Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11228
Peer-review started: June 21, 2021
First decision: September 28, 2021
Revised: September 29, 2021
Accepted: November 14, 2021
Article in press: November 14, 2021
Published online: December 26, 2021
Processing time: 185 Days and 4.1 Hours
The clinical manifestations of omphalomesenteric duct remnant (OMDR) can vary with the age at diagnosis, from asymptomatic incidental findings to symptoms related to gastrointestinal complications. The lifelong complication rates are reported as 4%-34%, and complications are more common in patients younger than 2 years of age. The authors attempted to identify different clinical features and management for the various pediatric age groups.
To find surgical perspectives for the pediatric age-related variants of OMDR and make recommendations for optimal management.
The medical records of pediatric patients diagnosed with OMDR were reviewed retrospectively. Fifteen patients diagnosed based on incidental findings during other surgeries were excluded. The patients were divided into two groups based on age: < 12 mo (infants) and > 12 mo (beyond infancy). We analyzed the demographic characteristics, clinical manifestations, diagnostic tools, surgical procedures, and clinical outcomes of the patients and compared them for the age groups. Chi-squared and Fisher's exact tests were used for nominal scales and a Mann-Whitney test was used for ratio scales.
A total of 35 patients (7 infants, 28 children beyond infancy) were finally included. In both groups, Meckel's diverticulum (MD) was the most common type of OMDR, while umbilical lesions were more common in the infant group (P = 0.006). Hematochezia and abdominal pain were common in the beyond infancy group, while umbilical lesions were the most frequent symptoms in the infant group. Several diagnostic tools were used, but Meckel's scan was most useful in diagnosing OMDR in patients with painless rectal bleeding. Minimally invasive surgery was more commonly performed for children than for infants (P = 0.016). Single-incision laparoscopic surgery (SILS) was performed for fifteen patients who underwent laparoscopic surgery. There were only three cases of postoperative complications, and all patients survived in good condition.
The clinical type of OMDR varies with age, umbilical lesions in infants, and MD beyond infancy. SILS is effective for managing children with MD regardless of age.
Core Tip: This is a retrospective study aimed at identifying surgical perspectives for variants of the omphalomesenteric duct remnant (OMDR) manifesting in different age groups. Meckel's diverticulum was the most common type of OMDR in all the subjects, while umbilical lesions were more common in the infant group. For management, minimally invasive surgery was more common beyond infancy, and single-incision laparoscopic surgery may be considered the preferred surgical procedure.
- Citation: Kang A, Kim SH, Cho YH, Kim HY. Surgical perspectives of symptomatic omphalomesenteric duct remnants: Differences between infancy and beyond. World J Clin Cases 2021; 9(36): 11228-11236
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11228.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11228
The omphalomesenteric duct is an embryologic connection between the yolk sac and primitive midgut. It normally disappears between the fifth and seventh weeks of fetal life[1,2], failure of this process results in various anomalies collectively called the omphalomesenteric duct remnant (OMDR), including Meckel's diverticulum (MD), umbilical fistula, sinus, polyp, and omphalomesenteric cysts[1-3]. The most common type of OMDR is MD (67%), which is also one of the most common congenital anomalies of the digestive tract in children (2%-4%)[1-4].
The clinical manifestations of OMDR can vary with age at diagnosis, from asymptomatic incidental findings to symptoms related to gastrointestinal complications[1-3]. Lifelong complication rates are reported as 4%-34%, and complications are more common in patients younger than 2 years of age[5,6].
This study aimed to identify surgical perspectives of OMDR by assessing its variants in different age groups (infants and children beyond infancy). The findings of this study may also provide useful information for the optimal management.
We retrospectively reviewed the medical records of pediatric patients aged < 18 years old with a histopathological diagnosis of OMDR after surgery who were treated in Pusan National University Children’s Hospital between November 2008 and December 2019. Fifty patients were histologically diagnosed with OMDR. We excluded 15 patients diagnosed with OMDR incidentally during other surgeries.
The patients were divided into two groups based on the age at surgery: Infants (< 12 month old) and children beyond infancy (> 12 month old). A total of 35 patients (7 infants and 28 children beyond infancy) were finally included.
We analyzed the data of the following variables: Sex, type of delivery, weight at birth and surgery, gestational age, and age at symptom onset, diagnosis, and surgical procedure. In addition, we investigated the symptom durations, associated anomalies, type of OMDR (MD, patent duct, umbilical polyp, duct cyst, and fibrous cord), clinical manifestations, diagnostic tools and sensitivity, surgical procedures performed, complications, and the final outcomes. We used medians with standard deviations (SDs) for representative values for ratio scales. Pain was measured using the Face-Legs-Activity-Cry-Consolability scale for the patients aged < 3 years old and the Wong-Baker Faces Pain scales for the older children; based on the instruction for each scale, scores of ≥ 1 were interpreted as abdominal pain[7,8].
All analyses were completed using IBM SPSS Statistics version 25 (SPSS Inc, Chicago, IL, United States). Chi-squared and Fisher's exact tests were used for nominal scales and a Mann-Whitney test was used for ratio scales. A P < 0.05 was considered statistically significant.
This study was approved by the Pusan National University Yangsan Hospital Institutional Review Board (IRB No. 05-2020-111) and carried out according to the recommendations of the IRB committee.
The subjects comprised 27 males and 8 females (male:female ratio = 3.4:1). There were no significant differences in delivery, birth weight, and symptomatic duration among the age groups (Table 1).
| Total (n = 35)1 | Infants (n = 7)1 | Children beyond infancy (n = 28)1 | P value | |
| Gender | 0.180 | |||
| Male | 27 (77.1) | 4 (57.1) | 23 (82.1) | |
| Female | 8 (22.9) | 3 (42.9) | 5 (17.9) | |
| Male:female ratio | 3.4:1 | 1.3:1 | 4.6:1 | |
| Delivery | 0.170 | |||
| NSVD | 22 (62.9) | 6 (85.7) | 16 (57.1) | |
| Caesarean section | 13 (37.1) | 1 (14.3) | 12 (42.9) | |
| Preterm | 0.171 | |||
| Preterm | 4 (11.4) | 2 (28.6) | 2 (7.1) | |
| Full term | 31 (88.6) | 5 (71.4) | 26 (92.9) | |
| Weight (kg) | ||||
| Birth | 3.40 ± 0.51 | 3.18 ± 0.34 | 3.41 ± 0.55 | 0.825 |
| (2.23–4.30) | (2.90–3.73) | (2.23–4.30) | ||
| Operation | 18.40 ± 21.53 | 6.64 ± 2.17 | 22.55 ± 21.39 | N/A |
| (2.95–77.45) | (2.95–8.85) | (7.25–77.45) | ||
| Age (mo) | N/A | |||
| Symptom onset | 53.0 ± 67.3 | 2.3 ± 4.2 | 85.4 ± 65.1 | |
| (0–201) | (0–11) | (0–201) | ||
| Diagnosis | 53.2 ± 65.9 | 2.4 ± 4.2 | 85.6 ± 62.5 | |
| (0–202) | (0–11) | (12–202) | ||
| Operation | 53.3 ± 65.9 | 2.6 ± 4.2 | 85.8 ± 62.5 | |
| (0–202) | (0–11) | (12–202) | ||
| Symptomatic duration (d) | 3.0 ± 292.0 | 7.0 ± 3.9 | 3.0 ± 325.1 | 0.246 |
| (1–1540) | (2–12) | (1–1540) |
In both groups, MD was the most frequent type of OMDR. While the infant group had other types of OMDR, most cases in the beyond infancy group were MD (P = 0.006). Hematochezia and abdominal pain were common in the beyond infancy group, while umbilical lesions were the most frequent in the infant group. Gastric-type ectopic mucosa was most common in the pathologic reports, but the presence of ectopic mucosa was not reported for several cases (Table 2).
| Total (n = 35)1 | Infant (n = 7)1 | Children beyond infancy (n = 28)1 | P value | |
| Clinical type | 0.006 | |||
| MD | 31 (88.6) | 4 (57.1) | 27 (96.4) | |
| Patent duct | 2 (5.7) | 2 (28.6) | 0 | |
| Duct cyst | 2 (5.7) | 1 (14.3) | 1 (3.6) | |
| Umbilical polyp | 0 | 0 | 0 | |
| Fibrous cord | 0 | 0 | 0 | |
| Clinical features2 | ||||
| Hematochezia | 20 (57.1) | 2 (28.6) | 18 (64.3) | 0.101 |
| Abdominal pain | 12 (34.3) | 0 | 12 (42.9) | 0.036 |
| Fever | 11 (31.4) | 2 (28.6) | 9 (32.1) | 0.619 |
| Bilous vomiting | 8 (22.9) | 1 (14.3) | 7 (25.0) | 0.484 |
| Umbilical lesion | 7 (20.0) | 4 (57.1) | 3 (10.7) | 0.018 |
| Abdominal distension | 4 (11.4) | 1 (14.3) | 3 (10.7) | 0.609 |
| Diarrhea | 3 (8.6) | 1 (14.3) | 2 (7.1) | 0.499 |
| Ectopic mucosa (in MD) | 0.283 | |||
| Gastric | 13 (41.9) | 0 | 13 (48.1) | |
| Pancreatic | 3 (9.7) | 1 (25.0) | 2 (7.4) | |
| Coexistent | 2 (6.5) | 0 | 2 (7.4) | |
| Absent | 1 (3.2) | 0 | 1 (3.7) | |
| Not mentioned | 12 (38.7) | 3 (75.0) | 9 (33.3) |
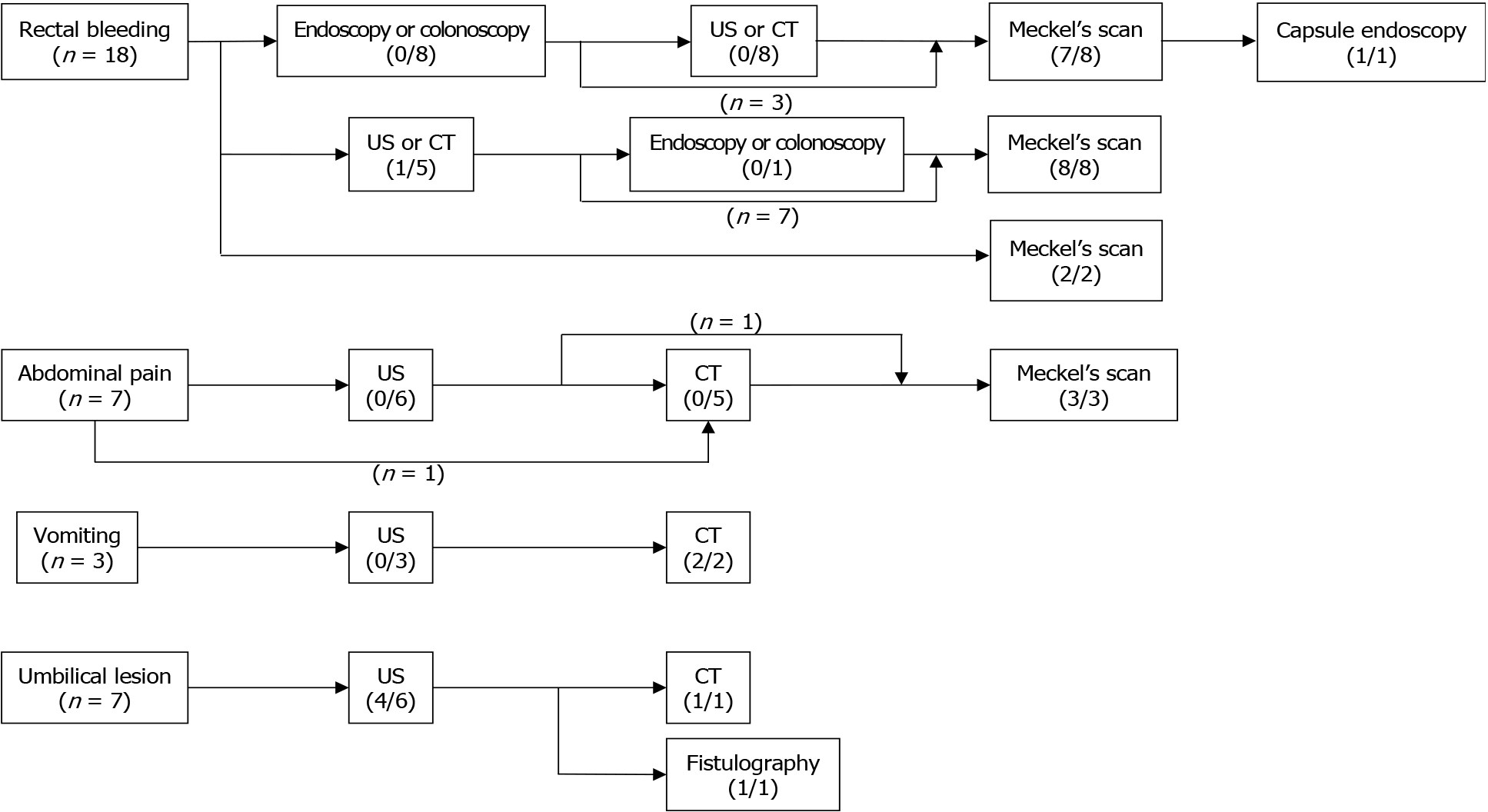
The diagnoses were based on the chief complaint (CCs) of the patients. The CCs of our patients were classified into four features: Painless rectal bleeding, abdominal pain, vomiting, and umbilical lesions. Patients with rectal bleeding underwent various diagnostic modalities, including abdominal ultrasonography (US), computed tomography (CT), endoscopy, or colonoscopy; most of these failed to show the bleeding focus. Technetium-99m pertechnetate scintigraphy, known as Meckel’s scan (MS), helped diagnose these patients with OMDR. For abdominal pain, US and CT were performed first. A MS was performed in patients with rectal bleeding-related conditions, such as anemia or melena. US was first performed for obstruction symptoms such as vomiting, but it could not be used to diagnose OMDR. Two of 3 patients with vomiting had CT findings of MD. The other patient underwent emergency operation for reduction-failed intussusception, and MD was found as the leading point of intussusception. Umbilical lesions can be diagnosed as OMDR based on US only. One child with umbilical discharge underwent only physical examinations before surgery. Figure 1 shows a flowchart for the diagnostic process of OMDR according to the CCs of patients.
The median operation time was not significantly different between age groups. The most common surgical procedure was segmental resection of the small bowel (24 patients, 68.6%). There were no significant differences between the surgical procedure for the groups. Minimally invasive surgery (MIS) was more commonly performed for children than for infants (P = 0.016). Fifteen patients who underwent laparoscopic surgery underwent a single-incision laparoscopic surgery (SILS) (Table 3).
| Total (n = 35)1 | Infant (n = 7)1 | Children beyond infancy (n = 28)1 | P value | |
| Time (min) | 80.0 ± 34.1 (15-205) | 78.0 ± 25.7 (45-120) | 80.0 ± 35.3 (15-205) | 0.171 |
| Operative method | 0.278 | |||
| Segmental resection of small bowel | 24 (68.6) | 4 (57.1) | 20 (71.4) | |
| Diverticulectomy | 7 (20.0) | 1 (14.3) | 6 (21.4) | |
| OMDR resection only | 4 (11.4) | 2 (28.6) | 2 (7.1) | |
| Minimal invasive surgery | 0.016 | |||
| Open | 15 (42.9) | 6 (85.7) | 9 (32.1) | |
| Laparoscopic | 20 (57.1) | 1 (14.3) | 19 (67.9) | |
| Single port (SILS) | 15 (42.9) | 1 (14.3) | 14 (50.0) | |
| Multiport | 5 (14.3) | 0 | 5 (17.9) |
The durations of hospitalization were similar for both groups. Complications were rare in both groups. One patient in the infant group, who underwent open segmental resection of the small bowel for an omphalomesenteric fistula, had a complication of superficial wound infection on the umbilicus. Two children in the beyond infancy group had a small bowel obstruction and pseudomembranous colitis, respectively. All patients survived and were discharged in good condition after surgery.
Fifteen cases were excluded due to incidental findings during other surgeries because their clinical course may have been affected by the underlying disease and not OMDR. The underlying problems of these patients included gastrointestinal (jejunal atresia, anorectal malformation, and Hirschsprung’s disease) and cardiovascular (coarctation of aorta with ventricular septum defect and total anomalous pulmonary venous connection) anomalies and other gastrointestinal diseases (acute appendicitis, small bowel obstruction, gastric polyp, hiatal hernia, and intussusception).
Previous investigators found that more than 50%-60% of the patients with symptoms were younger than 2 years of age[5,6,9]. Based on the previous studies, we categorized our patients by age into the infancy and beyond infancy groups. There were various types of OMDR in the infants, while MD was the most common clinical type in the children beyond infancy. Some previous studies reported that umbilical abnormalities associated with OMDR are usually present in infants[3,10-12]. In this study, three children in the beyond infancy group with umbilical lesions had symptoms onset during their infancy. Umbilical lesions developing after one year of age may have other diagnoses rather than OMDR. It is necessary to consider the age of diagnosis as well as the age of symptom onset for children with umbilical lesions. US also may be helpful for OMDR with umbilical lesions for the differential diagnosis of other umbilical lesions (umbilical granuloma, polyp, or urachal remnant), evaluation of connection with the bowel, and planning for surgical excision[1].
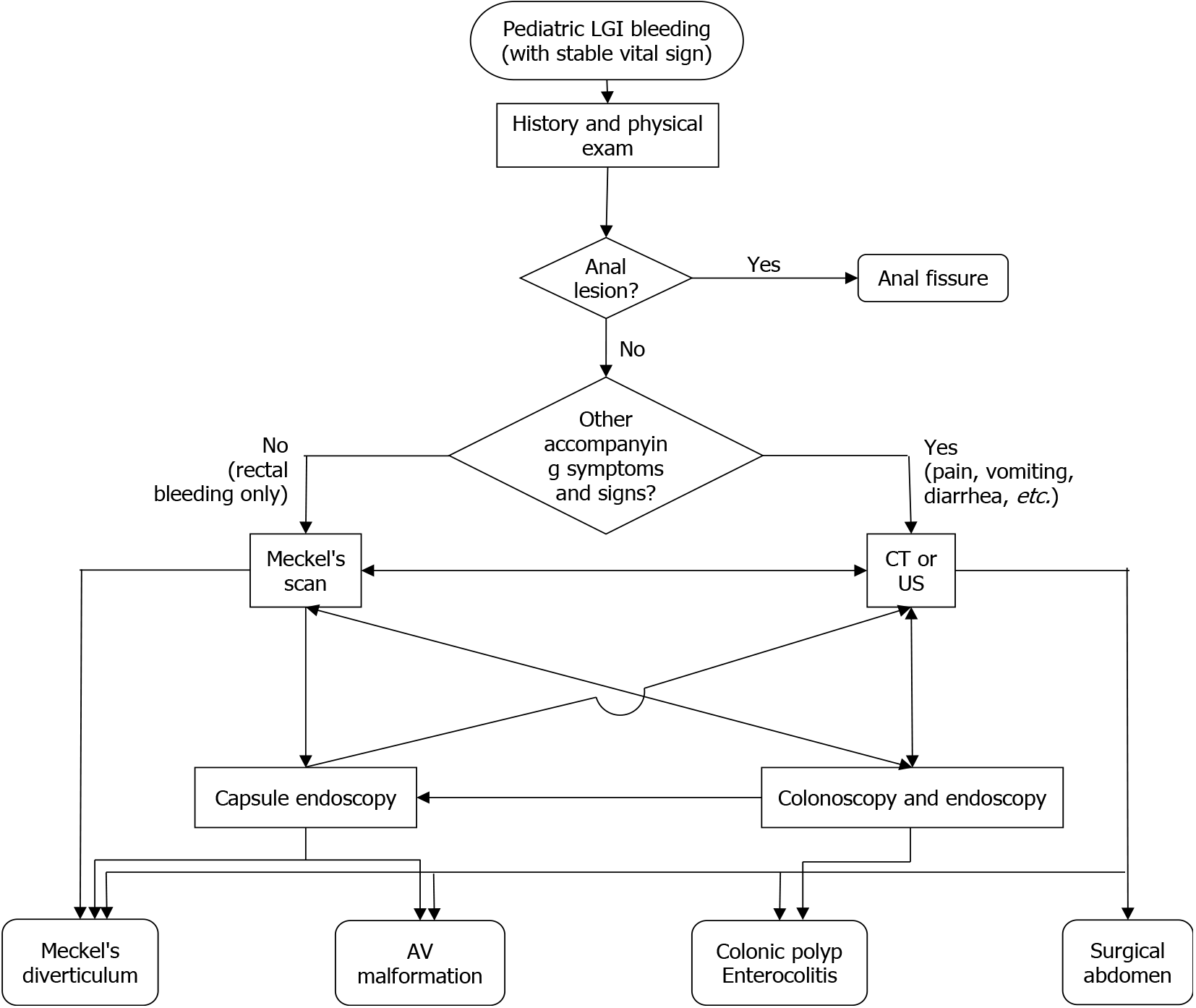
Besides umbilical lesions, the other main symptoms of OMDR included painless rectal bleeding, abdominal pain, and vomiting. Diagnostic tools were selected case by case according to the CCs. On evaluating the diagnosis process for patients in our study, MS was identified as the most useful modality for the diagnosis of OMDR. CT, endoscopy, and colonoscopy failed to detect OMDR. US may be helpful in infants, but it did not yield satisfactory results in this study, except for umbilical lesions. As described in previous studies, imaging studies such as CT or US, may help detect associated features such as bowel obstruction or perforation, but can seldom be used to locate the OMDR lesion itself[1-3,11]. The common causes of painless rectal bleeding in children aged > 12 month old include anal fissures, juvenile polyps, and MD. It is highly recommended that MD should be considered as the cause of pediatric lower gastrointestinal bleeding[13,14]. MS is a noninvasive diagnostic tool with high sensitivity for MD[1-4,11,15]. Its sensitivity in detecting MD is reported to be between 50% and 100% with an increased rate if bleeding is a presenting symptom[3]. In this study, MS had a high positive predictive value for the diagnosis of MD. Therefore, we recommend MS as the first diagnostic tool for prompt management of rectal bleeding in children. Symptoms such as abdominal pain or vomiting are nonspecific, as they can be associated with various diseases as gastroenteritis, intussusception, and other surgical abdomen. Several patients with CCs of abdominal pain and associated features of anemia or melena were finally diagnosed as OMDR using MS. Therefore, MS should be performed before other modalities such as colonoscopy or CT if the history and clinical features are suspicious of OMDR. Figure 2 shows an example of the evaluation process for pediatric lower gastrointestinal bleeding.
There was only one patient with negative findings on MS who was finally diagnosed with MD by capsule endoscopy. MS may have been negative if there was no ectopic mucosa in the lesion, but the gastric glandular epithelium was highlighted in the histopathologic report of this patient. We could not determine why MS revealed negative findings here.
Recently, MIS has become popular for the surgical treatment of OMDR. Several previous studies had demonstrated the advantages of MIS: Diagnosis without delay, fast recovery, and fewer complications[2,16,17]. In pediatric patients, especially small infants, there are restrictions for manipulating laparoscopic instruments due to the limited working space. Therefore, we prefer SILS with extracorporeal bowel resection to totally laparoscopic surgery using staplers. SILS also has the advantage of being able to directly modulate the lesion and achieve complete resection[17]. Our previous study showed that most lesions of the small bowel in pediatric patients can be managed with SILS, especially for MD and small bowel masses causing intussusception[18]. Patients in this study, especially those in the beyond infancy group, also had good outcomes of MIS. We, therefore, suggest MIS, especially SILS, as the first choice for the surgical treatment of OMDR.
This study has some limitations. It was a single-center retrospective observational study, and the sample of subjects was small. Further prospective multicenter studies with a large sample of patients will be needed.
We propose a work up protocol for the early diagnosis of OMDR and ruling out of other causes. Some highlights of the protocol are as follows. For pediatric massive rectal bleeding, MS should be the first diagnostic modality rather than US, CT, or endoscopy. For umbilical lesions in infants, US should be performed. This will be helpful for the rapid diagnosis and management of pediatric OMDR.
The most common type of OMDR differs among age groups; umbilical lesions are common in infancy, while MD is common in children with symptoms beyond infancy. US will be helpful for the differential diagnosis OMDR of with umbilical lesions. MS, a less invasive and accurate modality for OMDR, is recommended for patients with painless rectal bleeding for diagnosing MD. MIS, especially a SILS, is effective for managing children with MD regardless of age.
The omphalomesenteric duct remnant (OMDR) shows variable clinical manifestations according to the age at diagnosis with a lifelong complication rates of 4-34 which more common in patients younger than 2 years of age.
Identify the distinct clinical features and its management according to different age groups.
Variant clinical types of OMDR according to pediatric age were assessed and its results were analyzed, then tried to suggest a useful information for proper management by figuring out surgical perspectives.
A total of 35 patients (7 infants, 28 children beyond infancy) were reviewed. The patients were divided into two groups, infant and beyond infancy. The patients’ demographic characteristics, clinical manifestations, diagnostic tools, surgical procedures, and clinical outcomes were compared between two groups.
There were two different clinical patterns, Meckel's diverticulum (MD) was the most common clinical type and umbilical lesions were significantly common in the infant group (P = 0.006). Umbilical lesions were the most commonly presented symptom in infants, whereas hematochezia and abdominal pain were beyond infancy group. Meckel's scan was most useful in diagnosing OMDR in patients with painless rectal bleeding. Minimally invasive surgery (MIS), especially a single-incision laparoscopic surgery (SILS), was performed more frequently in children than infants (P = 0.016).
Considering the different clinical types, umbilical lesions in infants and MD beyond infancy, a MIS is effective for managing MD regardless of age.
SILS could be considered as a preferred method for managing OMDR regardless of age.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shi H S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Bagade S, Khanna G. Imaging of omphalomesenteric duct remnants and related pathologies in children. Curr Probl Diagn Radiol. 2015;44:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Chen Q, Gao Z, Zhang L, Zhang Y, Pan T, Cai D, Xiong Q, Shu Q, Qian Y. Multifaceted behavior of Meckel's diverticulum in children. J Pediatr Surg. 2018;53:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Durakbasa CU, Okur H, Mutus HM, Bas A, Ozen MA, Sehiralti V, Tosyali AN, Zemheri IE. Symptomatic omphalomesenteric duct remnants in children. Pediatr Int. 2010;52:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Pepper VK, Stanfill AB, Pearl RH. Diagnosis and management of pediatric appendicitis, intussusception, and Meckel diverticulum. Surg Clin North Am. 2012;92:505-526, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Blevrakis E, Partalis N, Seremeti C, Sakellaris G. Meckel's diverticulum in paediatric practice on Crete (Greece): a 10-year review. Afr J Paediatr Surg. 2011;8:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Onen A, Ciğdem MK, Oztürk H, Otçu S, Dokucu AI. When to resect and when not to resect an asymptomatic Meckel's diverticulum: an ongoing challenge. Pediatr Surg Int. 2003;19:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293-297. [PubMed] |
| 8. | Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17. [PubMed] |
| 9. | St-Vil D, Brandt ML, Panic S, Bensoussan AL, Blanchard H. Meckel's diverticulum in children: a 20-year review. J Pediatr Surg. 1991;26:1289-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 127] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Das A. Umbilical Lesions: A Cluster of Known Unknowns and Unknown Unknowns. Cureus. 2019;11:e5309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Hansen CC, Søreide K. Systematic review of epidemiology, presentation, and management of Meckel's diverticulum in the 21st century. Medicine (Baltimore). 2018;97:e12154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 12. | Heymann WR. Contemplating the navel: Omphalomesenteric duct remnant disorders. J Am Acad Dermatol. 2019;81:1072-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Boyle JT. Gastrointestinal bleeding in infants and children. Pediatr Rev. 2008;29:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Romano C, Oliva S, Martellossi S, Miele E, Arrigo S, Graziani MG, Cardile S, Gaiani F, de'Angelis GL, Torroni F. Pediatric gastrointestinal bleeding: Perspectives from the Italian Society of Pediatric Gastroenterology. World J Gastroenterol. 2017;23:1328-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (4)] |
| 15. | Irvine I, Doherty A, Hayes R. Bleeding meckel's diverticulum: A study of the accuracy of pertechnetate scintigraphy as a diagnostic tool. Eur J Radiol. 2017;96:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Chan KW, Lee KH, Wong HY, Tsui SY, Wong YS, Pang KY, Mou JW, Tam YH. Laparoscopic excision of Meckel's diverticulum in children: what is the current evidence? World J Gastroenterol. 2014;20:15158-15162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Duan X, Ye G, Bian H, Yang J, Zheng K, Liang C, Sun X, Yan X, Yang H, Wang X, Ma J. Laparoscopic vs. laparoscopically assisted management of Meckel's diverticulum in children. Int J Clin Exp Med. 2015;8:94-100. [PubMed] |
| 18. | Lee KH, Kim SH, Cho YH, Kim HY. Efficacy and safety of single-site umbilical laparoscopic surgery for small bowel resection in pediatric patients. Adv Pediatr Surg. 2018;24:44-50. [DOI] [Full Text] |