's decision. Loss of response was defined as active disease after a primary response following at least the first four infusions of the biologic drug.
The CD activity index (CDAI) was determined in patients with Crohn's disease at baseline and at each of the evaluations. The Truelove-Witts index was used in patients with UC.
The safety variable was the number of adverse effects described in the follow-up.
Adverse events: Type, severity (low, mild, severe), whether they were related to CT-P13 (Remsima®) or not, duration (days), hospitalization (yes or no), and need for surgery (if yes, the reasons for surgery). Variables were collected in a CRF every three months.
The statistical analyses in this study were performed by Joaquin Peña Siles and Jose Antonio Parejo Maestre from the University of Seville. Sample size was not defined. The choice of treatment was based on usual clinical practice. A descriptive analysis of all the variables of interest was made. The results of continuous variables are presented as mean ± SD. The results for categorical variables are presented as frequencies and percentages. The statistical analysis included appropriate measures for statistical significance (Student’s paired two-sample t-test) using the standard cutoff for significance of P < 0.05 via Microsoft Excel.
A total of 220 IBD patients treated with CT-P13 (Remsima®) were included in the study: 87 (40%) with UC and 133 (60%) with CD. At the time of starting CT-P13 (Remsima®) treatment, the mean age was 41.47 (SD 15.74) years. Patients were evenly distributed by sex: 42% were male and 58% were female. Most patients (140, 63.6%) were non-smokers, 37 (16.8%) were smokers, and 28 (12.8%) were former smokers.
Table 2 shows the clinical characteristics of the study patients at baseline, including previous and current treatments. Before their inclusion in the study, some of the patients had previously been treated with biologic agents: 35 patients (21 with CD and 14 with UC) had received treatment with infliximab, and 22 (15 with CD and seven with UC) with adalimumab. At the time of the study, 74 (35%) patients were concomitantly treated with corticosteroids. The disease phenotypes of CD and UC according to the Montreal classification[39] are shown in Table 3.
| Overall (n = 220) | CD (n = 133) | UC (n = 87) | |
| Endoscopic findings (presence and size of ulcers) | |||
| Unaffected | 9 (4) | 7 (5) | 2 (2) |
| Mild (1-5 mm) | 7 (3) | 1 (1) | 6 (7) |
| Moderate (5-20 mm) | 54 (25) | 21 (16) | 33 (38) |
| Severe (> 20 mm) | 41 (19) | 25 (19) | 16 (18) |
| Fibrotic stenosis | 7 (3) | 7 (5) | 0 (0) |
| Not performed | 102 (46) | 72 (54) | 30 (34) |
| Ultrasound findings (hyperemia of the bowel wall assessed by color Doppler ultrasound) | |||
| Unaffected | 5 (2) | 5 (4) | - |
| Mild (2 signal dots/cm2) | 7 (3) | 7 (5) | - |
| Moderate | 34 (26) | - | |
| (3-5 signal dots/cm2) | 34 (15) | ||
| Severe (> 5 signal dots/cm2) | 22 (10) | 22 (17) | - |
| Not performed | 152 (69) | 65 (49) | 87 (100) |
| Previous treatments | |||
| Mesalazine | 97 (44) | 37 (28) | 60 (69) |
| Corticosteroids | 131 (60) | 73(55) | 58 (67) |
| AZA/6-MP | 135 (61) | 82 (62) | 53 (61) |
| MTX | 12 (5) | 10 (8) | 2 (2) |
| Remicade® | 35 (16) | 21 (16) | 14 (16) |
| Humira® | 22 (10) | 15 (11) | 7 (8) |
| Concomitant treatments | |||
| Mesalazine | 77 (35) | 28 (21) | 49 (56) |
| Corticosteroids | 74 (35) | 37 (28) | 37 (43) |
| AZA/6-MP | 115 (52) | 67 (50) | 48 (55) |
| MTX | 15 (7) | 15 (11) | 0 (0) |
| Montreal classification of CD | CD (n = 133) |
| Age of diagnosis | |
| A1 (below 16 yr) | 15 (11) |
| A2 (between 17 and 40 yr) | 90 (68) |
| A3 (above 40 yr) | 18 (14) |
| - | 10 (8) |
| Location | |
| L1 (ileal) | 62 (47) |
| L2 (colonic) | 16 (12) |
| L3 (ileocolonic) | 47 (35) |
| - | 8 (6) |
| Location L41 (concomitant UGI disease) | |
| Yes | 4 (3) |
| No | 87 (65) |
| - | 42 (32) |
| Behavior | |
| B1 (non-stricturing, non-penetrating) | 60 (45) |
| B2 (stricturing) | 24 (18) |
| B3 (penetrating) | 38 (29) |
| - | 11 (8) |
| P2 (concomitant perianal disease) | |
| Yes | 38 (29) |
| No | 84 (63) |
| - | 11 (8) |
| Montreal classification of UC | UC (n = 87) |
| Extent | |
| E1 (Ulcerative proctitis) | 10 (11) |
| E2 (Left-sided UC - distal UC) | 18 (21) |
| E3 (Extensive UC - pancolitis) | 48 (55) |
| - | 11 (13) |
| Severity | |
| S1 (Mild UC) | 8 (9) |
| S2 (Moderate UC) | 54 (62) |
| S3 (Severe UC) | 15 (17) |
| - | 10 (11) |
Only 19 (9%) patients started treatment with CT-P13 (Remsima®) after switching from infliximab. Of the remaining 201 patients, 142 (65%) were naïve to biologic agents; 25 (11%) started treatment with CT-P13 due to failure to respond to previous biological treatments [21 (84%) to adalimumab, 1 (4%) to certolizumab, 1 (4%) to vedolizumab, and 2 (8%) unspecified]; 20 (9%) had been treated with Inflectra®, another commercial name for CT-P13; and in 14 (6%) cases, treatment was not specified. These patients had pharmacological and clinical reasons for starting CT-P13 (Remsima®). Among the pharmacological reasons, we found dependence on or resistance to corticosteroids, intolerance or resistance to IMM, and combinations of these reasons. The most frequent reasons were resistance to IMM (15%), dependence on corticosteroids (12%), and intolerance to IMM (10%). The main clinical reason for starting CT-P13 (Remsima®) was moderate exacerbation (42%). Other clinical reasons included severe exacerbation (12%), mild exacerbation (5%), perianal disease (5%), extraintestinal manifestations (1%), and combinations of some of these reasons.
Of the 19 patients who switched from infliximab original (Remicade®), 12 had already completed 12 mo of follow-up at the time of the study. Of these, 10 patients (83.3%) remained in remission.
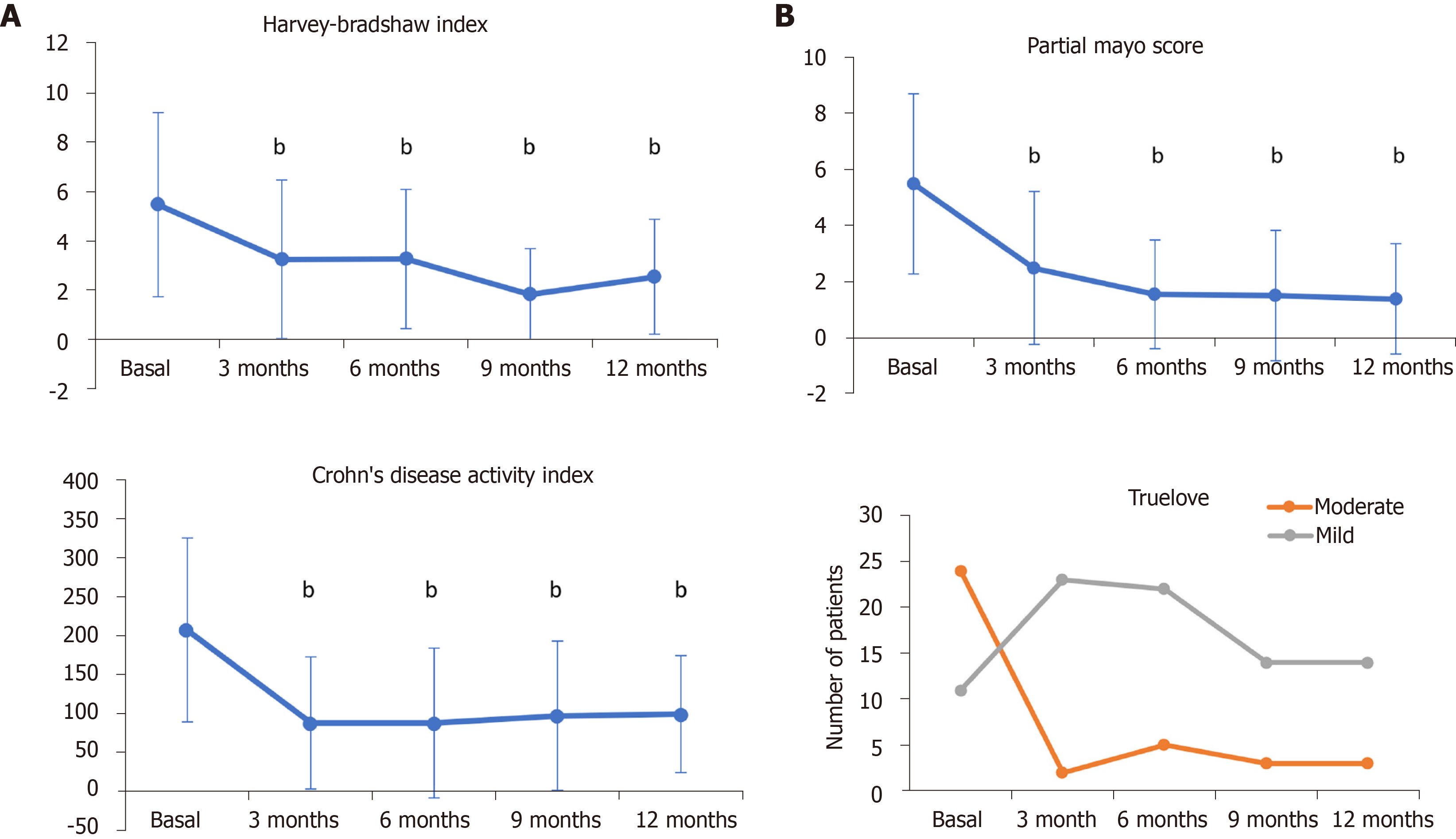
Of the remaining 201 patients, 81 had already completed 12 mo of follow-up at the time of the study. At baseline, 68.6% (n = 138/201) of patients presented with active disease; the remainder were in remission after having started treatment with corticosteroids (see Table 2, concomitant treatments). After one year of treatment with CT-P13 (Remsima®), the proportion of patients with active disease was 14.8% (n = 12/81), and the proportion of patients who achieved clinical remission (without corticosteroids) was 64.2% (n = 52/81) (Table 4). At the time of the study, 152 patients had completed 3 mo of follow-up. As early as 3 mo, 75.5% (n = 115/152) of patients treated with CT-P13 (Remsima®) had a clinical response or achieved clinical remission, which was sustained during one year of treatment (85.2%; n = 69/81) (Table 4). A statistically significant decrease was observed in specific CD and UC indices [HBI and CDAI for CD and PMS for UC] after 3, 6, 9, and 12 mo of treatment with CT-P13 (Remsima®) (P < 0.001) (Figure 1). As shown in Figure 1B, according to the Truelove-Witts index, the number of patients with moderate UC decreased after three months of treatment, which was sustained for 12 mo; the number of patients with mild disease increased consistently.
| Active disease | Clinical remission | Clinical response | |
| Baseline (n = 201) | 138 (68.6) | - | - |
| 3 mo (n = 152) | 37 (24.4) | 81 (53.2) | 34 (22.3) |
| 6 mo (n = 122) | 21 (17.2) | 74 (60.7) | 27 (22.1) |
| 9 mo (n = 84) | 8 (9.5) | 61 (72.6) | 15 (17.9) |
| 12 mo (n = 81) | 12 (14.8) | 52 (64.2) | 17 (21) |
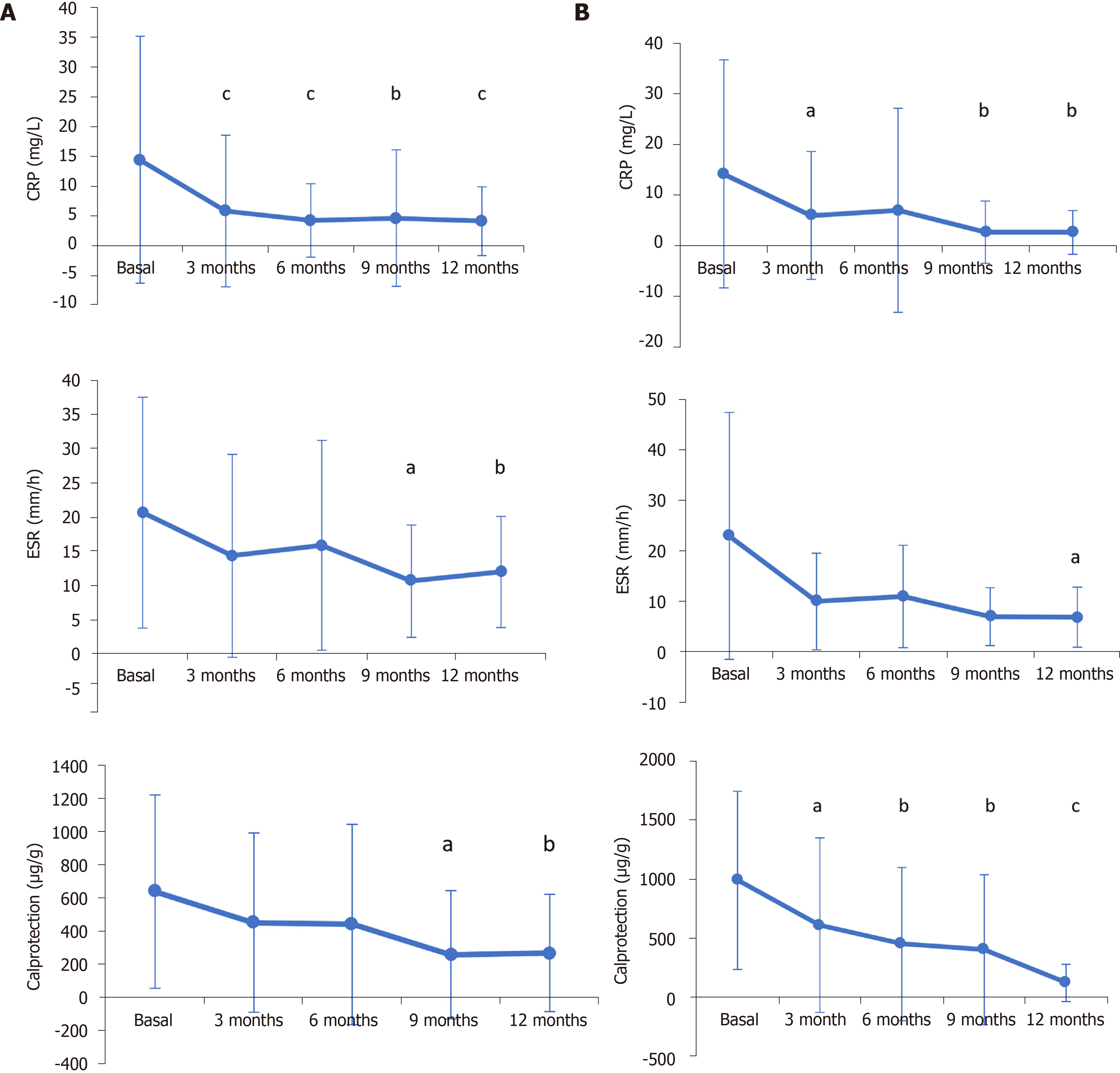
General inflammatory blood biomarkers decreased after 12 mo of CT-P13 (Remsima®) treatment in CD and UC patients. In CD patients, blood CRP levels decreased from 14.51 mg/L (SD 20.78; n = 108) at baseline to 4.17 mg/L (SD 5.80; n = 50) (P < 0.001); fecal calprotectin levels decreased from 638.99 µg/g (SD 584.32; n = 57) to 268.58 µg/g (SD 354.65; n = 31) (P = 0.0018); and the erythrocyte sedimentation rate (ESR) decreased from 20.65 mm/h (SD 16.91; n = 49) to 11.97 mm/h (SD 8.16; n = 30) (P = 0.010) (Figure 2A). In UC patients, CRP decreased from 14.20 mg/L (SD 22.56; n = 62) at baseline to 2.64 mg/L (SD 4.31; n = 33) (P = 0.0045); fecal calprotectin levels decreased from 990.112 µg/g (SD 755.46; n = 43) to 121.67 µg/g (SD 157.58; n = 18) (P < 0.001); and ESR decreased from 23.00 mm/h (SD 24.50; n = 17) to 6.83 mm/h (SD 5.95; n = 12) (P = 0.0344) (Figure 2B).
At the beginning of the study, 11 patients had active perianal disease (5.4%) and had started treatment for this reason. Perianal disease remained active after one year of follow-up in 3 patients (3.7%, 3/81). The low number of patients with active perianal disease did not allow us to draw conclusions from the analysis.
Considering patients who switched and those who did not, antibodies to CT-P13 were measured in 22.2% (38/171), 17.9% (25/139), 15.3% (15/98), and 24.7% (23/93) of patients at 3, 6, 9, and 12 mo, respectively. At three months, four (10.5%) patients had detectable antibodies, one (4%) at six months, one (6.7%) at nine months, and six (26.1%) at 12 mo.
Mean CT-P13 trough levels were 5.74 µg/mL (SD 3.64), 5.26 µg/mL (SD 3.74), 3.83 µg/mL (SD 3.46), and 3.45 µg/mL (SD 3.63) at 3, 6, 9, and 12 mo, respectively. At 3, 6, and 9 mo, the CT-P13 level was 0 µg/mL in those patients with detectable antibodies. At 12 mo, five (83.3%) patients with detectable antibodies had CT-P13 levels of 0 µg/mL, and one (26.7%) had a CT-P13 level of 2 µg/mL.
A total of 34 (15.45%) adverse events (AEs) were reported by 27 patients; of these, nine (26.5%) were serious. Of all the AEs reported, 19 (55.9%) were probably associated with treatment, five (14.7%) were possibly associated with treatment, one (2.9%) was probably not associated with treatment, and one (2.9%) was not related to treatment. Ten (29.4%) of the 34 adverse events reported required hospitalization (they were all flares of the disease). The most frequent AEs were due to infections (23.5%) (mainly urinary and upper respiratory tract) and mild acute infusion-related reactions (26.5%) (Table 5). No patient discontinued treatment due to an AE. No patient required surgery during the study.
In this real-life practice study, we analyzed the efficacy and safety of infliximab biosimilar, CT-P13 (Remsima®) for the treatment of patients with IBD. At 12 mo of treatment, the proportion of patients with active disease was only 14.8%. Similarly, the proportion of patients in clinical remission or with a clinical response was 85.2% after one year of treatment. Moreover, the decrease in the number of patients with active disease and the increase in clinical remission rates were already observed as early as after three months of treatment with CT-P13 (Remsima®). Thus, this biosimilar can be considered to have an acceptable safety profile, as no serious AEs proved to be treatment-related.
As this was a real-life practice study, not all patients had active disease when CT-P13 (Remsima®) was started. This is due to the fact that a percentage of patients had started other treatments (for example corticosteroids) to induce remission immediately before initiation of Remsima® (sometimes initiation is delayed due to lack of a Mantoux result for example). Another example of this situation would be a corticosteroid-dependent patient with no response to immunosuppressants starting infliximab who is probably in remission due to concomitant treatment with corticosteroids. It is for this reason that at baseline, only 68.6% of the patients had active disease and not 100%.
Remission rates in our study varied from 64.2% (observed at 12 mo) to 53% (at three months), which are similar to those reported in other studies (remission rates ranging from 32% to 56%)[26,36,40-46]. Some studies found much higher remission rates, such as the randomized NOR-SWITCH study, in which a 52-wk remission rate of 65% and 93% for CD and UC, respectively, was observed[35].
This study has the advantages of a real-life-practice study in that the patients were similar to those clinicians find in medical settings; thus, they are more heterogeneous and present with more comorbidities than the patients usually studied in clinical trials[47]. In real life, some hospitals change the brand name of CT-P13 (Remsima® for Inflectra® and vice versa), and this situation is reflected in our study. Similarly, this work shows the limitations of a real-life study: As the patients were not assigned beforehand to any therapeutic strategy, it makes the interpretation of results difficult. A reflection of this is the change in the CT-P13 brand that the patients take when they leave some hospitals. In this sense, it would have been interesting to compare results from patients who started treatment with CT-P13 (Remsima®) after switching from Remicade® with those who were naïve to biological treatment. However, the number of patients in each group was unequal — only 19 (9%) patients included in the study had switched from adalimumab, and 142 (65%) were naïve to biological treatment — thus making the comparison between groups difficult.
In 2018, Bergqvist et al[34] published a large series on switched IBD patients and demonstrated that Remicade® can be switched to CT-P13 with preserved therapeutic efficacy and safety in both CD and UC.
Another limitation was that available data were heterogeneous across visits; moreover, some parameters could not be included because they had not been measured in routine practice (for example endoscopy or ultrasound). CT-P13 has been shown to achieve mucosal healing both in patients with UC and in those with CD[48,49]. However, in our real-world study, not all patients were evaluated by endoscopy if mucosal healing was observed.
In a systematic review published in 2018, Gisbert and Chaparro pointed out, as a limitation of most of studies, that the effectiveness of CT-P13 had been evaluated through clinical assessments, or sometimes using additional biological parameters, although most authors did not perform endoscopic evaluations[10]. In our study, we used disease-specific indices (HBI and CDAI for CD, and PMS and Truelove for UC) and measured biological parameters indicative of inflammation, such as CRP, fecal calprotectin, and ESR.
Safety data with original and biosimilar infliximab have been variable, sometimes due to different definitions. Our finding of 15.45% for AEs is similar to that reported in other clinical practice series[50].
Finally, immunogenicity is also a main concern when switching patients from a reference product to its biosimilar. The development of antibodies against the drug is one of the main factors that can cause loss of response to a biological agent. We observed a correlation between the presence of anti-drug antibodies and low CT-P13 trough levels. In those patients with detectable anti-drug antibodies, drug levels were 0 µg/mL, with the exception of one patient. However, the CT-P13 trough level in this patient at 12 mo was lower than the mean level at that time point (2 µg/mL vs 3.63 µg/mL).
It is important to note that the physicians had no incentive to switch from original to biosimilar or to start a biosimilar in naive patients. In our area, physicians had total freedom of prescription in relation to reference biological drugs and biosimilars, although there was a guideline to start infliximab-naive patients with biosimilars.
In summary, CT-P13 (Remsima®) is an effective and safe biosimilar of infliximab for the treatment of CD and UC in real-life practice. It is a valid and attractive alternative for the treatment of IBD, as it reduces healthcare costs and facilitates access to biological treatments for more patients.
Authorization of infliximab biosimilars has revolutionized the treatment of inflammatory bowel disease (IBD), in terms of both clinical practice and cost reduction. Although several studies have evaluated the safety and efficacy of infliximab biosimilar, some have retrospective designs and small study populations.
There is a need to evaluate the effectiveness and safety of these drugs in daily clinical practice in patients with IBD.
The objective of the present study was to evaluate the efficacy and safety of infliximab biosimilar in real-world practice.
The authors performed a multicenter, observational, prospective study in Spanish hospitals based on the real-world clinical practice of the participating physicians.
After 12 mo of treatment, 64.2% (n = 52/81) of patients were in clinical remission without corticosteroids. After 3 mo, 75.5% (n = 115/152) had achieved a clinical response or clinical remission, which was sustained for 12 mo (85.2%; n = 69/81). There was a decrease in specific IBD indices at 3, 6, 9, and 12 mo (P < 0.001). A total of 34 adverse events were reported by 27 (12.3%) patients; of these, nine (26.5%) were serious.
CT-P13 (Remsima®) is an effective and safe infliximab biosimilar for the treatment of Crohn's disease and ulcerative colitis in real-life practice. It is a valid and attractive alternative for the treatment of IBD, as it reduces healthcare costs and facilitates access to biological treatments for more patients.
New studies will be necessary to provide more in-depth information on the man
Medical writing assistance was provided by Dr. Paloma Goñi Oliver and Dr. Pablo Rivas on behalf of Springer Healthcare Communications with the support of Kern Pharma Laboratories.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gazouli M, Parra RS, Sassaki LY, Wen XL S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Yu HG
| 1. | Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 2. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 950] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 3. | Keller R, Mazurak N, Fantasia L, Fusco S, Malek NP, Wehkamp J, Enck P, Klag T. Quality of life in inflammatory bowel diseases: it is not all about the bowel. Intest Res. 2021;19:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Parra RS, Chebli JMF, Amarante HMBS, Flores C, Parente JML, Ramos O, Fernandes M, Rocha JJR, Feitosa MR, Feres O, Scotton AS, Nones RB, Lima MM, Zaltman C, Goncalves CD, Guimaraes IM, Santana GO, Sassaki LY, Hossne RS, Bafutto M, Junior RLK, Faria MAG, Miszputen SJ, Gomes TNF, Catapani WR, Faria AA, Souza SCS, Caratin RF, Senra JT, Ferrari MLA. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J Gastroenterol. 2019;25:5862-5882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 5. | Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2744] [Article Influence: 119.3] [Reference Citation Analysis (2)] |
| 7. | Argollo M, Fiorino G, Hindryckx P, Peyrin-Biroulet L, Danese S. Novel therapeutic targets for inflammatory bowel disease. J Autoimmun. 2017;85:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, D'Haens G; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463-8; quiz e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 641] [Article Influence: 42.7] [Reference Citation Analysis (35)] |
| 9. | Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Gisbert JP, Chaparro M. Switching from an originator anti-TNF to a biosimilar in patients with inflammatory bowel disease: Can it be recommended? Gastroenterol Hepatol. 2018;41:389-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 423] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Kuek A, Hazleman BL, Ostör AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009;15:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 531] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 14. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-659, quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 15. | Annese V, Duricova D, Gower-Rousseau C, Jess T, Langholz E. Impact of New Treatments on Hospitalisation, Surgery, Infection, and Mortality in IBD: a Focus Paper by the Epidemiology Committee of ECCO. J Crohns Colitis. 2016;10:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Jha A, Upton A, Dunlop WC, Akehurst R. The Budget Impact of Biosimilar Infliximab (Remsima®) for the Treatment of Autoimmune Diseases in Five European Countries. Adv Ther. 2015;32:742-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Cohen RD, Thomas T. Economics of the use of biologics in the treatment of inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:867-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, Mahmmod N, van de Meeberg PC, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk CJ, Vermeijden JR, Siersema PD, van Oijen MG, Oldenburg B; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 19. | Scheinberg MA, Kay J. The advent of biosimilar therapies in rheumatology--"O brave new world". Nat Rev Rheumatol. 2012;8:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | World Health Organization. Expert Committee on Biological Standardization: guidelines on evaluation of similar biotherapeutic products (SBPs). [cited 31 August 2019] Available from: https://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.2009. |
| 21. | Danese S, Bonovas S, Peyrin-Biroulet L. Biosimilars in IBD: from theory to practice. Nat Rev Gastroenterol Hepatol. 2017;14:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Danese S, Fiorino G, Raine T, Ferrante M, Kemp K, Kierkus J, Lakatos PL, Mantzaris G, van der Woude J, Panes J, Peyrin-Biroulet L. ECCO Position Statement on the Use of Biosimilars for Inflammatory Bowel Disease-An Update. J Crohns Colitis. 2017;11:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 23. | Rodríguez Gonzalez GE, Arranz Hernández L, Vela González M, Rodríguez Días CY, Pérez Hernández F, Diáz Hernández L, Morales Barrios JA, Tardillo Marín CA, Hernández Camba A, Merino Alonso FJ, Viña Romero MM. P629 Efficacy, safety and economic impact of the switch to biosimilar of infliximab in inflammatory bowel disease patients in clinical practice: results of one year. J Crohn’s Colitis. 2017;11:S402-S402. [PubMed] [DOI] [Full Text] |
| 24. | Sieczkowska J, Jarzębicka D, Banaszkiewicz A, Plocek A, Gawronska A, Toporowska-Kowalska E, Oracz G, Meglicka M, Kierkus J. Switching Between Infliximab Originator and Biosimilar in Paediatric Patients with Inflammatory Bowel Disease. Preliminary Observations. J Crohns Colitis. 2016;10:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Park KY, Ko EJ, Kim BJ, Kim MN, Hong CK, Chang SE, Won CH, Lee YW. A multicenter, randomized, double-blind clinical study to evaluate the efficacy and safety of PP-501-B in correction of nasolabial folds. Dermatol Surg. 2015;41:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Jung YS, Park DI, Kim YH, Lee JH, Seo PJ, Cheon JH, Kang HW, Kim JW. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: A retrospective multicenter study. J Gastroenterol Hepatol. 2015;30:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Kang B, Lee Y, Lee K, Choi YO, Choe YH. Long-term Outcomes After Switching to CT-P13 in Pediatric-Onset Inflammatory Bowel Disease: A Single-Center Prospective Observational Study. Inflamm Bowel Dis. 2018;24:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Nugent S, Nugent M, Mullane D, Kelly C. P430 EirSwitch echoes of NorSwitch: switching biosimilar therapy in an IBD cohort an Irish experience. J Crohns Colitis 2017; 11(suppl_1): S295-S295. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Smits LJT, Grelack A, Derikx LAAP, de Jong DJ, van Esch AAJ, Boshuizen RS, Drenth JPH, Hoentjen F. Long-Term Clinical Outcomes After Switching from Remicade® to Biosimilar CT-P13 in Inflammatory Bowel Disease. Dig Dis Sci. 2017;62:3117-3122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Smits LJT, van Esch AAJ, Derikx LAAP, Boshuizen R, de Jong DJ, Drenth JPH, Hoentjen F. Drug Survival and Immunogenicity After Switching From Remicade to Biosimilar CT-P13 in Inflammatory Bowel Disease Patients: Two-year Follow-up of a Prospective Observational Cohort Study. Inflamm Bowel Dis. 2019;25:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, Vilches-Arenas A, Castro Laria L, Maldonado Pérez B, Chaaro Benallal D, Benítez Roldán A, Merino V, Ramirez G, Calleja-Hernández MA, Caunedo Álvarez A, Romero Gómez M. Switching from reference infliximab to CT-P13 in patients with inflammatory bowel disease: 12 mo results. Eur J Gastroenterol Hepatol. 2017;29:1290-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, Vilches-Arenas A, Castro Laria L, Maldonado Pérez B, Chaaro D, Benítez Roldán A, Merino V, Ramírez G, Caunedo Álvarez A, Romero Gómez M. Effectiveness and Safety of CT-P13 (Biosimilar Infliximab) in Patients with Inflammatory Bowel Disease in Real Life at 6 Months. Dig Dis Sci. 2017;62:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Kolar M, Duricova D, Bortlik M, Hruba V, Machkova N, Mitrova K, Malickova K, Lukas M Jr, Lukas M. Infliximab Biosimilar (Remsima™) in Therapy of Inflammatory Bowel Diseases Patients: Experience from One Tertiary Inflammatory Bowel Diseases Centre. Dig Dis. 2017;35:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Bergqvist V, Kadivar M, Molin D, Angelison L, Hammarlund P, Olin M, Torp J, Grip O, Nilson S, Hertervig E, Lillienau J, Marsal J. Switching from originator infliximab to the biosimilar CT-P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018;11:1756284818801244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, Lundin KEA, Mørk C, Jahnsen J, Kvien TK; NOR-SWITCH study group. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389:2304-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 593] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 36. | Ilias A, Gonczi L, Kurti Z, Lakatos PL. Biosimilars in ulcerative colitis: When and for who? Best Pract Res Clin Gastroenterol. 2018;32-33:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1434] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 38. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 863] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 39. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2328] [Article Influence: 122.5] [Reference Citation Analysis (2)] |
| 40. | Komaki Y, Yamada A, Komaki F, Micic D, Ido A, Sakuraba A. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:1043-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Fiorino G, Manetti N, Armuzzi A, Orlando A, Variola A, Bonovas S, Bossa F, Maconi G, DʼIncà R, Lionetti P, Cantoro L, Fries W, Annunziata ML, Costa F, Terpin MM, Biancone L, Cortelezzi CC, Amato A, Ardizzone S, Danese S, Guidi L, Rizzuto G, Massella A, Andriulli A, Massari A, Lorenzon G, Ghione S, Kohn A, Ventra A, Annese V; PROSIT-BIO Cohort. The PROSIT-BIO Cohort: A Prospective Observational Study of Patients with Inflammatory Bowel Disease Treated with Infliximab Biosimilar. Inflamm Bowel Dis. 2017;23:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 42. | Gonczi L, Gecse KB, Vegh Z, Kurti Z, Rutka M, Farkas K, Golovics PA, Lovasz BD, Banai J, Bene L, Gasztonyi B, Kristof T, Lakatos L, Miheller P, Nagy F, Palatka K, Papp M, Patai A, Salamon A, Szamosi T, Szepes Z, Toth GT, Vincze A, Szalay B, Molnar T, Lakatos PL. Long-term Efficacy, Safety, and Immunogenicity of Biosimilar Infliximab After One Year in a Prospective Nationwide Cohort. Inflamm Bowel Dis. 2017;23:1908-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 43. | Jahnsen J, Detlie TE, Vatn S, Ricanek P. Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: A Norwegian observational study. Expert Rev Gastroenterol Hepatol. 2015;9 Suppl 1:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Kaniewska M, Moniuszko A, Rydzewska G. The efficacy and safety of the biosimilar product (Inflectra®) compared to the reference drug (Remicade®) in rescue therapy in adult patients with ulcerative colitis. Prz Gastroenterol. 2017;12:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Keil R, Wasserbauer M, Zádorová Z, Hajer J, Drastich P, Wohl P, Beneš M, Bojková M, Svoboda P, Konečný M, Falt P, Vaňásek T, Pešta M, Pešek F, Bouchner L, Koželuhová J, Novotný A, Bartůsková L, Špičák J. Clinical monitoring: infliximab biosimilar CT-P13 in the treatment of Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 2016;51:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Park SH, Kim YH, Lee JH, Kwon HJ, Lee SH, Park DI, Kim HK, Cheon JH, Im JP, Kim YS, Lee SY, Lee SJ. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol. 2015;9 Suppl 1:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 47. | Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther. 2018;35:1763-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 482] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 48. | Farkas K, Rutka M, Golovics PA, Végh Z, Lovász BD, Nyári T, Gecse KB, Kolar M, Bortlik M, Duricova D, Machkova N, Hruba V, Lukas M, Mitrova K, Malickova K, Bálint A, Nagy F, Bor R, Milassin Á, Szepes Z, Palatka K, Lakatos PL, Molnár T. Efficacy of Infliximab Biosimilar CT-P13 Induction Therapy on Mucosal Healing in Ulcerative Colitis. J Crohns Colitis. 2016;10:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Tursi A, Mocci G, Faggiani R, Allegretta L, Valle ND, Forti G, Franceschi M, Ferronato A, Gallina S, Larussa T, Luzza F, Lorenzetti R, Penna A, Rodino S, Sebkova L, Lauria A, Piergallini S, Pranzo G, Ricciardelli C, Zampaletta C, Elisei W, Picchio M. Infliximab biosimilar CT-P13 is effective and safe in treating inflammatory bowel diseases: a real-life multicenter, observational study in Italian primary inflammatory bowel disease centers. Ann Gastroenterol. 2019;32:392-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Ye BD, Pesegova M, Alexeeva O, Osipenko M, Lahat A, Dorofeyev A, Fishman S, Levchenko O, Cheon JH, Scribano ML, Mateescu RB, Lee KM, Eun CS, Lee SJ, Lee SY, Kim H, Schreiber S, Fowler H, Cheung R, Kim YH. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn's disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393:1699-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |