Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11115
Peer-review started: July 30, 2021
First decision: September 1, 2021
Revised: September 6, 2021
Accepted: October 21, 2021
Article in press: October 21, 2021
Published online: December 16, 2021
Processing time: 133 Days and 3 Hours
Spermatocytic tumor is a rare, malignant neoplasm of the testes. Since the prognosis for this tumor type is favorable, accurate diagnosis and differentiation from other malignant testicular neoplasms (classic seminoma and lymphoma) are crucial. To add to the existing literature on the diagnosis of spermatocytic tumor, herein we report the detailed clinical and histopathologic findings for a case that we encountered.
A 60-year-old Chinese man presented with a solid mass in the right scrotum. The mass was surgically removed and spermatocytic tumor was diagnosed. On microscopy, the tumor cells displayed an unusual arrangement in lobules, presenting a pseudo-glandular appearance. To summarize and compare the diagnostic features of this tumor and those of the differential diagnoses, we report our case findings and those mentioned in the literature for various testicular tumors. Although imaging methods can detect masses early in development, their diagnostic capabilities are limited. Biopsy, histopathology, and immunohistochemistry are necessary for confirmatory diagnosis.
It is important to identify and review the key diagnostic features of spermatocytic tumor.
Core Tip: Spermatocytic tumor is a rare malignant testicular tumor. At present, there are few reported cases in the world. The understanding of clinicians and pathologists about this disease is not comprehensive enough. Here we report a case of spermatocytic tumor, emphasizing the morphological features of spermatocytic tumor and the key points of differentiation from classic seminoma and lymphoma, so as to avoid misdiagnosis.
- Citation: Hao ML, Li CH. Spermatocytic tumor: A rare case report. World J Clin Cases 2021; 9(35): 11115-11121
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11115.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11115
Spermatocytic tumor is a rare malignancy that accounts for 0.61% of testicular germ cell tumors[1]. Tumor development is independent of ethnicity and a history of cryptorchidism. Older men with an average age of 52 years are often the most affected[2]. At present, as spermatocytic tumors are extremely rare, there is a paucity of data for clinicians and pathologists to make differential diagnosis. Herein, we present a case of spermatocytic tumor, and review and consult the relevant literature. We summarize the morphological characteristics of spermatocytoma, hoping to provide clinicians and pathologists with more reliable diagnostic data and diagnostic ideas to help them serve patients better, reduce patients' pain, and avoid unnecessary, if not harmful, treatments.
A 60-year-old Chinese male farmer presented with a 3-mo history of right scrotal enlargement.
The patient presented with right scrotal enlargement and had no scrotal tenderness, chills, fever, or other discomfort. No abnormality of the left testis, epididymis, or spermatic cord was discerned. The patient did not self-medicate or seek alternative therapies. He reported no lumbar or abdominal pain and no increased frequency, urgency, or pain associated with urination, but had a slight weight loss.
The patient had no history of trauma, tuberculosis, or other relevant infectious disease.
The patient denied any family history.
A 4 cm × 5 cm, slightly moveable, solid mass was palpated in the right scrotum, which drooped and was pale in color. No normal testicular or epididymal structures were palpated in the affected testis. No abnormality of the left testis, epididymis, or spermatic cord was discerned.
The results such as routine hematological testing, blood sedimentation rate, vascular endothelial growth factor, human chorionic gonadotropin (HCG), serum carbohydrate antigen (CA)199, CA125, CA153, alpha-fetoprotein (AFP), thymidine kinase 1, and carcinoembryonic antigen (CEA) were normal.
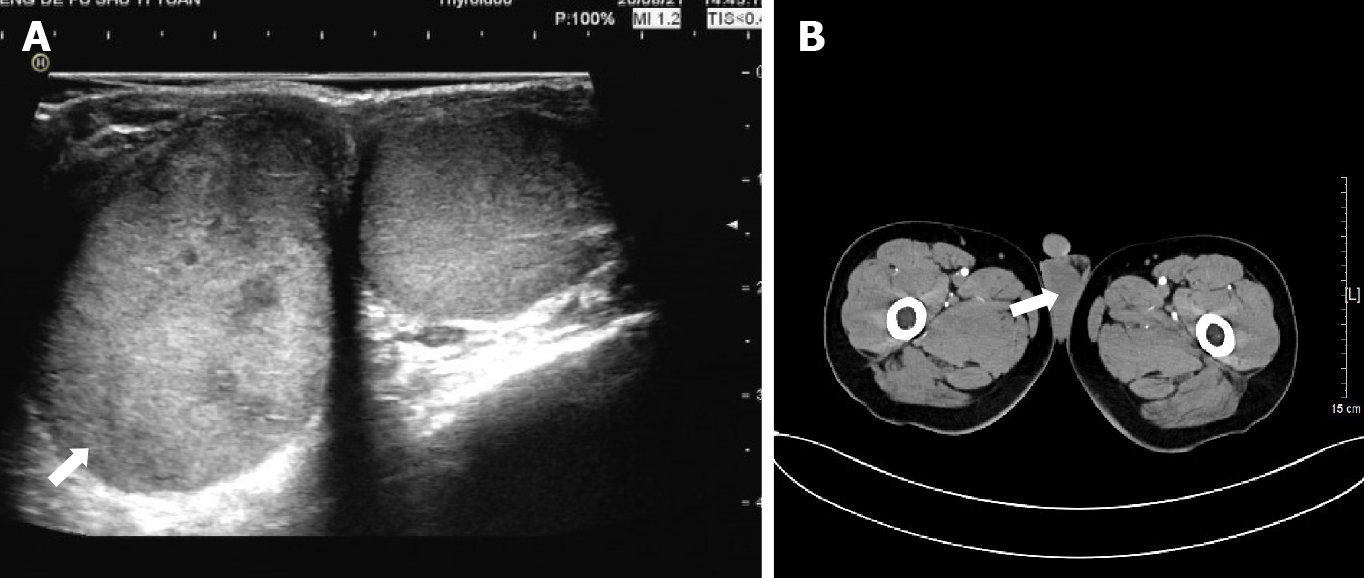
Within 1 wk from presentation, the patient underwent scrotal ultrasound showing a 4.5 cm × 2.7 cm × 3.7 cm oval-shaped hypoechoic mass, with uneven internal echogenicity in the right testicle (Figure 1A). Color Doppler showed scattered color blood flow signals within the lesion. Meanwhile, the patient underwent computed tomography (CT), which revealed an enlarged right testis, with indistinct contour, uneven density, and uniform nodular change (Figure 1B). No normal testicular or epididymal structures were palpated in the affected testis.
Spermatocytic tumor.
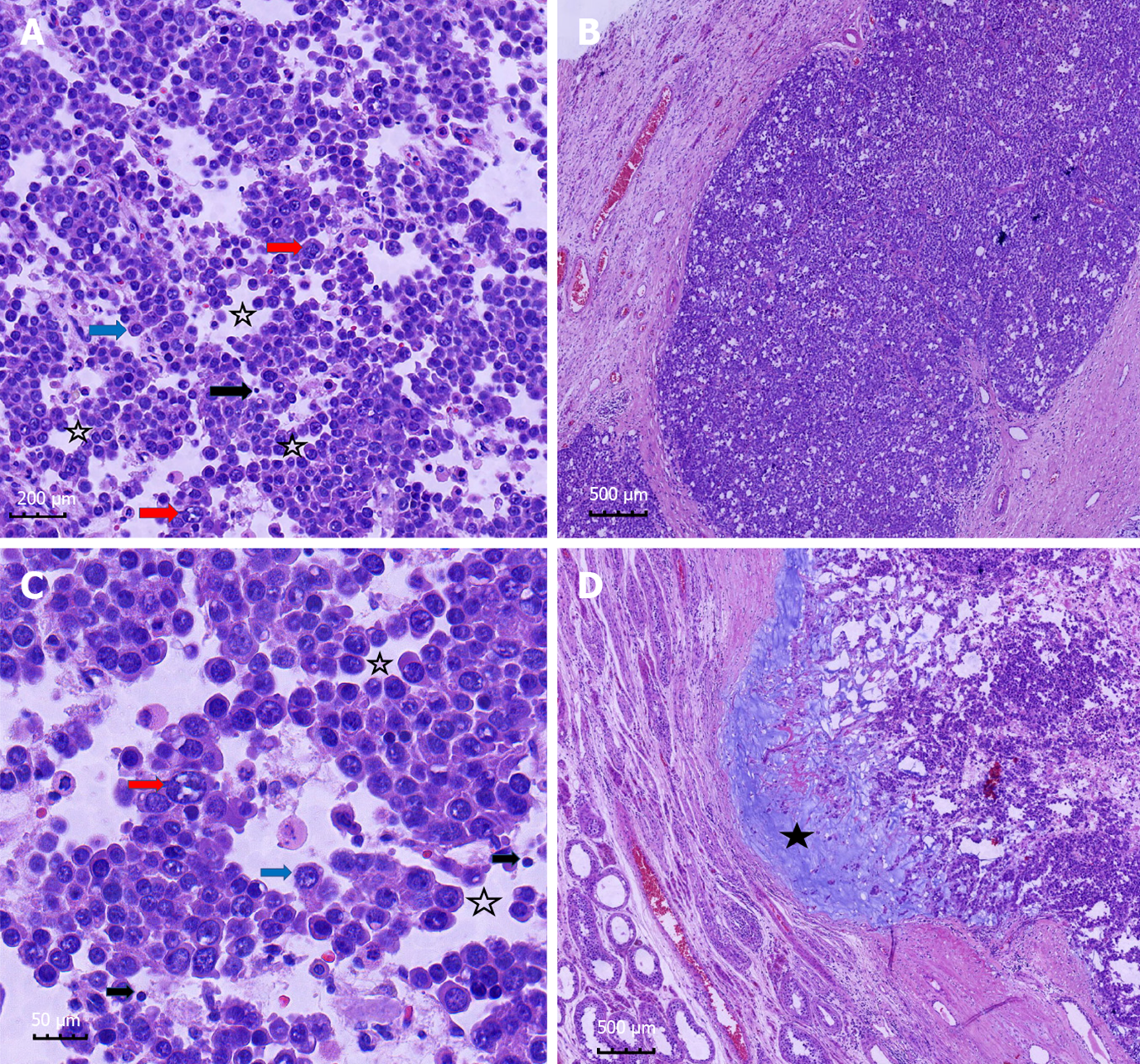
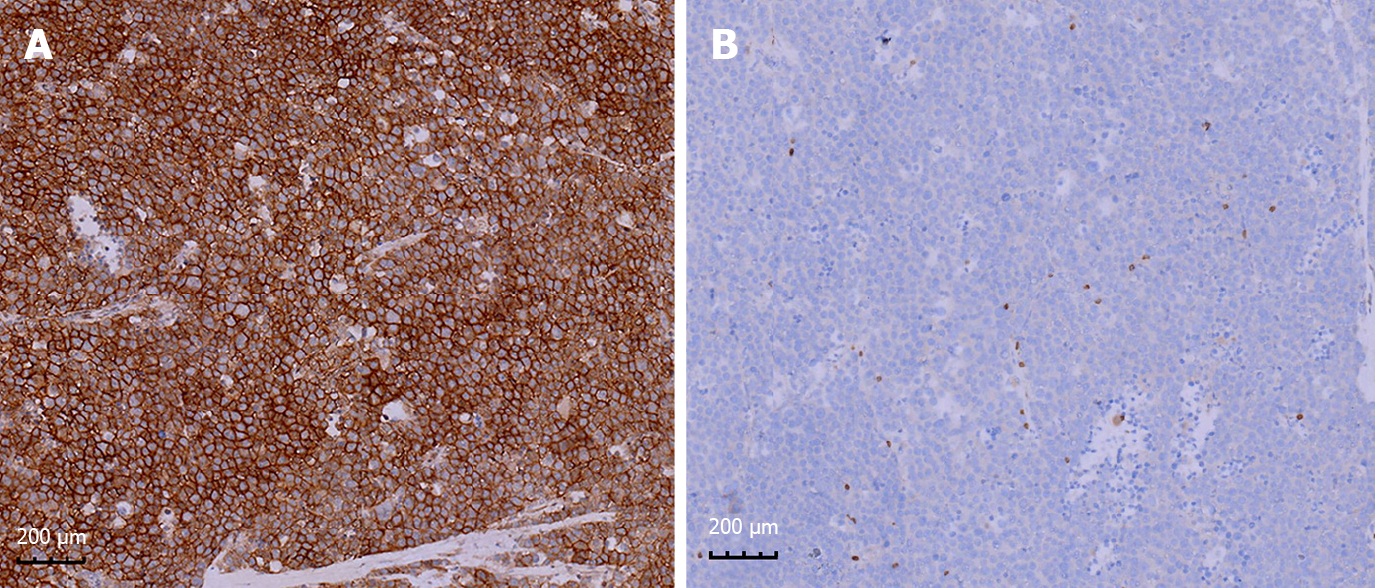
The patient agreed and voluntarily underwent orchiectomy after 1 wk. The testicular specimens were fixed with 4% formalin for 24 h. Gross examination of the right testis identified a well-demarcated, solid, lobulated, expansile, yellow-gray nodule that was 3 cm × 3 cm × 2 cm in size. The mass contained multiple areas of myxoid degeneration. The postoperative specimens were made into wax blocks and observed after hematoxylin-eosin staining. Microscopy revealed a pseudo-glandular appearance of the tumor tissue, in which aggregates of tumor cells were arranged in an edematous stroma containing scant fibrous tissue (Figure 2A and C). The tumor cells formed diffuse sheets and nests (Figure 2B). Large, small, and medium-sized tumor cells were identified. The medium-sized cell type predominated and was characterized by eosinophilic cytoplasm, suggesting decreased glycogen, round nuclei, and a filamentous chromatin pattern similar to that of spermatocytes (Figure 2A and C). The small cells resembled lymphocytes and had no obvious cytoplasm (Figure 2A and C). The large mononuclear tumor cells had round, oval, or indented nuclei, with thick chromatin and multiple nucleoli (Figure 2A and C). No mitotic figures were observed. Mucinous degeneration was observed in some areas (Figure 2D). No lymphocyte infiltration or granulomatous inflammatory response was noted. Thereafter, we performed indirect immunohistochemical staining and used mouse anti-human primary antibody and rabbit anti-mouse secondary antibody. The results revealed: CD117(+) (Figure 3A), PLAP(-) (Figure 3B), CD30(-), HCG(-), SOX-2(-), CK(-), CD45RO(-), CD3(-), D2-40(-), CK8/18(-), AFP(-), and GPC3(-). The proliferation index (Ki-67) was 80%.
The patient only underwent orchiectomy without radiotherapy or chemotherapy. No recurrence or metastasis has been observed till date (12 mo post surgery).
The theory that spermatocytic tumor precursor cells originate in embryogenesis is disputed[3]. Some scholars[1] believe that spermatocytic tumor develops from mature cells such as pachytene spermatocytes. A recent study found that these tumor cells express reproductive cell-specific markers[4]. Morphological and immunohistochemical features of the tumor cells suggest derivation from spermatogonial stem cells[5].
Clinical symptoms associated with spermatocytic tumor include painless and slowly progressive testicular swelling[6,7] and low back pain in cases with a poor prognosis due to retroperitoneal metastasis. Spermatocytic tumors range from 2 cm to 20 cm in diameter, with an average diameter of 7 cm. Grossly, Hu et al[7] reported that these lobulated tumors have a homogeneous parenchymal appearance and most tumors were pink-tan, brown-tan, or white-tan and typically soft and lobulated, mucinous. They often contain areas of edema, hemorrhage, and necrosis. The majority of these tumors are contained within the testis and do not breach the testicular sheath to infiltrate surrounding tissues[8-10]. The histomorphologic spectrum of spermatocytic tumor[11] is characterized by several points as follows: (1) At low magnification, the tumors are mainly multinodular or diffuse. All tumors have typical cell populations of three different sizes; (2) spermatocytic tumors often show edematous or myxoid degeneration with edematous stroma forming slit like structures and follicular like or irregular patterns, which are seen in some classical seminomas and rare in lymphomas; (3) tumor nodules focally show fibrous margins, and closely anastomosing connected island like structures; and (4) there is marked lymphocytic infiltration with granulomatous inflammation. It is important to recognize all the above characteristics, which will help the pathologist to diagnose and make a differential diagnosis[5]. Notably, its immunohistochemical markers are also very special. Although it belongs to germ cell tumors of the testis, it does not express useful markers in other germ cell tumors, such as OCT3/4, PLAP, AFP, HCG, and CD30. It often shows positive or weak positive expression of a key marker, CD117, and the proliferation index (Ki-67) tended to be very high. This adds challenges to our diagnostic work and requires pathologists to constantly expand their diagnostic ideas.
Spermatocytic tumor must be distinguished from classic and anaplastic seminoma. Classic seminoma is characterized by an earlier average age of onset (30 years)[2] and tumor cells that are often rich in glycogen with clear cytoplasm. The tumor cells form nests that are rimmed by fibrous tissue bands. Lymphocytic infiltration and a granulomatous inflammatory response are seen in the stroma. Classic seminoma tumor cells are positive for the immunohistochemical markers such as PLAP, CD117, vimentin, LDH, ferritin, and germ cell antigen, but usually negative for high molecular weight keratin and CD30. Anaplastic seminoma cells display obvious heteromorphism and increased mitotic rate, but do not stain positively for the abovementioned immunohistochemical markers[12]. Some experts proposed that classic seminoma and anaplastic seminoma are variants of the same tumor[13]. An elevated serum HCG level and/or tumor features, such as hemorrhage, necrosis, and vascular infiltration, are seen in patients with invasive, late clinical stage anaplastic seminoma that has a poor prognosis[14]. The spermatocytic tumor we identified in our patient contained small, medium, and large tumor cells that were slightly separated within an edematous stroma, creating a pseudo-glandular tissue appearance. The lack of lymphocytic infiltration, granulomatous inflammatory response, increased level of mitosis, and sarcomatous components were features of our patient’s tumor that were consistent with this diagnosis. The tumor in our patient’s case lacked markers (OCT3/4, AE1, AE3, and CD30) that are absent in spermatocytic tumor, but present in other germ cell tumors. Moreover, immunohistochemical staining for CD117 was positive in our patient’s tumor; this marker is a key feature for identifying spermatocytic tumor.
The clinical features and imaging findings of testicular spermatocytic tumor are not distinct from those of classic spermatoplasm cytomaspermatocytic tumor and other types of testicular tumors. Laboratory test results for serum LDH, HCG, and AFP levels are usually not elevated. Ultrasound is useful for early testicular tumor detection and is a preferred imaging method due to its non-invasive nature[14-18]. CT provides more information regarding features such as tumor boundary, internal architecture, involvement of surrounding structures, and lymph node metastasis, and is useful for establishing a preoperative presumptive diagnosis and for postoperative follow-up[19-22]. However, biopsy and histopathology are required to confirm the diagnosis. Currently, orchiectomy is the preferred treatment for testicular spermatocytic tumor. The scope of surgical resection should include the testicular epididymis and a portion of the spermatic cord to ensure complete excision for the 10%-15% of testicular tumors that invade these structures[23-26]. Most spermatocytic tumors exhibit benign behavior, with a low potential for invasion and metastasis. Given the favorable prognosis for this tumor type, no adjuvant therapy is usually required following excision by orchidectomy[27,28]. However, tumors that are larger than 4 cm in diameter, involve the epididymis or spermatic cord, or have sarcomatous features may have an increased risk for malignant behavior and recurrence. Long term follow-up is necessary in such cases.
In summary, we report a typical case of spermatocytic tumor, review its epidemiology and various examined features, as well as pathological features such as three typical cellular morphologies and interstitial mucinous degeneration, and make a differential diagnosis with its similar counterpart. The first line of therapy for spermatocytic tumor is orchiectomy, which does not require other adjuvant therapy unless there is an incomplete or spermatic cord, or recurrent features. Long-term follow-up may be recommended to identify potential postoperative recurrence.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Andrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Grogg JB, Schneider K, Bode PK, Wettstein MS, Kranzbühler B, Eberli D, Sulser T, Beyer J, Hermanns T, Fankhauser CD. A systematic review of treatment outcomes in localised and metastatic spermatocytic tumors of the testis. J Cancer Res Clin Oncol. 2019;145:3037-3045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Facchini G, Rossetti S, Berretta M, Cavaliere C, D'Aniello C, Iovane G, Mollo G, Capasso M, Della Pepa C, Pesce L, Facchini S, Imbimbo C, Pisconti S. Prognostic and predictive factors in testicular cancer. Eur Rev Med Pharmacol Sci. 2019;23:3885-3891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Menon S, Karpate A, Desai S. Spermatocytic seminoma with rhabdomyosarcomatous differentiation: a case report with a review of the literature. J Cancer Res Ther. 2009;5:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Haroon S, Tariq MU, Fatima S, Kayani N. Spermatocytic seminoma: a 21 years' retrospective study in a tertiary care hospital in Pakistan. Int J Clin Exp Pathol. 2013;6:2350-2356. [PubMed] |
| 5. | Waheeb R, Hofmann MC. Human spermatogonial stem cells: a possible origin for spermatocytic seminoma. Int J Androl. 2011;34:e296-305; discussion e305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Patel PM, Patel HD, Koehne EL, Doshi C, Belshoff A, Seffren CM, Baker M, Gorbonos A, Gupta G. Contemporary Trends in Presentation and Management of Spermatocytic Seminoma. Urology. 2020;146:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Hu R, Ulbright TM, Young RH. Spermatocytic Seminoma: A Report of 85 Cases Emphasizing Its Morphologic Spectrum Including Some Aspects Not Widely Known. Am J Surg Pathol. 2019;43:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Jha RK, Mathur S, Saidha NK. A case of spermatocytic seminoma in young individual. Med J Armed Forces India. 2018;74:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Pandey V, Khatib Y, Khade AL, Pandey R, Khare MS. Spermatocytic seminoma with rhabdomyoblastic differentiation: Case report and review of literature. Indian J Pathol Microbiol. 2018;61:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Stueck AE, Grantmyre JE, Wood LA, Wang C, Merrimen J. Spermatocytic Tumor With Sarcoma: A Rare Testicular Neoplasm. Int J Surg Pathol. 2017;25:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Mikuz G. [Spermatocytic seminoma. A tumor with many faces]. Pathologe. 2014;35:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Lieng H, Warde P, Bedard P, Hamilton RJ, Hansen AR, Jewett MAS, O'malley M, Sweet J, Chung P. Recommendations for followup of stage I and II seminoma: The Princess Margaret Cancer Centre approach. Can Urol Assoc J. 2018;12:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Marko J, Wolfman DJ, Aubin AL, Sesterhenn IA. Testicular Seminoma and Its Mimics: From the Radiologic Pathology Archives. Radiographics. 2017;37:1085-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Azizi M, Peyton CC, Boulware DC, Gilbert SM, Sexton WJ. Primary tumor size thresholds in stage IA testicular seminoma: Implications for adjuvant therapy after orchiectomy and survival. Urol Oncol. 2020;38:7.e9-7.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | He Y, Liao H, Xiang X, Cai D, Qiu L. High-frequency ultrasonography and contrast-enhanced ultrasound for the evaluation of testicular capillary hemangioma: A case report. Medicine (Baltimore). 2019;98:e14779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Huang H, Ling W, Qiu T, Luo Y. Ultrasonographic features of testicular metastasis from renal clear cell carcinoma that mimics a seminoma: A case report. Medicine (Baltimore). 2018;97:e12728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Fu J, Yang W, Wang S, Bai J, Wu H, Wang H, Yan K, Chen M. Clinical value of contrast-enhanced ultrasound in improving diagnostic accuracy rate of transthoracic biopsy of anterior-medial mediastinal lesions. Chin J Cancer Res. 2016;28:617-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Saba L. The primitive extratesticular seminoma: diagnosis of a rare pathology. Acta Biomed. 2017;88:82-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Lwin TT, Yoneyama A, Maruyama H, Takeda T. Visualization Ability of Phase-Contrast Synchrotron-Based X-Ray Imaging Using an X-Ray Interferometer in Soft Tissue Tumors. Technol Cancer Res Treat. 2021;20:15330338211010121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Bantis A, Sountoulides P, Metaxa L, Pavlidis P, Aggelonidou E, Arif H, Zisimopoulos A. The diagnostic yield of fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in recurrent testicular seminoma. Urol Ann. 2016;8:496-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Gossner J. Computed tomography as a problem solving tool in non-radiopaque central venous port systems - A report of three cases. Interv Med Appl Sci. 2014;6:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | von Amsberg G, Sehovic M, Hartmann M, Bokemeyer C. [Diagnosis and treatment of rare testicular tumors using the example of malignant mesothelioma of the tunica vaginalis testis and Sertoli cell tumors]. Urologe A. 2021;60:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Bois JI, Vagni RL, de Badiola FI, Moldes JM, Losty PD, Lobos PA. Testis-sparing surgery for testicular tumors in children: a 20 year single center experience and systematic review of the literature. Pediatr Surg Int. 2021;37:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Al-Obaidy KI, Idrees MT. Testicular Tumors: A Contemporary Update on Morphologic, Immunohistochemical and Molecular Features. Adv Anat Pathol. 2021;28:258-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Miao X, Li Y, Zhou T, Lv M. Testis-sparing surgery in children with testicular tumors: A systematic review and meta-analysis. Asian J Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Michalová K, Michal M, Hora M, Hes O. Practices recommendations in the applications of immunohistochemistry and molecular genetics in testicular tumors. Review article. Cesk Patol. 2020;56:153-160. [PubMed] |
| 27. | Schwarze V, Marschner C, Sabel B, de Figueiredo GN, Marcon J, Ingrisch M, Knösel T, Rübenthaler J, Clevert DA. Multiparametric ultrasonographic analysis of testicular tumors: a single-center experience in a collective of 49 patients. Scand J Urol. 2020;54:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 28. | Fankhauser CD, Roth L, Kranzbühler B, Eberli D, Bode P, Moch H, Oliveira P, Ramani V, Beyer J, Hermanns T. The Role of Frozen Section Examination During Inguinal Exploration in Men with Inconclusive Testicular Tumors: A Systematic Review and Meta-analysis. Eur Urol Focus. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |