Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11193
Peer-review started: May 3, 2021
First decision: June 2, 2021
Revised: June 16, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: December 26, 2021
Processing time: 234 Days and 4.2 Hours
Previous nomograms for hepatocellular carcinoma (HCC) did not include the neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR). This study aimed to establish an effective nomogram capable of estimating the association between preoperative inflammatory factors and overall survival (OS) of HCC patients after hepatectomy.
To analyse the factors affecting the prognosis of HCC and establish a nomogram.
A total of 626 HCC patients (410 training set patients from the First Affiliated Hospital of Anhui Medical University and 216 validation set patients from the First Affiliated Hospital of University of Science and Technology of China) underwent hepatectomy from January 2014 to December 2017 and were followed up every 3–6 mo. The nomogram was based on OS-related independent risk factors identified by Cox regression analysis. The C-index, calibration curve, and area under the curve (AUC) were used to evaluate the nomogram’s accuracy.
The 1-, 2- and 3-year OS rates were 79.0%, 68.0% and 45.4% in the training cohort (median OS = 34 mo) and 92.1%, 73.9% and 51.2% in the validation cohort (median OS = 38 mo). Higher α-fetoprotein [hazard ratio (HR) = 1.812, 95% confidence interval (CI): 1.343–2.444], NLR (HR = 2.480, 95%CI: 1.856–3.312) and PLR (HR = 1.974, 95%CI: 1.490–2.616), tumour size ≥ 5 cm (HR = 1.323, 95%CI: 1.002–1.747), and poor differentiation (HR = 3.207, 95%CI: 1.944–5.290) were significantly associated with shortened OS. The developed nomogram integrating these variables showed good reliability in both the training (C-index = 0.71) and validation cohorts (C-index = 0.75). For predicting 1-, 2- and 3-year OS, the nomogram had AUCs of 0.781, 0.743 and 0.706 in the training cohort and 0.789, 0.815 and 0.813 in the validation cohort. The nomogram was more accurate in predicting prognosis than the AJCC TNM staging system.
The prognostic nomogram combining pathological characteristics and inflammation indicators could provide a more accurate individualized risk estimate for the OS of HCC patients with hepatectomy.
Core tip: Inflammation is a systemic process involving multicell participation, multipathway activation, and multifunctional execution. Prognosis of hepatocellular carcinoma (HCC) depends on progression of liver malignancy but is also affected by inflammation. Nomograms, as a visualization method of statistical models, gradually improve individualization with the inclusion of risk factors, providing clinicians and patients with information to effectively stratify liver malignancy patients and formulate personalized treatments. The nomograms developed in the past focused on pathological characteristics. In this study, we developed a nomogram that could predict the prognosis of HCC patients after hepatectomy based on tumour pathological characteristics and inflammation indicators.
- Citation: Pu T, Li ZH, Jiang D, Chen JM, Guo Q, Cai M, Chen ZX, Xie K, Zhao YJ, Liu FB. Nomogram based on inflammation-related markers for predicting survival of patients undergoing hepatectomy for hepatocellular carcinoma. World J Clin Cases 2021; 9(36): 11193-11207
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11193.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11193
Hepatocellular carcinoma (HCC) is a common malignancy that accounts for more than 80% of primary liver malignancies worldwide[1]. It is the third leading cause of cancer-related death around the world and has become a major health concern in most countries[2]. The incidence of HCC is closely related to hepatitis virus infection. As a wide epidemic area for hepatitis B, China contributes to three quarters of the world’s hepatitis B infections and nearly half of HCC patients[3]. In recent decades, effective treatments such as surgical resection, liver transplantation, radiofrequency ablation (RFA) and transcatheter arterial embolization or transcatheter arterial chemoembolization (TACE) have been developed. However, in the context of a shortage of liver donor resources and the inefficiency of palliative care, liver resection is still the most common treatment for HCC[4]. Therefore, the accuracy of cancer staging is of importance since it is conducive to the formulation of surgical plans and prognostic evaluation, balancing effective treatment and overtreatment so that patients could fully benefit.
The current mainstream cancer staging systems include the American Joint Committee on Cancer (AJCC), Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), and China liver cancer staging (CNLC) systems[5]. However, the above staging systems are mainly based on tumour pathological indicators and group characteristics, adopting similar treatments for people at the same stage and ignoring individual heterogeneity. Therefore, in the context of precision medicine, nomograms, as linear result output methods based on statistical models, combine traditional AJCC TNM staging and personal characteristics to comprehensively evaluate individual risk levels and provide evidence for personalized treatment intervention and prognosis evaluation[6]. Previous studies also indicated that the prognosis of HCC patients with hepatectomy was related to several indicators, such as age, sex, drinking status, tumour size, α-fetoprotein (AFP), and inflammation[7]. AFP is a common blood test method to monitor HCC, and the potential mechanism of inflammation in the development and metastasis of cancer was proposed in the last century and gradually incorporated into clinical consensus[8]. However, the nomograms developed in the past focused on pathological characteristics, and it was difficult to provide a universally applicable predictive model to clinicians. In addition, the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were not concurrently included, which may have reduced the accuracy of prognostic evaluation. The aim of this study was to develop a nomogram that is suitable for HCC patients and comprehensively reflects pathological factors and inflammatory factors. According to the calibration and receiver operating characteristic (ROC) curves in the training cohort and the validation cohort, we explored whether the model’s postoperative prediction was accurate.
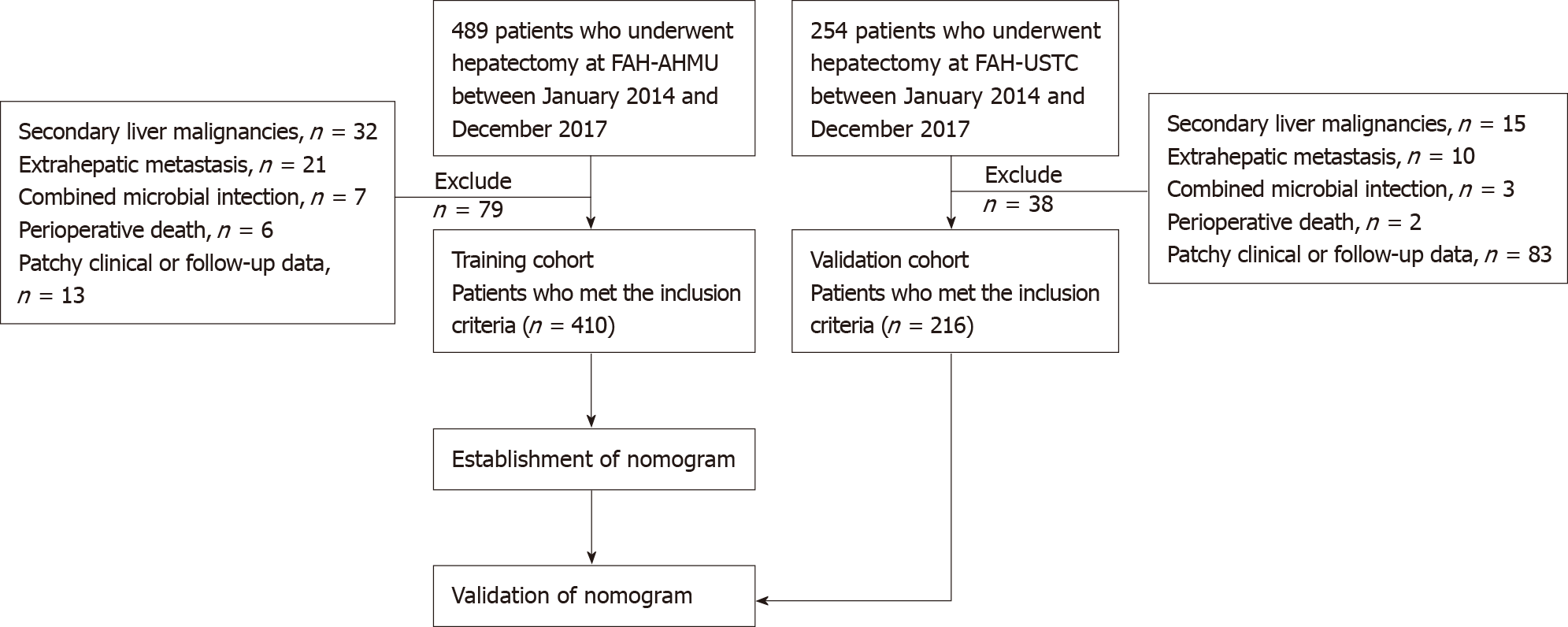
This study included 410 HCC patients who underwent hepatectomy at the First Affiliated Hospital of Anhui Medical University (FAH-AHMU) from January 2014 to December 2017 as the training group; based on the same criteria, 216 patients enrolled from the First Affiliated Hospital of University of Science and Technology of China (FAH-USTC) served as the external verification group (Figure 1). Both hospitals are high-volume surgical centres. The therapeutic approach to patients with HCC was similar. Liver malignancy was diagnosed by two clinically experienced liver pathologists based on biochemical indicators, imaging indicators, and pathological tissue samples. If any disagreements between the two experts arose, a third liver pathologist made the final diagnosis. The diagnosis of HCC was based on the standards of the European Association for the Study of the Liver (EASL)[9]: A needle biopsy confirmed that the lesion was cancerous or an imaging examination [computed tomography (CT) or magnetic resonance imaging (MRI)] suggested an intrahepatic space, which was accompanied by increased levels of AFP, hepatitis virus infection history, etc. Participants needed to meet the following inclusion criteria: (1) Primary liver cancer; (2) Meet the treatment criteria for liver resection; (3) No adjuvant chemotherapy or radiotherapy before surgery; (4) No other infections or autoimmune diseases; and (5) No extrahepatic metastasis. The exclusion criteria were as follows: (1) Presence of extrahepatic metastases or failure to achieve R0 resection; (2) Secondary or metastatic liver malignancies; (3) History of malignant tumours of other organs; (4) Combined microbial infection; and (5) Unavailability of any key research variables or follow-up data. Written or verbal informed consent was obtained from the subjects, and sensitive data were deleted before analysis. This study was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki and was approved by the ethical review committees at FAH-AHMU and FAH-USTC (ID: Quick-PJ 2021-01-22). All included patients or their relatives provided written informed consent before the data were analysed.
Sociodemographic and clinical data were extracted from the electronic medical record system, including age (< 50/≥ 50 years), sex (male/female), past medical history, smoking history (no/yes), and alcohol consumption history (no/yes). Regarding laboratory data, the patients received routine biochemical and immunological examinations before surgery, and the nurses collected blood samples and sent them to the laboratory within 3 h. Laboratory physicians used an automatic blood cell analyser to detect the neutrophil count, platelet count, and lymphocyte count. After blood coagulation, the samples were centrifuged at 3000 r/min for 10 min, and the upper layer of serum was collected to detect the levels of biomarkers, including AFP (< 400/≥ 400 μg/L), hepatitis B surface antigen (HBsAg), alanine aminotransferase (ALT), aspartate aminotransferase (AST), prothrombin time (PT), carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). The tumour-related indicators collected after surgery included tumour size, tumour location, microvascular invasion (MVI), and tumour differentiation grade. NLR and PLR were calculated as follows: NLR = neutrophil count/lymphocyte count; PLR = platelet count/lymphocyte count. According to the median values in the training cohort, the patients were divided into a low NLR group (< 2.22) and a high NLR group (≥ 2.22), and PLR (< 102.92, ≥ 102.92) followed the same rule. Tumour staging was performed according to the TNM staging system published by the AJCC in 2017 (AJCC 8th edition).
The in-hospital treatment and follow-up plans were communicated to the patients to improve patient compliance and follow-up response rates. Follow-up visits were conducted every 3 mo for the first year and every 6 mo for the next 2 years. The follow-up content included a physical examination, abdominal CT or MRI, and AFP test. Patients with high risk of early recurrence were underwent preventative TACE to detect and treat intrahepatic metastases 1 mo postoperatively. Once intrahepatic recurrence was confirmed, various locoregional treatments including TACE, RFA, or repeated liver resection were performed. Patients with unsatisfactory responses to the above therapies were finally treated with systemic chemotherapy including sorafenib or regorafenib. Trained nurses asked about the survival of patients who did not follow the plan according to the scheduled time. Furthermore, we inquired about the date of death when the family members had reported the death of the patient. Patients who changed their mobile phone number, refused to be followed up, or died due to other diseases or accidents were recorded as left-censored data; patients still alive after 3 years were recorded as right-censored data. Overall survival (OS) in months was calculated as follows: OS = (date of patient death or last follow-up date-discharge date)/30.
This study used SPSS version 25.0 (IBM, Chicago, IL, USA) and R (http://www.r-project.org, version 4.0.3) software for statistical analysis. SPSS was used to describe the population characteristics and compare the differences between the training cohort and validation cohort. Continuous variables were described as means and standard deviations and were compared by independent sample t-test. Categorical variables were represented by numbers (n) or percentages (%) and were compared by the χ2 test. The Kaplan–Meier method was used to determine the average OS, median OS, and OS trends of the training group. Univariate and multivariate Cox regression analyses were performed based on the training data, and the independent risk factors associated with OS were incorporated into the nomogram[10].
The Cph_() and Nomogram_() functions of the rms package in R were used to develop a nomogram to predict 1-year, 2-year and 3-year OS. The patient characteristics vertically corresponded to the scale (points, 0–100), and the sum of all characteristic scores was the total points, which corresponded to the survival probability at 1, 2 and 3 years at the bottom of the nomogram. The C-index (C-index = Dxy × 0.5 + 0.5) and Kaplan–Meier-based calibration (calibrate_() and plot_() functions) were used to test the predictive power of the nomogram. The range of the C-index was 0–1; 0 meant that the model incorrectly predicted the survival outcome of all patients, and 1 meant that it predicted the survival outcome completely correctly. It is generally believed that a C-index > 0.7 indicates better prediction discrimination. The external Cox regression model was based on the total points, which were calculated by the established nomogram (formula_lp_() and points_cal_() functions of the NomogramFormula package), and the C-index and calibration curve were calculated. Finally, the ROC curve was used to compare the prediction accuracy between the nomogram and the TNM staging system, with the prediction effect increasing with the area under the curve (AUC). A two-sided P value < 0.05 was considered statistically significant.
The clinicopathological data of 489 consecutive patients who underwent hepatectomy for HCC at FAH-AHMU between January 2014 and December 2017 were collected consecutively. Of the 489 patients, 79 (16.2%) who did not fulfil the inclusion criteria were excluded: Six died in the perioperative period, 21 had extrahepatic metastasis, 32 had a secondary liver malignancy, seven had combined microbial infection and 13 had incomplete clinical or follow-up data. Ultimately, 410 (83.8%) patients were identified and established as the training cohort. Based on the same screening criteria, an independent group consisting of 216 patients from FAH-USTC in the same period was included in the present study and served as an external validation cohort (Figure 1). The characteristics of the training group and the validation group are shown in Table 1. The average age of the training group was 57.29 years (SD = 11.51), the proportion of patients ≥ 50 years was 75.4%, and the proportion of men was close to 80%. Overall, 71.7% and 52.2% of patients had a history of smoking and alcohol consumption, respectively. There were 221 patients (53.9%) with albumin–bilirubin (ALBI) grade of 1, and 189 (46.1%) with grade 2 or 3. A total of 295 patients (72.0%) were positive for hepatitis B surface antibody, and 121 (30.9%) had preoperative AFP ≥ 400 μg/L. Of all tumours, 60.1% were located on the right and 57.9% had a diameter ≥ 5 cm; because there was no significant difference between < 2 cm and 2–5 cm groups, we chose 5 cm as the cut-off value. The tumour differentiation grade was mainly moderate (61.7%), followed by well differentiated (24.9%). There were 103 (26.8%) patients with microvascular invasion (MVI) and only 14 (3.7%) had lymph node metastasis (LNM). Regarding the TNM staging system, I, II, III and IV accounted for 20.1%, 14.3%, 61.3% and 4.4%, respectively. In the validation cohort, the average age was 58.75 years (SD = 10.59), 78% of patients were > 50 years, 77.8% were men, and 69.0% and 57.9% had a history of smoking and alcohol consumption, respectively. There were 121 patients (56.0%) with ALBI grade 1 and 160 (74.1%) had hepatitis B virus infection. Based on the median cut-off value of NLR and PLR in the training cohort, high AFP, NLR and PLR levels accounted for 27.1%, 45.6% and 45.6%, respectively. A total of 57.0% of tumours were located in the right liver. Long-diameter tumours and poorly differentiated tumours accounted for 54.8% and 6.9%, res
| Training cohort (n = 410) | Validation cohort (n = 216) | P value | |||
| n | % | n | % | ||
| Age, yr | |||||
| < 50 | 101 | 24.6 | 47 | 21.8 | 0.421 |
| ≥ 50 | 309 | 75.4 | 169 | 78.2 | |
| Sex | |||||
| Male | 327 | 79.8 | 168 | 77.8 | 0.563 |
| Female | 83 | 20.2 | 48 | 22.2 | |
| Smoking | |||||
| No | 116 | 28.3 | 67 | 31.0 | 0.476 |
| Yes | 294 | 71.7 | 149 | 69.0 | |
| Drinking | |||||
| No | 196 | 47.8 | 91 | 42.1 | 0.176 |
| Yes | 214 | 52.2 | 125 | 57.9 | |
| ALBI grade | |||||
| 1 | 221 | 53.9 | 121 | 56.0 | 0.998 |
| 2 and 3 | 189 | 46.1 | 95 | 44.0 | |
| HBsAg | |||||
| Negative | 115 | 28.0 | 56 | 25.9 | 0.571 |
| Positive | 295 | 72.0 | 160 | 74.1 | |
| Tumour location | |||||
| Left | 88 | 28.8 | 42 | 28.2 | 0.335 |
| Right | 184 | 60.1 | 85 | 57.0 | |
| Others | 34 | 11.1 | 22 | 14.8 | |
| Tumour size, cm | |||||
| < 5 | 157 | 42.1 | 94 | 45.2 | 0.469 |
| ≥ 5 | 216 | 57.9 | 114 | 54.8 | |
| Grade | |||||
| Well differentiated | 78 | 24.9 | 49 | 24.0 | 0.050 |
| Moderately differentiated | 193 | 61.7 | 141 | 69.1 | |
| Poorly differentiated | 42 | 13.4 | 14 | 6.9 | |
| MVI | |||||
| No | 282 | 73.2 | 142 | 71.0 | 0.564 |
| Yes | 103 | 26.8 | 58 | 29.0 | |
| AFP, μg/L | |||||
| < 400 | 270 | 69.1 | 145 | 72.9 | 0.338 |
| ≥ 400 | 121 | 30.9 | 54 | 27.1 | |
| NLR | |||||
| Low | 201 | 49.9 | 117 | 54.4 | 0.282 |
| High | 202 | 50.1 | 98 | 45.6 | |
| PLR | |||||
| Low | 202 | 50.0 | 117 | 54.4 | 0.295 |
| High | 202 | 50.0 | 98 | 45.6 | |
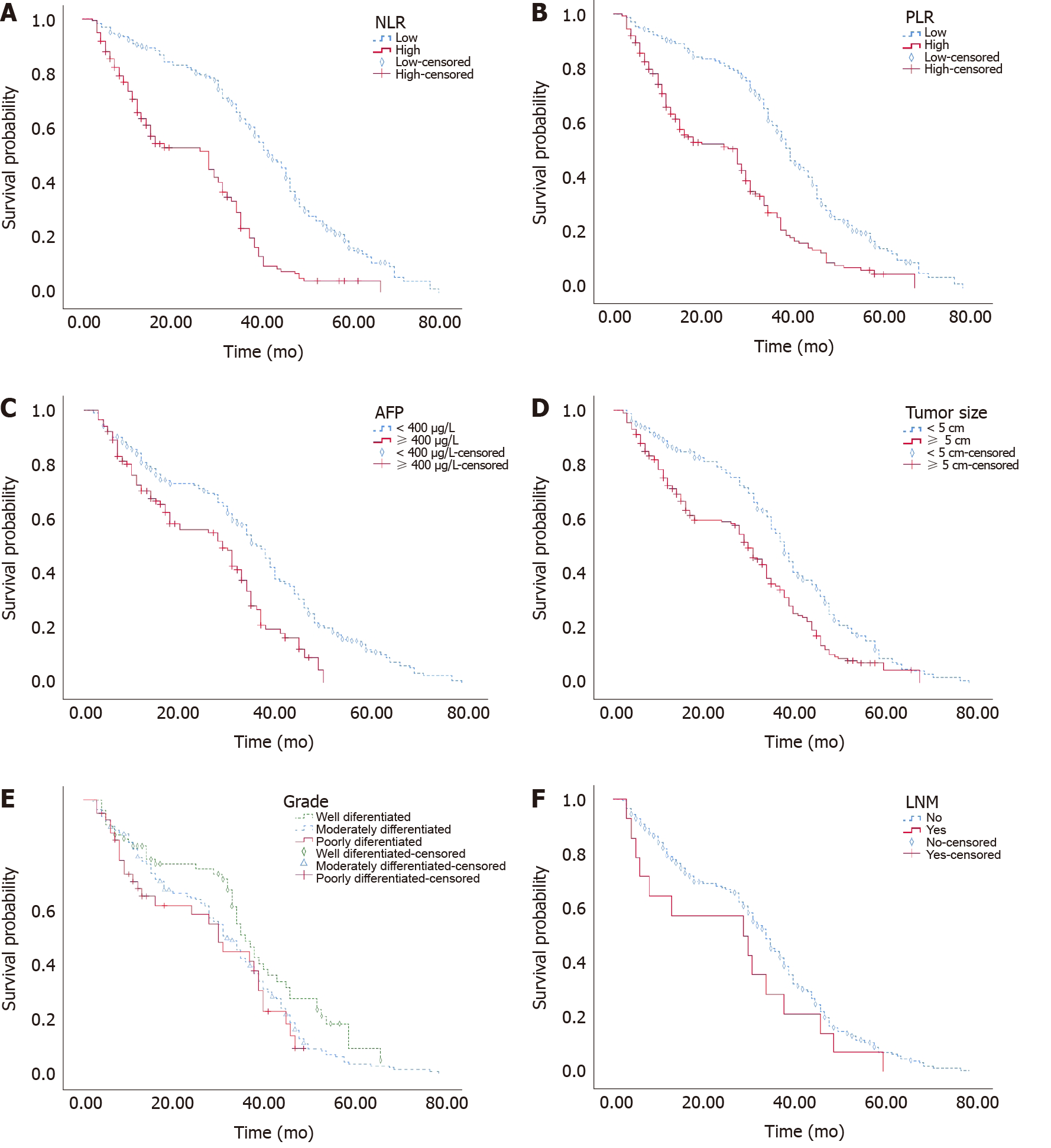
The average OS times of the training and validation cohorts were 33.71 and 39.19 mo, respectively, and the median OS times were 34 and 38 mo. The 1-, 2- and 3-year OS rates were 79.0%, 68.0% and 45.4% in the training cohort and 92.1%, 73.9% and 51.2% in the validation cohort, respectively. Kaplan–Meier survival analysis showed that NLR ≥ 2.22, PLR ≥ 102.92, AFP ≥ 400, and tumour diameter ≥ 5 cm were related to shorter OS (P < 0.001). OS was shortened with decreased tumour differentiation (P = 0.042), but it was not significantly associated with LNM (P = 0.164) (Figure 2).
The results of the univariate and multivariate Cox regression analyses are presented in Table 2. The univariate results suggested that OS was related to tumour size, differentiation grade, and preoperative AFP, NLR and PLR levels (P < 0.05), but there were no significant differences for age, HBsAg, MVI, smoking history, or alcohol consumption history. The significant variables were included in the multivariate Cox model (n = 296), and their associations with OS were still significant after adjustment. Compared to that of patients with low levels, the OS of patients with high levels of AFP [hazard ratio (HR) = 1.812, 95% confidence interval (CI): 1.343–2.444], NLR (HR = 2.480, 95%CI: 1.856–3.312), and PLR (HR = 1.974, 95%CI: 1.490–2.616) was significantly shortened. The postoperative OS of patients with tumour size ≥ 5 cm was significantly lower than that of patients with tumour size < 5 cm (HR = 1.323, 95%CI: 1.002–1.747). Compared with patients with well-differentiated cancer, those with moderately differentiated (HR = 1.752, 95%CI: 1.246–2.463) and poorly differentiated (HR = 3.207, 95%CI: 1.944–5.290) cancer had a significantly increased risk of death.
| Univariate Model | Multivariate Model | |||||||
| HR | 95%CI | P value | HR | 95%CI | P value | |||
| Age, yr | ||||||||
| < 50 | Ref. | |||||||
| ≥ 50 | 0.837 | 0.642 | 1.090 | 0.187 | ||||
| Sex | ||||||||
| Male | Ref. | |||||||
| Female | 1.065 | 0.809 | 1.401 | 0.655 | ||||
| Smoking | ||||||||
| No | Ref. | |||||||
| Yes | 0.959 | 0.748 | 1.230 | 0.741 | ||||
| Drinking | ||||||||
| No | Ref. | |||||||
| Yes | 0.986 | 0.787 | 1.234 | 0.900 | ||||
| ALBI Grade | ||||||||
| 1 | Ref. | |||||||
| 2 and 3 | 1.032 | 0.821 | 1.290 | 0.820 | ||||
| HBsAg | ||||||||
| Negative | Ref. | |||||||
| Positive | 1.063 | 0.833 | 1.365 | 0.639 | ||||
| Tumour location | ||||||||
| Left | Ref. | |||||||
| Right | 1.263 | 0.942 | 1.693 | 0.119 | ||||
| Others | 1.359 | 0.855 | 2.159 | 0.195 | ||||
| Tumour size, cm | ||||||||
| < 5 | Ref. | |||||||
| ≥ 5 | 1.595 | 1.255 | 2.027 | < 0.001 | 1.323 | 1.002 | 1.747 | 0.048 |
| Grade | ||||||||
| Well differentiated | Ref. | Ref. | ||||||
| Moderately differentiated | 1.421 | 1.031 | 1.959 | 0.032 | 1.752 | 1.246 | 2.463 | 0.001 |
| Poorly differentiated | 1.669 | 1.047 | 2.661 | 0.031 | 3.207 | 1.944 | 5.290 | < 0.001 |
| MVI | ||||||||
| No | Ref. | |||||||
| Yes | 1.171 | 0.905 | 1.515 | 0.230 | ||||
| AFP, μg/L | ||||||||
| < 400 | Ref. | |||||||
| ≥ 400 | 1.757 | 1.347 | 2.293 | < 0.001 | 1.812 | 1.343 | 2.444 | < 0.001 |
| NLR | ||||||||
| Low | Ref. | |||||||
| High | 2.744 | 2.152 | 3.498 | < 0.001 | 2.480 | 1.856 | 3.312 | < 0.001 |
| PLR | ||||||||
| Low | Ref. | |||||||
| High | 2.212 | 1.748 | 2.800 | < 0.001 | 1.974 | 1.490 | 2.616 | < 0.001 |
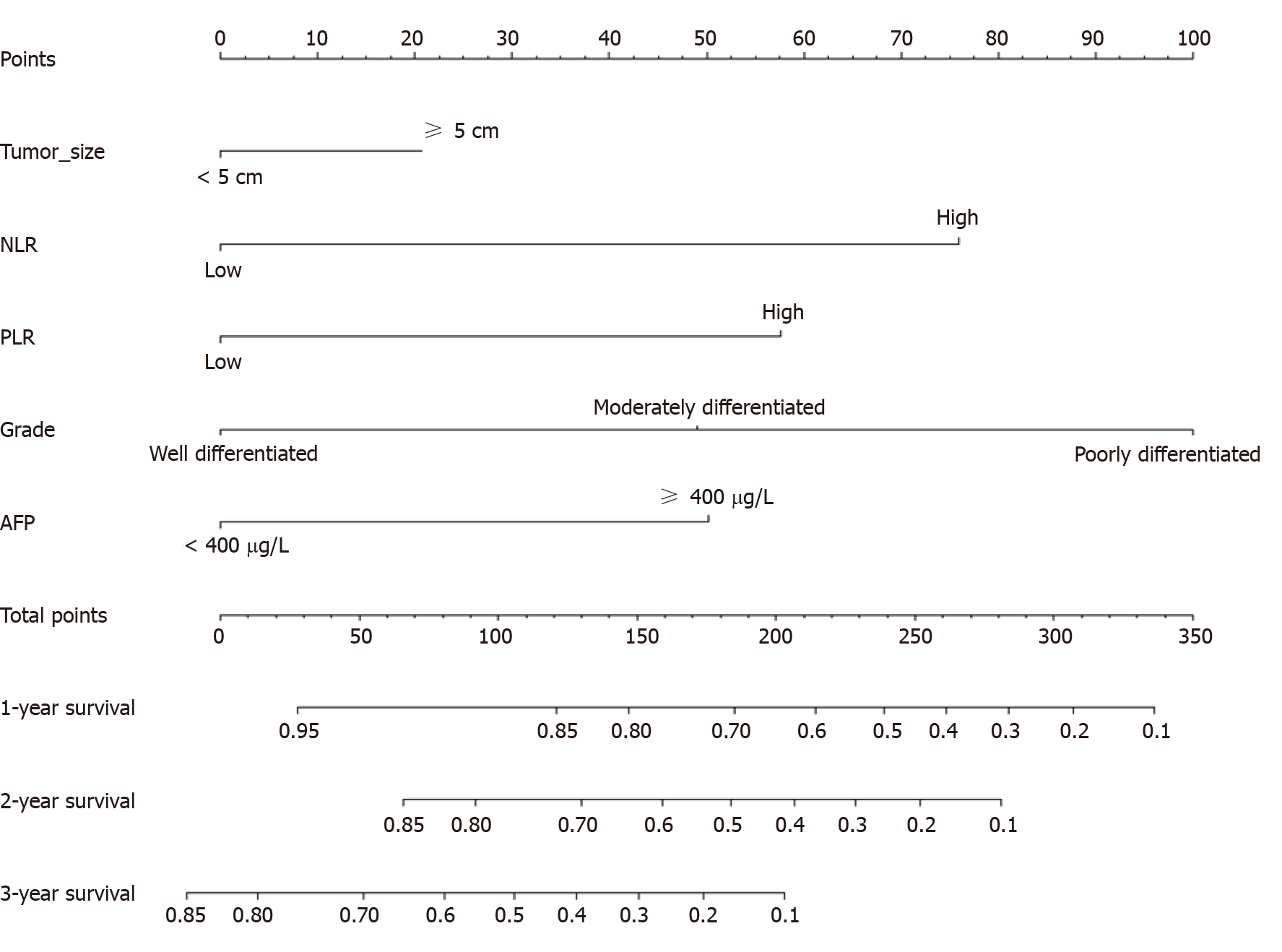
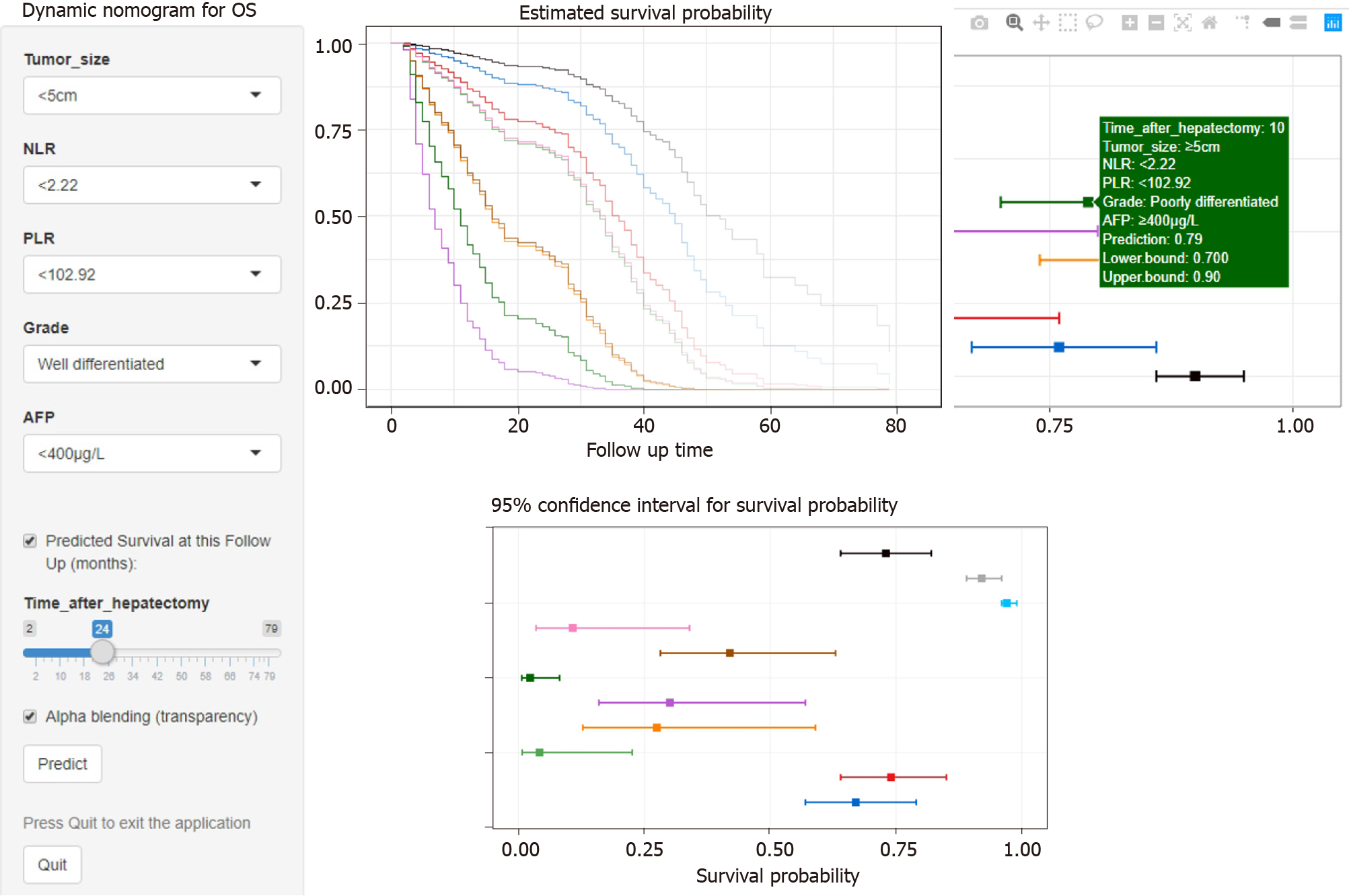
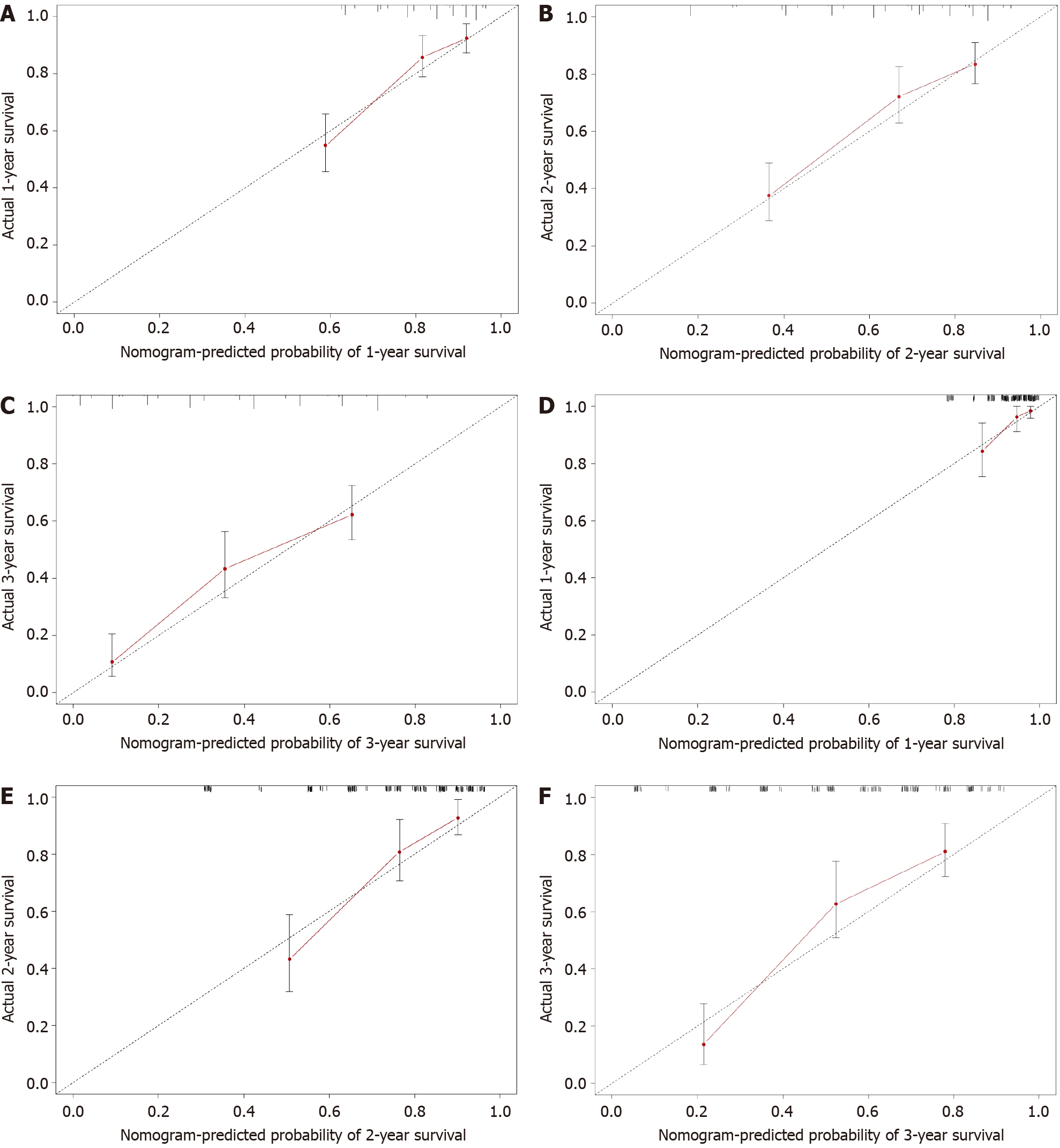
A Cox model-based nomogram was constructed to predict 1-, 2- and 3-year patient survival probabilities (Figure 3), which reflected the survival probabilities in this population through indicators including inflammation-related markers. The nomogram-predicted values were consistent with the actual observations, and the C-indexes were 0.71 (95%CI: 0.70–0.73) and 0.75 (95%CI: 0.73–0.77) in the training and validation groups, respectively, which revealed that the original nomogram remained valid for use in the external set. Further, an online calculator was developed (screenshots in Figure 4), and the nomogram for OS is freely available at https://ahmuptt.shinyapps.io/NLPL/. The nomogram model parameters were applied to the external validation cohort, and an external Cox model was established based on OS and the total points. The calibration curves were drawn for OS at 1, 2 and 3 years. The x-axis shows the nomogram-predicted probabilities, and the y-axis shows the actual survival observations. All the predicted curves approximatively overlapped with the reference curves, indicating that the nomogram performed well in both cohorts (Figure 5).
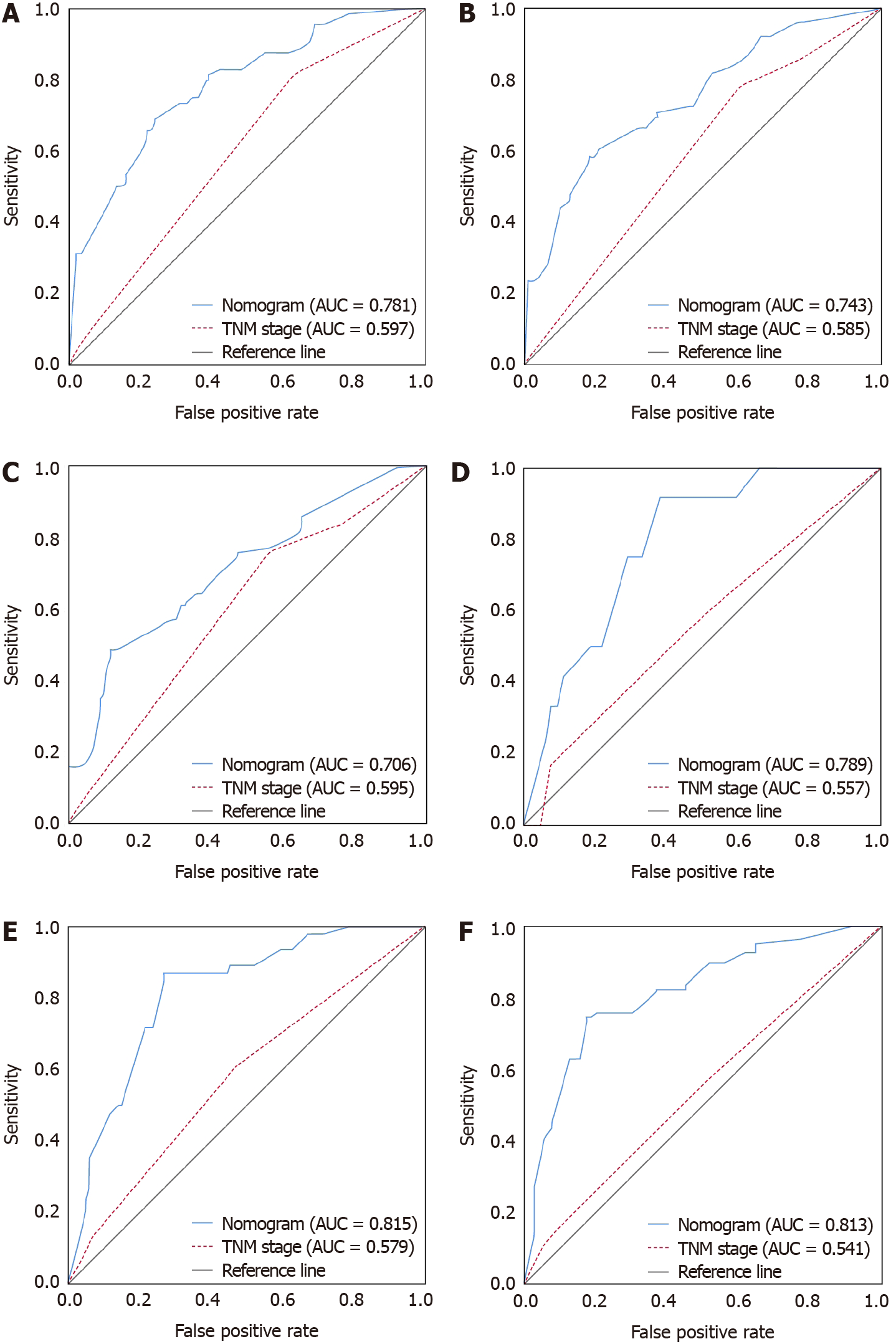
The nomogram developed in this study was more accurate than the AJCC TNM staging system in predicting the survival rate. In the training cohort, the AUCs of the nomogram-predicted OS at 1, 2 and 3 years were 0.781, 0.743 and 0.706, respectively, while the AUCs of the TNM staging system were 0.597, 0.585 and 0.595 (Figure 6A–C). In the external validation cohort, for OS predictions at 1, 2 and 3 years, the nomogram-based AUCs were 0.789, 0.815 and 0.813, respectively (Figure 6D–F), which were also higher than the TNM-based AUCs of 0.557, 0.579 and 0.541.
The main findings of this study were as follows: (1) High levels of AFP, NLR and PLR were associated with shorter OS. A lower differentiation grade and tumour size > 5 cm significantly increased the risk of death; (2) The nomogram could accurately and precisely divide patients into different risk subgroups, and the predicted OS was close to the actual observation; and (3) Compared with the AJCC TNM staging system, the nomogram combined tumour pathological characteristics and biochemical indicators and provided more prognostic information, which is conducive to individualized treatment plan formulation and postoperative consultation.
This study found that AFP[11], NLR[12], PLR[13], tumour size[14] and tumour differentiation[15] were independent risk factors for OS, which is consistent with the findings of previous studies. Tumour size and tumour differentiation were used as key variables in the AJCC TNM staging system. The lower the degree of tumour differentiation is, the greater the degree of malignancy and cell heterogeneity[16], and a larger tumour might increase intraoperative blood loss, which affects patient recovery. AFP is a glycoprotein produced by the yolk sac and liver, and it gradually decreases after reaching a peak in the foetal period. Increased AFP levels in adulthood mainly stem from the canceration of liver cells; therefore, AFP is often used for HCC monitoring and diagnosis. However, false-negative results might emerge with low AFP levels due to the regulation of genes. Wang et al[17] also proposed combining other blood test indicators to improve the sensitivity and prognostic prediction accuracy of AFP-negative HCC. Wang et al[17] focused on direct indicators such as liver function and hepatitis virus infection, while this study focused on two systemic inflammation biomarkers, NLR and PLR. Inflammation is a systemic process involving multicell participation, multipathway activation, and multifunctional execution. An increased neutrophil count indicates bacterial infection, an increased platelet count indicates acute and chronic inflammation and chronic blood loss, and a decreased lymphocyte count indicates that the immune mechanism is abnormal and that the antiviral and antitumour abilities are disturbed. Increased NLR also indicates that the body might have a cytokine storm and might release more reactive oxygen species to damage DNA; oncocytes also produce plenty of inflammatory cytokines, which induce leukocytes to accumulate around the tumour[18]. Elevated PLR levels indicate that platelets are involved in tumour development through the process of epi
The prognosis of HCC not only depends on the progression of liver malignancy but is also affected by inflammation. When biomarkers and potential risk factors are gradually detected and the monitoring and intervention window gradually advance, a prediction model with wide applicability and sensitivity is crucial. With the traditional TNM staging system, only pathological characteristics are considered, the classification and treatment measures of patients are relatively simple, and the predictive effect of OS has gradually deviated from clinical needs. Clinical medicine and preventive medicine advocate detection, treatment and intervention in the early stage. The literature has reported that the effect of early treatment on HCC is better than that of late treatment[5,22,23]. Nomograms, as a visualization method of statistical models, gradually improve individualization with the inclusion of risk factors, providing clinicians and patients with much information to understand short-term and long-term outcomes. Taking into account collinearity, the nomogram contains three tumour-related indicators (AFP, tumour size and tumour differentiation) and two inflammatory indicators (NLR and PLR). The C-index, calibration and ROC results support that the predictive accuracy of the nomogram is better than that of the AJCC TNM staging system, and the nomogram could effectively stratify liver malignancy patients and formulate personalized treatments. Compared with other nomograms[17,23-25], the nomogram developed in this study could fully reflect the immune status of the body, is economical and convenient, and has excellent accuracy.
As a two-centre retrospective study, the reliability and the level of evidence for causal inference were high, but this study still had several limitations. First, although the nomogram performed well in terms of its accuracy and consistency for prognostic prediction, the effective sample size was small, which might have affected the extrapolation to people with different characteristic distributions. Second, to balance convenience and model stability, objective indicators such as hepatitis virus infection, liver function, and cirrhosis were not included, which might have had an impact on the model. In addition, due to the lack of postoperative biochemical index information in the validation group, the effect of biomarker alterations during the perioperative period could not be discussed. Finally, it was difficult to avoid the influence of behavioural deviations and potential confounding factors.
We developed a nomogram that could predict the prognosis of HCC patients after hepatectomy based on tumour pathological characteristics and inflammation indicators. External validation and comparison with the AJCC TNM staging system showed that the nomogram had accurate discrimination and prognostic evaluation capabilities. Through this nomogram, clinicians could fully consider an individual's risk level and postoperative survival rate and provide guidance for monitoring frequency, treatment, and nursing.
Hepatocellular carcinoma (HCC) is a common malignancy. The prognosis of HCC is poor and affected by many factors.
In this study, a nomogram model was established to predict the prognosis of patients by inflammatory and pathological indicators. Through the online version of dynamic nomogram, it is more convenient for clinical use to help evaluate the prognosis of patients.
The nomogram included inflammatory markers, tumour markers and pathological indicators, ensuring that the model can comprehensively assess the prognosis of patients.
The data of HCC patients in the two largest hepatobiliary centres in Anhui province were collected retrospectively, and the independent risk factors included in the nomogram were obtained by COX analysis. Patients from the two centres were divided into training and validation cohorts. The reliability of the models was verified through internal validation and external validation.
Higher α-fetoprotein, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), tumour size ≥ 5 cm and poor differentiation were significantly associated with shortened overall survival. The nomogram was more accurate in predicting prognosis than the American Joint Committee on Cancer TNM staging system.
Inflammation-related markers (such as NLR and PLR) play a predictive role in the prognosis of patients with HCC, and should not be ignored in clinical practice. The application of online dynamic nomogram can improve clinical practicability.
The inherent limitations of retrospective studies require prospective cohort validation.
The authors would like to thank Professor Faming Pan (Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University), who had full access to all the data in the present study, for his responsibility for the integrity and accuracy of the data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakano H S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Liu JH
| 1. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2839] [Article Influence: 473.2] [Reference Citation Analysis (17)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11825] [Article Influence: 844.6] [Reference Citation Analysis (4)] |
| 3. | Liao Y, Pan S, Cao B, Luo M. Effects of blood neutrophil-to-lymphocyte ratio on predicting survival in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Practical Med. 2018;. |
| 4. | Zaydfudim VM, Vachharajani N, Klintmalm GB, Jarnagin WR, Hemming AW, Doyle MB, Cavaness KM, Chapman WC, Nagorney DM. Liver Resection and Transplantation for Patients With Hepatocellular Carcinoma Beyond Milan Criteria. Ann Surg. 2016;264:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Wan G, Gao F, Chen J, Li Y, Geng M, Sun L, Liu Y, Liu H, Yang X, Wang R, Feng Y, Wang X. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer. 2017;17:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Kattan MW, Scardino PT. Evidence for the usefulness of nomograms. Nat Clin Pract Urol. 2007;4:638-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Brunetti O, Gnoni A, Licchetta A, Longo V, Calabrese A, Argentiero A, Delcuratolo S, Solimando AG, Casadei-Gardini A, Silvestris N. Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5737] [Article Influence: 239.0] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1127] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 10. | Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |
| 11. | Menahem B, Duvoux C, Ganne N, Mallat A, Seror O, Calderaro J, Launoy G, Alves A, Cherqui D, Luciani A, Laurent A. Liver Resection for Solitary Transplantable Hepatocellular Carcinoma: The Role of AFP-Score. World J Surg. 2019;43:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Sprinzl MF, Kirstein MM, Koch S, Seib ML, Weinmann-Menke J, Lang H, Düber C, Toenges G, Zöller D, Marquardt JU, Wörns MA, Galle PR, Vogel A, Pinter M, Weinmann A. Improved Prediction of Survival by a Risk Factor-Integrating Inflammatory Score in Sorafenib-Treated Hepatocellular Carcinoma. Liver Cancer. 2019;8:387-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zheng J, Cai J, Li H, Zeng K, He L, Fu H, Zhang J, Chen L, Yao J, Zhang Y, Yang Y. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cell Physiol Biochem. 2017;44:967-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 14. | Yang A, Xiao W, Chen D, Wei X, Huang S, Lin Y, Zhang C, Lin J, Deng F, Wu C, He X. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients. Cancer Med. 2018;7:6040-6050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Zhao J, Mao J, Li W. Association of Tumor Grade With Long-Term Survival in Patients With Hepatocellular Carcinoma After Liver Transplantation. Transplant Proc. 2019;51:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 17. | Wang X, Mao M, He Z, Zhang L, Li H, Lin J, He Y, Dai S, Hu W, Liu W. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Lu Y, Xin D, Wang F. Predictive Significance Of Preoperative Systemic Immune-Inflammation Index Determination In Postoperative Liver Metastasis Of Colorectal Cancer. Onco Targets Ther. 2019;12:7791-7799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Bihari C, Rastogi A, Shasthry SM, Bajpai M, Bhadoria AS, Rajesh S, Mukund A, Kumar A, Sarin SK. Platelets contribute to growth and metastasis in hepatocellular carcinoma. APMIS. 2016;124:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Wang D, Bai N, Hu X, OuYang XW, Yao L, Tao Y, Wang Z. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Du Z, Dong J, Bi J, Bai R, Zhang J, Wu Z, Lv Y, Zhang X, Wu R. Predictive value of the preoperative neutrophil-to-lymphocyte ratio for the development of hepatocellular carcinoma in HBV-associated cirrhotic patients after splenectomy. PLoS One. 2018;13:e0195336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4497] [Article Influence: 224.9] [Reference Citation Analysis (0)] |
| 23. | Wang YY, Xiang BD, Ma L, Zhong JH, Ye JZ, Wang K, Xing BC, Li LQ. Development and Validation of a Nomogram to Preoperatively Estimate Post-hepatectomy Liver Dysfunction Risk and Long-term Survival in Patients With Hepatocellular Carcinoma. Ann Surg. 2021;274:e1209-e1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu J, Zou R, Shen J, Li B, Yuan Y. Nomogram to Predict Survival of Patients With Recurrence of Hepatocellular Carcinoma After Surgery. Clin Gastroenterol Hepatol. 2018;16:756-764.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Shen J, He L, Li C, Wen T, Chen W, Lu C, Yan L, Li B, Yang J. Nomograms to Predict the Individual Survival of Patients with Solitary Hepatocellular Carcinoma after Hepatectomy. Gut Liver. 2017;11:684-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |