Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11208
Peer-review started: May 16, 2021
First decision: June 15, 2021
Revised: June 28, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: December 26, 2021
Processing time: 220 Days and 23.5 Hours
Frailty is prevalent in elderly patients with cardiovascular diseases. However, the association between frailty and in-hospital outcomes for elderly patients with heart failure and reduced ejection (HFrEF) remains unknown.
To evaluate the predictive efficacy of frailty, compared with pre-frailty, for adverse events in these patients.
Elderly patients (≥ 60 years) with HFrEF were assessed. Frailty was evaluated with the Fried phenotype criteria, and physical performance was evaluated based on handgrip strength and the short physical performance battery (SPPB). The composite incidence of adverse events, including all-cause death, multiple organ failure, cardiac shock, and malignant arrhythmia, during hospitalization was recorded.
Overall, 252 elderly individuals with HFrEF [mean age: 69.4 ± 6.7 years, male: 169 (67.0%)] were included. One hundred and thirty-five (53.6%) patients were frail and 93 (36.9%) were pre-frail. Frail patients were older, more likely to be female, to have a lower blood pressure, and to present with left ventricular thrombosis (P all < 0.05). Frail patients with HFrEF had a higher incidence of in-hospital mortality (11.9% vs 4.3%, P = 0.048). Multivariate analyses showed that female gender (OR = 0.422), aging (OR = 1.090), poor cardiac functional class (OR = 2.167), frailty (OR = 2.379), and lower handgrip strength (OR = 1.106) were independent predictors of in-hospital adverse events (P all < 0.05).
Frailty may be associated with poor in-hospital outcomes for elderly patients with HFrEF. The influence of frailty on long-term prognosis in these patients deserves further investigation.
Core Tip: This retrospective study included 252 elderly individuals with heart failure and reduced ejection who showed a high prevalence of frailty (53.6%) and pre-frailty (36.9%). Female gender, aging, poor cardiac functional class, frailty, and lower handgrip strength were found to be independent predictors of in-hospital adverse events in these patients. Taken together, frailty is prevalent in elderly individuals with heart failure and reduced ejection, and frailty may be associated with poor in-hospital outcomes for these patients.
- Citation: Kang YP, Chen LY, Zhu JJ, Liu WX, Ma CS. Association of frailty with in-hospital outcomes in elderly patients with heart failure. World J Clin Cases 2021; 9(36): 11208-11219
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11208.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11208
Heart failure (HF) is a clinical syndrome that presents as the end stage of various cardiovascular diseases[1], and the incidence has increased in recent decades[2-4]. Despite significant improvements in the management of patients with HF, this disease, particularly HF with reduced ejection fraction (HFrEF), remains one of the leading causes of morbidity and mortality for people all over the world[5]. Accumulating evidence suggests that aging is an important risk factor for the development of HF[6]. Many mechanisms have been proposed to relate aging with the development of HF, among which frailty is suggested to play an important role[7,8]. Frailty, defined as a clinical syndrome characterized by reduced functional capacity, multiple system disorder, impaired regulatory efficacy for homeostasis, and increased vulnerability to stimuli, becomes more prevalent with age[7,9]. People with frailty are vulnerable to diseases and frailty has been associated with increased mortality[10]. Interestingly, previous studies also showed that frailty is prevalent in patients with cardiovascular diseases, including those with ischemic heart disease, atrial fibrillation (AF), and HF[11,12]. Additionally, previous studies showed that frailty is associated with poor prognosis in patients with HF[13]. A previous meta-analysis with 20 observational studies showed that frailty significantly increased the risk of all-cause mortality and hospitalization in HF patients[14]. However, whether frailty, compared to pre-frailty, in elderly patients with HFrEF is also related to an increased risk of adverse events during hospitalization remains unknown[15]. Therefore, we aimed to determine the differences in the clinical characteristics and clinical implications of frailty and pre-frailty in elderly patients with HFrEF in our center.
Elderly patients with a confirmed diagnosis of HFrEF who were admitted to the Department of Cardiac Care Unit at the Beijing Anzhen Hospital for HF decompensation between January 1st, 2014 and December 30th, 2019 were included. The protocol for the study was approved by the Ethics Committee of Beijing Anzhen Hospital before the initiation of the study. Due to the retrospective nature of this study, the requirement for informed consent was waived.
Patients were included if they met all of the following criteria: (1) Age ≥ 60 years; (2) Ambulant patients with a confirmed diagnosis of HFrEF according to the criteria of the 2016 European Society of Cardiology Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure (with symptoms and signs of HF and LVEF < 40%)[16]; and (3) Complete data on the clinical characteristics, treatment details, and outcome records during hospitalization. All of the included patients were Chinese and the enrolment of patients started in 2014. According to the Chinese laws on the protection of the rights and interests of the elderly population, 60 is defined as the cut-off age for elderly individuals. Accordingly, we used 60 years-old as the cut-off to define elderly patients rather than 75 years-old in this study. The following exclusion criteria were applied: (1) Patients with difficulties in language communication, cognitive impairment, or mental disorders that prevented the comprehensive evaluation of frailty status and physical performance; (2) Long-term bedridden individuals with severe musculoskeletal or neurological diseases that prevented standing or walking; (3) Incomplete clinical or physical performance assessment data; and (4) Patients with unstable hemodynamic parameters, unstable vital signs, or other conditions that prevented the assessment of frailty or physical performance.
Data regarding the demographic and clinical characteristics of the included patients at admission were extracted from their medical records, including age, gender, systolic and diastolic blood pressure (SBP and DBP), resting heart rate (HR), and the New York Heart Association (NYHA) functional classification. The medical histories of the patients including hypertension, diabetes mellitus (DM), dyslipidemia, AF, peripheral artery disease (PAD), and previous coronary percutaneous intervention (PCI) and coronary artery bypass grafting (CABG) procedures were also recorded. Serum biochemical parameters were obtained on the second day of admission from blood samples collected under a fasting condition. The following parameters were extracted: alanine aminotransferase (ALT), serum creatinine (SCr), fasting plasma glucose (FPG), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and type B natriuretic peptide (BNP). Blood gas analysis (BGA) was performed for each patient on admission and the partial pressure of oxygen (PaO2) was extracted. On admission, echocardiography was performed for each patient by an experienced physician to evaluate cardiac structure and function. The left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic dimension (LVESD), LVEF, and the presence of left ventricular (LV) thrombosis were also recorded. All of the patients received evidenced-based medications for HF, which included diuretics, digitalis, beta-blockers, angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), and aldosterone receptor antagonists (ARAs)[16].
A strict evaluation system has been applied for a long time for stage 1 cardiac rehabilitation of patients with HF at the CCU at our institution. Patients who undergo rehabilitation assessment and training must meet the following conditions: (1) Resting heart rate < 120 beats/min; (2) Resting respiratory rate < 30 breaths/min; (3) SpO2 ≥ 90%; (4) Systolic blood pressure (SBP) < 180 mmHg, diastolic blood pressure (DBP) < 110 mmHg; (5) Weight change within 72 h less than ± 1.8 kg; (6) Random blood glucose within 5-18 mmol/L; (6) No evidence of new-onset ischemia on the electrocardiogram at rest; (7) No angina attack; (8) No malignant arrhythmia leading to hemodynamic instability; (9) No shock; (10) No acute heart failure due to severe valve disease; (11) No neurological and motor system diseases; and (12) Patients agreed to be evaluated. The assessment was performed on the basis of sufficient treatment and on patients in a stable condition.
Frailty was evaluated within 72 h after admission when the patients were confirmed to be hemodynamically stable by the physicians. In order to reduce bias and ensure consistency during the evaluation, the frailty and physical performance of all included patients were evaluated by the same clinical rehabilitation specialist, who had been trained before the assessment.
The frailty phenotype proposed by Fried et al[17] was used to evaluate the frailty status of the included patients with HFrEF as previously described. Briefly, this tool includes the following five components for evaluating the frailty status of an individual[17]: (1) Underweight or sarcopenia: defined as weight loss of ≥ 4.5 kg in the past 12 mo; (2) Reduced handgrip strength: defined as handgrip strength < 20% of the average value for the population with the same age, sex, and body mass index (BMI) and assessed according to the reference range for reduced grip strength in different genders and BMIs in the Fried frailty assessment table; (3) Reduced exercise capacity: defined as self-reported symptoms of fatigue; (4) Reduced gait speed: assessed based on the 4.5 m walking time for people of different genders and heights listed in the Fried frailty assessment table; and (5) Low physical activity: defined as low daily consumption of calories (< 20% of the average level), < 383 kcals/wk for men and < 270 kcals/wk for women. The patients were categorized as frail if they fulfilled ≥ three of the components and as pre-frail if they satisfied one or two components[17]. Patients were considered non-frail if they fulfilled none of the components[17].
For the physical performance assessment, the International Physical Activity Questionnaire (IPAQ), the handgrip strength test, and the short physical performance battery (SPPB) were used.
The IPAQ evaluates the degree of self-reported physical activity decline via a questionnaire with good reliability and validity[18]. The weekly caloric consumption of patients was evaluated according to their physical activity status within one week before admission. The data were collected by a trained cardiologist using the IPAQ short questionnaire, and the BMI and body surface area were calculated at the same time. The contents of the IPAQ short questionnaire included exercise intensity, exercise form, and exercise time within 7 d. Exercise intensity was determined according to the patient’s responses and expressed as the metabolic equivalent (MetS, 1 Mets = 1.05 kcal/kg per hour). The energy consumption for physical activity in 7 d was estimated.
The CAMRY electronic grip strength meter was used for the measurement of handgrip power and the recommended method in the "Chinese National Physique Measurement Standard Manual" was adopted. Briefly, the patients remained in a standing position with both feet together and the arms drooping vertically. During the measurement, the grip force meter did not contact the body or clothes and the patients were not allowed to bend the arm or bend down when exerting force. Both hands were tested twice and the maximum value was recorded.
The SPPB includes three tests, namely a balance test, a strength test (chair standing test), and a movement test (4-m gait test)[19]. The balance test was conducted during the first phase and the second phase consisted of a 4-m walking speed test. The time required to walk at a normal walking speed for 4 min was recorded. In the third phase, the chair standing test was conducted. The participants crossed their arms in front of their chest and attempted to stand up from the chair once. The time to stand was recorded five times. Each component of the SPPB was scored from 0 to 4, with a total score of 0 to 12[19].
The in-hospital mortality of patients in each group was recorded, and the primary causes of in-hospital mortality were categorized as cardiac shock, malignant arrhythmia, and multiple organ failure (MOF). The diagnosis of cardiac shock was in accordance with the definition in the 2016 ESC Guidelines on the Diagnosis and Treatment of Acute Heart Failure[20]. Malignant arrhythmia included high-grade (Mobitz II or third degree) atrioventricular block[21], sustained ventricular tachycardia, and ventricular fibrillation[22]. MOF was defined as physiological dysfunction of more than one organ or system.
Continuous variables were presented as means and SDs if normally distributed, and the differences between groups were analyzed with the independent t test. Continuous variables with skewed distribution were presented as medians and interquartile range (IQR) and compared with the Mann-Whitney U test. Categorized variables were presented as numbers and proportions, and comparisons between groups were analyzed with the Chi-square test. The composite incidence of adverse events, including all-cause death, multiple organ failure, cardiac shock, and malignant arrhythmia, during hospitalization was recorded. Patient characteristics, frailty status, and physical performance were compared in a univariate analysis between patients with and without adverse events during hospitalization. Multivariate logistic regression analyses were performed to evaluate the potential predictors of in-hospital adverse events, focusing on the frailty status and parameters related to physical performance. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 22.0 Software.
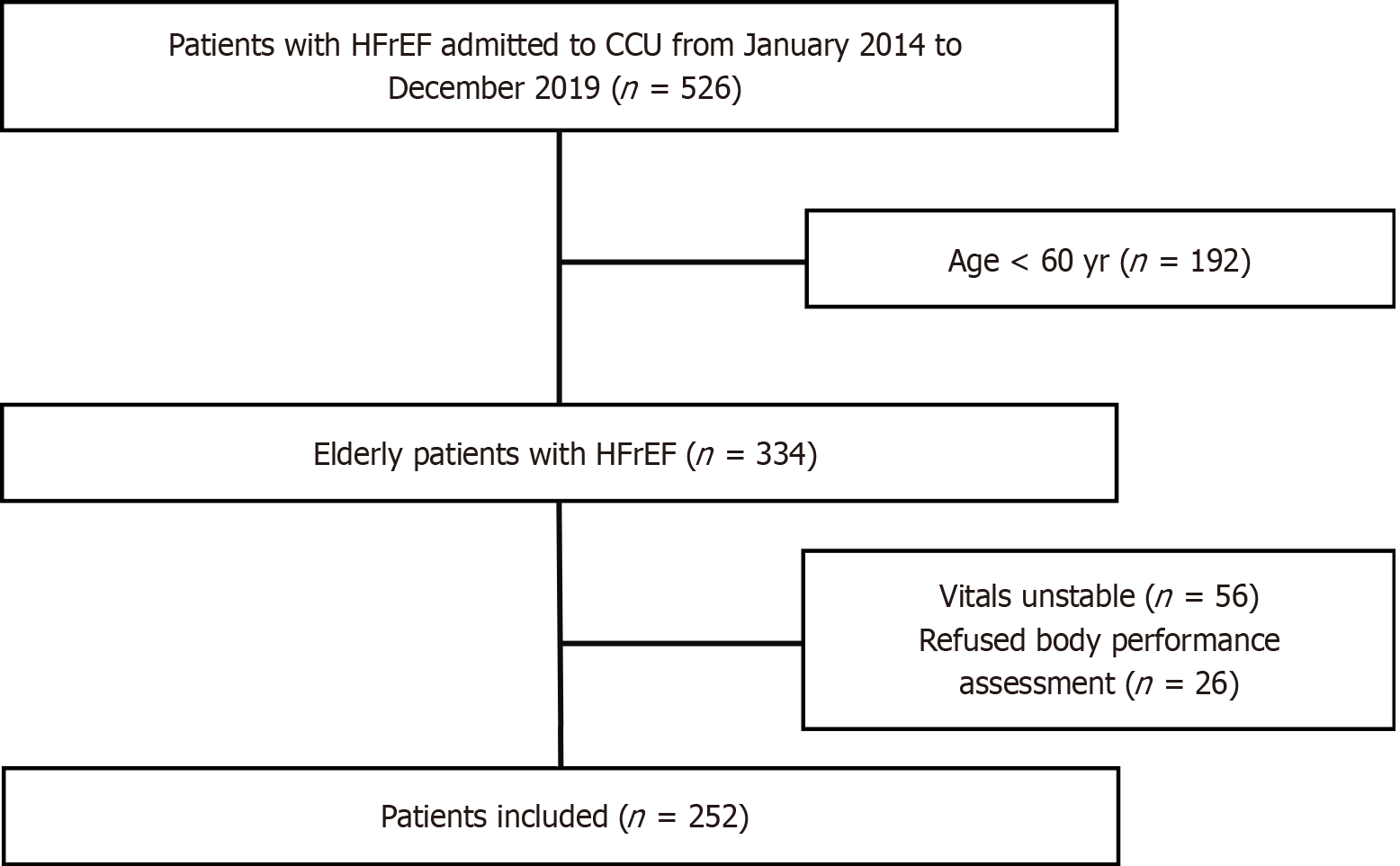
Overall, 526 patients were screened, and 252 elderly [mean age: 69.4 ± 6.7 years, male: 169 (67.0%)] patients with HFrEF were included in the final analyses. The flowchart of patient enrollment is shown in Figure 1. According to the definition of frailty by Fried et al[17], 135 (53.6%) patients were categorized as frail, 93 as pre-frail (36.9%), and 24 (9.5%) as non-frail. Since the number of non-frail patients was limited, we compared the characteristics of patients with frailty and pre-frailty. As shown in Table 1, patients with frailty were older (70.7 ± 7.2 years vs 68.8 ± 5.8 years, P = 0.039), were more likely to be female (32.6% vs 26.9%, P = 0.022), had lower SBP (107.71 ± 21.03 mmHg vs 114.01 ± 23.39 mmHg, P = 0.035) and DBP (66.90 ± 13.57 vs 72.03 ± 12.06, P = 0.004), had larger LVEDD (56.81 ± 9.01 mm vs 54.46 ± 7.66 mm, P = 0.036), and were more likely to present with LV thrombosis (20% vs 9.7%, P = 0.036) than those with pre-frailty. Other characteristics were not statistically different between frail and pre-frail patients with HFrEF, including NYHA class, previous medical history of cardiovascular diseases, and biochemical parameters.
| Frail (n = 135) | Pre-frail (n = 93) | P value | |
| Demographic factors | |||
| Age (yr) | 70.7 ± 7.2 | 68.8 ± 5.8 | 0.039 |
| Female, n (%) | 44 (32.6) | 25 (26.9) | 0.022 |
| NYHA Class | |||
| III, n (%) | 70 (51.9) | 57 (61.3) | 0.249 |
| IV, n (%) | 44 (32.6) | 23 (24.7) | 0.010 |
| Medical history | |||
| Hypertension, n (%) | 78 (57.8) | 48 (51.6) | 0.358 |
| DM, n (%) | 57 (42.2) | 29 (31.2) | 0.091 |
| Dyslipidemia, n (%) | 34 (25.2) | 17 (18.3) | 0.219 |
| AF, n (%) | 40 (29.6) | 18 (19.4) | 0.080 |
| PAD, n (%) | 9 (6.7) | 8 (8.6) | 0.585 |
| CABG, n (%) | 15 (11.1) | 11 (11.8) | 0.867 |
| PCI, n (%) | 24 (17.8) | 18 (19.4) | 0.763 |
| Clinical parameters | |||
| SBP (mmHg) | 107.71 ± 21.03 | 114.01 ± 23.39 | 0.035 |
| DBP (mmHg) | 66.90 ± 13.57 | 72.03 ± 12.06 | 0.004 |
| HR (bpm) | 88.16 ± 20.41 | 89.39 ± 19.77 | 0.653 |
| ALT (U/L)1 | 39 (23, 79) | 35 (20, 71) | 0.347 |
| SCr (μmol/L) | 109.70 ± 65.63 | 102.67 ± 54.03 | 0.395 |
| FPG (mmol/L) | 9.01 ± 6.32 | 8.49 ± 4.18 | 0.499 |
| TG (mmol/L)1 | 1.4 (0.9, 1.8) | 1.5 (1.0, 2.1) | 0.082 |
| LDL-C (mmol/L) | 2.59 ± 0.77 | 3.44 ± 1.51 | 0.455 |
| HDL-C (mmol/L) | 1.05 ± 0.31 | 0.99 ± 0.31 | 0.901 |
| PaO2 (mmHg) | 96.85 ± 33.03 | 101.12 ± 38.93 | 0.374 |
| BNP (μmol/L)1 | 712 (329, 1422) | 542 (307, 925) | 0.189 |
| Echocardiography | |||
| LVEDD (mm) | 56.81 ± 9.01 | 54.46 ± 7.66 | 0.036 |
| LVESD (mm) | 41.97 ± 11.03 | 41.89 ± 9.98 | 0.956 |
| LVEF (%) | 33.79 ± 6.06 | 34.03 ± 5.64 | 0.753 |
| LV thrombosis, n (%) | 27 (20) | 9 (9.7) | 0.036 |
| Medications | |||
| Diuretics, n (%) | 101 (74.8) | 73 (78.5) | 0.521 |
| ARAs, n (%) | 90 (66.7) | 55 (59.1) | 0.246 |
| ACEIs/ARBs, n (%) | 75 (55.6) | 53 (57.0) | 0.830 |
| Beta-blockers, n (%) | 114 (84.4) | 76(81.7) | 0.588 |
| Digitalis, n (%) | 51 (37.8) | 23 (24.7) | 0.039 |
| Anticoagulants, n (%) | 65 (48.1) | 33 (35.5) | 0.058 |
Compared to pre-frail patients, frail patients with HFrEF showed lower handgrip strengths stratified by sex (men: 23.8 ± 4.1 kg vs 25.1 ± 4.2 kg, P = 0.056, and women: 17.9 ± 3.4 kg vs 21.2 ± 4.5 kg, P = 0.003; Table 2). Moreover, frail patients with HFrEF showed poor performance in the balance, walking, and strength portions of the SPPB evaluation (all P < 0.001, Table 2). Finally, the overall SPPB score was lower in frail patients with HFrEF than in those with pre-frailty (7.24 ± 1.72 vs 8.95 ± 1.44, P < 0.001, Table 2), indicating the poor physical performance of frail patients compared to pre-frail patients with HFrEF.
| Frail (n = 135) | Pre-frail (n = 93) | P value | |
| Handgrip strength (kg) | |||
| Men | 23.8 ± 4.1 | 25.1 ± 4.2 | 0.056 |
| Women | 17.9 ± 3.4 | 21.2 ± 4.5 | 0.003 |
| Balance test | 3.08 ± 0.68 | 3.42 ± 0.61 | < 0.001 |
| Four-meter gait test | 2.19 ± 0.82 | 2.70 ± 0.76 | < 0.001 |
| Chair stand test | 1.96 ± 0.77 | 2.83 ± 0.70 | < 0.001 |
| Overall SPPB score | 7.24 ± 1.72 | 8.95 ± 1.44 | < 0.001 |
The overall in-hospital mortality of elderly patients with HFrEF in this study was 8.7%, including four patients with pre-frailty and 16 patients with frailty (11.9% vs 4.3%, P = 0.048; Table 3). Further analysis of the causes of hospital death showed that 10 patients with frailty died of MOF, and only one patient with pre-frailty died of MOF (7.4% vs 1.1%, P = 0.028; Table 3). There was no significant difference in cardiogenic shock and malignant arrhythmia between patients with pre-frailty and frailty (P = 0.971 and 0.517).
| Frail (n = 135) | Pre-frail (n = 93) | P value | |
| In-hospital death | 16 (11.9) | 4 (4.3) | 0.048 |
| Cardiac shock | 3 (2.2) | 2 (2.4) | 0.971 |
| Malignant arrhythmia | 3 (2.2) | 1 (1.1) | 0.517 |
| MOF | 10 (7.4) | 1 (1.1) | 0.028 |
Comparisons of the clinical characteristics and physical performance in patients with and without adverse events are shown in Table 4. Univariate analysis showed that patients with adverse events during hospitalization were older, more likely to have a poor NYHA class (all P < 0.05) compared to those without in-hospital adverse events. Moreover, patients with adverse events were more likely to be frail (72.1% vs 51.4%, P = 0.002; Table 4), to have lower handgrip (22.26 ± 5.34 kg vs 23.04 ± 4.44 kg, P = 0.030), and to have lower total SPPB scores (7.50 ± 2.04 vs 8.18 ± 1.61; P = 0.006).
| With AEs (n = 86) | Without AEs (n = 142) | P value | |
| Female, n (%) | 30 (34.9) | 39 (27.5) | 0.279 |
| NYHA Class III/IV, n (%) | 79 (91.9) | 115 (80.9) | 0.010 |
| Frailty, n (%) | 62 (72.1) | 73 (51.4) | 0.002 |
| Hypertension, n (%) | 54 (62.8) | 72 (50.7) | 0.075 |
| DM, n (%) | 33 (38.4) | 53 (37.3) | 0.874 |
| Dyslipidemia, n (%) | 18 (20.9) | 33 (23.2) | 0.685 |
| AF, n (%) | 28 (32.6) | 30 (21.1) | 0.055 |
| Age (yr) | 72.05 ± 7.46 | 68.65 ± 5.90 | < 0.001 |
| Handgrip strength (kg) | 22.26 ± 5.34 | 23.04 ± 4.44 | 0.030 |
| Overall SPPB score | 7.50 ± 2.04 | 8.18 ± 1.61 | 0.006 |
| SBP (mmHg) | 110.87 ± 23.24 | 109.92 ± 21.62 | 0.759 |
| DBP (mmHg) | 69.34±15.13 | 68.78 ± 11.92 | 0.772 |
| PaO2 (mmHg) | 99.23 ± 47.56 | 98.20 ± 25.87 | 0.853 |
| LVEF (%) | 33.74 ± 6.45 | 33.97 ± 5.53 | 0.786 |
Multivariate logistic analyses were performed using SBP and the significant variables in Table 4 as well as female gender and LVEF, two of the established prognostic parameters for HFrEF. The results showed that female gender [odds ratio (OR) = 0.422, 95%CI: 0.189–0.943, P = 0.035], aging (OR = 1.090, 95%CI: 1.029–1.154, P = 0.003), poor NYHA class (OR = 2.167, 95%CI: 1.320–3.559, P = 0.002), frailty (OR = 2.379, 95%CI: 1.196–4.733, P = 0.014), and lower handgrip strength (OR = 1.010, 95%CI: 1.020–1.212, P = 0.030) were independent predictors of in-hospital adverse events in elderly patients with HFrEF (Table 5), but not the total SPPB score, SBP, or LVEF (all P > 0.05).
| SE | OR | 95%CI | P value | |
| Female | 0.410 | 0.422 | 0.189-0.943 | 0.035 |
| Age | 0.029 | 1.090 | 1.029-1.154 | 0.003 |
| NYHA Class III/IV | 0.253 | 2.167 | 1.320-3.559 | 0.002 |
| Frailty | 0.351 | 2.379 | 1.196-4.733 | 0.014 |
| Overall SPPB Score | 0.100 | 0.945 | 0.776-1.150 | 0.570 |
| Handgrip strength | 0.047 | 1.106 | 1.010-1.212 | 0.030 |
| SBP | 0.007 | 1.003 | 0.989-1.017 | 0.656 |
| LVEF | 0.027 | 0.990 | 0.939-1.042 | 0.694 |
In this study of admitted elderly patients with HFrEF, we found that frailty and pre-frailty were common in this population, with a prevalence of 53.6% and 36.9%. Moreover, compared to patients with pre-frailty, frail patients with HFrEF were more likely to be female, to have lower blood pressure, and to present with LV thrombosis. Importantly, frail patients with HFrEF had a higher composite incidence of in-hospital adverse events. Additionally, compared with pre-frailty, frailty was associated with poor in-hospital outcomes in elderly patients with HFrEF. Although the results should be validated in multicenter prospective cohort studies, these results suggest that frailty may be associated with poor prognosis during hospitalization in elderly patients with HFrEF. Future studies are needed to determine whether early rehabilitation targeting frailty can improve the clinical outcomes in elderly patients with HFrEF.
Previous studies have shown that the prevalence of frailty varies considerably in patients with HF. A systematic review of eight studies with 5522 HF patients aged between 70 and 79 years showed that the prevalence of frailty in these studies ranged from 18%-54%, which may be related to the different populations studied and different criteria used for the diagnosis of frailty[23]. A recent study including 467 consecutive HF patients assessed with three frailty tools showed that the prevalence of frailty varies from 30% to 52% according to the tools used[24]. Regardless of the diagnostic tools used, the prevalence of frailty in HF patients is significant higher than that in controls[24]. A previous meta-analysis of 26 studies involving 6896 patients with HF showed an overall estimated prevalence of frailty in 44.5% of HF patients. The prevalence was slightly lower among studies that used Physical Frailty measures (42.9%, Z = 9.05; P < 0.001) and slightly higher among studies that used Multidimensional Frailty measures (47.4%, Z = 5.66; P < 0.001)[25]. The Fried frailty phenotype used to diagnose frailty and pre-frailty[17] in our study is a well-validated tool and has been comprehensively used in the era of geriatric medicine. Moreover, the Fried frailty phenotype has been recommended as a diagnostic tool for frailty in the Chinese population by the Chinese Geriatric Society[26]. Our results showed that the prevalence of frailty was 53.6% in our cohort, which is similar to the findings in previous studies. We found that the prevalence of pre-frailty in elderly patients with HFrEF was 36.9%. A previous study that also used the Fried frailty phenotype showed that in old patients with stable HF (mean age 85.2 years)[27], the prevalence of pre-frailty was 40.1%, which is slightly higher than the value in our population. In addition, we found that compared to patients with pre-frailty, patients with frailty were more likely to be female. Female gender has been identified as an independent risk factor for the incidence of in-hospital adverse events in elderly patients with HFrEF. These findings are also consistent with previous studies showing that women may have higher odds for frailty than men of the same age[28].
Our study showed that compared to HFrEF patients with pre-frailty, patients with frailty had a higher incidence of in-hospital mortality. The difference between the incidences of in-hospital mortality in the two groups was mainly driven by death related to MOF, which may reflect the overall poor clinical status of patients with frailty not limited to the cardiovascular system. Regarding the clinical characteristics of HFrEF patients with frailty and pre-frailty, no significant difference was observed for LVEF, renal and liver function at admission, or the use of evidence-based medications for HF. This finding suggests that frailty could predict the in-hospital outcome for these patients independent of the above clinical characteristics. The presence of frailty, characterized by impaired mobility, fatigue, underweight, and sarcopenia, may overlap with the symptoms of some chronic diseases, particularly in patients with HF[29]. Currently, the mechanisms for the association between frailty and poor outcomes in HF patients remain unknown. However, experimental studies demonstrated that frailty is characterized by overactivated inflammation, which is also recognized as a key pathophysiological feature of ventricular remodeling and myocardial injury[30]. Pathophysiologically, frailty may lead to cardiac overload and an overactivated inflammatory response, thereby worsening the cardiac function[31,32]. In addition, patients with HF exhibit reduced peripheral perfusion, sarcopenia, and reduced functional preservation of multiple organs, all of which are key features of frailty[31,32]. Future studies are warranted to determine the key molecular mechanisms underlying this association. More importantly, from the clinical perspective, future studies are needed to determine whether early rehabilitation targeting frailty can improve the clinical outcomes for elderly patients with HFrEF.
We used handgrip strength and SPPB to evaluate the physical performance of the elderly patients with HFrEF. Although we found that patients with frailty had lower handgrip strength and SPPB scores compared to those with pre-frailty, only lower handgrip strength was an independent predictor of increased risk of in-hospital adverse events in our study population. The limitation of SPPB evaluation is that it focuses on lower-extremity function. For patients with severe clinical conditions such as decompensated HF, the discrimination of SPPB for physical performance may be less sensitive to handgrip strength. Our results are consistent with those from the PURE study, which included 139691 patients and showed that for every 5 kg reduction in handgrip strength, the risk of all-cause death increased by 16% and the risk of cardiovascular disease increased by 7%[33].
Our study has limitations. Firstly, no consensus has been reached regarding the optimal tool for the evaluation of frailty. Although the Fried frailty phenotype tool has been well validated and recommended, the tool includes physical domains only and does not include components related to cognitive function, which has been confirmed as an important determinant of functional performance in elderly individuals[34]. Secondly, although we used multivariate analyses to adjust the potential confounding factors, we could not exclude the existence of unadjusted residual factors, which may confound the association between frailty and in-hospital outcomes for the patients. In addition, the patients were evaluated during hospitalization with no subsequent follow-up. The influence of frailty on the long-term outcomes for these patients should be investigated in the future. Moreover, the proportion of patients using digitalis was significantly higher in the frail group than in the pre-frail group, and it has been reported that digitalis increased death in women with HF, which may confound the results. In addition, the blood concentration levels of digitalis were not available for the included patients, although none of the included patients suffered from digitalis toxicity.
Finally, a causative relationship between frailty and poor in-hospital outcomes in elderly patients with HFrEF could not be established based on our results since the study is observational. Clinical trials are needed to determine the influence of early rehabilitation targeting frailty on the clinical outcomes for these patients.
In conclusion, the results of our study showed that frailty and pre-frailty are common in elderly patients with HFrEF, and that frailty, compared to pre-frailty, may be associated with poor in-hospital outcomes. These results should be validated in multicenter prospective cohort studies, and studies are needed to determine whether early rehabilitation targeting frailty can improve the clinical outcomes for elderly patients with HFrEF.
Frailty is prevalent in elderly patients with cardiovascular diseases. However, the association between frailty and in-hospital outcomes for elderly patients with heart failure and reduced ejection (HFrEF) remains unknown.
Frailty has been included in geriatric comprehensive assessment, which may not only be useful for evaluation of the functional status of elderly patients, but also contribute to the prognostic efficacy for patients with HFrEF.
To evaluate the predictive efficacy of frailty, compared with pre-frailty, for adverse events in these patients.
Elderly patients (≥ 60 years) with HFrEF were assessed. Frailty was evaluated with the Fried phenotype criteria, and physical performance was evaluated based on handgrip strength and the short physical performance battery (SPPB). The composite incidence of adverse events, including all-cause death, multiple organ failure, cardiac shock, and malignant arrhythmia, during hospitalization was recorded.
Frailty and pre-frailty were common in this population, with a prevalence of 53.6% and 36.9%. Moreover, compared to patients with pre-frailty, frail patients with HFrEF were more likely to be female, to have lower blood pressure, and to present with left ventricular thrombosis. Importantly, frail patients with HFrEF had a higher composite incidence of in-hospital adverse events. Additionally, compared with pre-frailty, frailty was associated with poor in-hospital outcomes in elderly patients with HFrEF.
Frailty may be associated with poor prognosis during hospitalization in elderly patients with HFrEF.
The results should be validated in multicenter prospective cohort studies. Future studies are needed to determine whether early rehabilitation targeting frailty can improve the clinical outcomes in elderly patients with HFrEF.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peltrini R S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 589] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 2. | Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1560] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 3. | Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56-e528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 5839] [Article Influence: 973.2] [Reference Citation Analysis (5)] |
| 4. | Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 1241] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 5. | Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. 2019;393:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 244] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 6. | Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 7. | Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Chen H, Reeves GR. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Heart Fail. 2019;7:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Warmoth K, Tarrant M, Abraham C, Lang IA. Relationship between perceptions of ageing and frailty in English older adults. Psychol Health Med. 2018;23:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ribeiro AR, Howlett SE, Fernandes A. Frailty-A promising concept to evaluate disease vulnerability. Mech Ageing Dev. 2020;187:111217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | McDonagh J, Ferguson C, Newton PJ. Frailty Assessment in Heart Failure: an Overview of the Multi-domain Approach. Curr Heart Fail Rep. 2018;15:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 819] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 12. | Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019;7:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 13. | Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019;21:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and Clinical Outcomes in Heart Failure: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2018;19:1003-1008.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Crow RS, Lohman MC, Titus AJ, Bruce ML, Mackenzie TA, Bartels SJ, Batsis JA. Mortality Risk Along the Frailty Spectrum: Data from the National Health and Nutrition Examination Survey 1999 to 2004. J Am Geriatr Soc. 2018;66:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4368] [Cited by in RCA: 4903] [Article Influence: 544.8] [Reference Citation Analysis (4)] |
| 17. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13384] [Cited by in RCA: 15730] [Article Influence: 655.4] [Reference Citation Analysis (1)] |
| 18. | Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11293] [Cited by in RCA: 13543] [Article Influence: 615.6] [Reference Citation Analysis (0)] |
| 19. | Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1448] [Cited by in RCA: 1767] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 20. | O'Connor CM, Rogers JG. Evidence for overturning the guidelines in cardiogenic shock. N Engl J Med. 2012;367:1349-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Auffret V, Loirat A, Leurent G, Martins RP, Filippi E, Coudert I, Hacot JP, Gilard M, Castellant P, Rialan A, Delaunay R, Rouault G, Druelles P, Boulanger B, Treuil J, Avez B, Bedossa M, Boulmier D, Le Guellec M, Daubert JC, Le Breton H. High-degree atrioventricular block complicating ST segment elevation myocardial infarction in the contemporary era. Heart. 2016;102:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Mehta RH, Yu J, Piccini JP, Tcheng JE, Farkouh ME, Reiffel J, Fahy M, Mehran R, Stone GW. Prognostic significance of postprocedural sustained ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention (from the HORIZONS-AMI Trial). Am J Cardiol. 2012;109:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Jha SR, Ha HS, Hickman LD, Hannu M, Davidson PM, Macdonald PS, Newton PJ. Frailty in advanced heart failure: a systematic review. Heart Fail Rev. 2015;20:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of Frailty in Chronic Heart Failure. JACC Heart Fail. 2019;7:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 25. | Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2017;236:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 26. | Hao Q, Li J, Dong B, Li X. Chinese experts consensus on assessment and intervention for elderly patients with frailty. Zhonghua Laonian Yixue Zazhi. 2017;36:251-256. [DOI] [Full Text] |
| 27. | Rodríguez-Pascual C, Paredes-Galán E, Ferrero-Martínez AI, Gonzalez-Guerrero JL, Hornillos-Calvo M, Menendez-Colino R, Torres-Torres I, Vilches-Moraga A, Galán MC, Suarez-Garcia F, Olcoz-Chiva MT, Rodríguez-Artalejo F. The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: A prospective study in six Spanish hospitals. Int J Cardiol. 2017;236:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol. 2017;89:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 29. | Uchmanowicz I, Młynarska A, Lisiak M, Kałużna-Oleksy M, Wleklik M, Chudiak A, Dudek M, Migaj J, Hinterbuchner L, Gobbens R. Heart Failure and Problems with Frailty Syndrome: Why it is Time to Care About Frailty Syndrome in Heart Failure. Card Fail Rev. 2019;5:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, Frailty and Cardiovascular Disease. Adv Exp Med Biol. 2020;1216:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 31. | Uchmanowicz I, Nessler J, Gobbens R, Gackowski A, Kurpas D, Straburzynska-Migaj E, Kałuzna-Oleksy M, Jankowska EA. Coexisting Frailty With Heart Failure. Front Physiol. 2019;10:791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Butts B, Gary R. Coexisting Frailty, Cognitive Impairment, and Heart Failure: Implications for Clinical Care. J Clin Outcomes Manag. 2015;22:38-46. [PubMed] |
| 33. | Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S; Prospective Urban Rural Epidemiology (PURE) Study investigators. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 1241] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 34. | Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |