Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11085
Peer-review started: June 28, 2021
First decision: July 26, 2021
Revised: August 15, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: December 16, 2021
Processing time: 165 Days and 6.1 Hours
Acute myocarditis is an acute myocardium injury that manifests as arrhythmia, dyspnea, and elevated cardiac enzymes. Acute myocarditis is usually caused by a viral infection but can sometimes be caused by autoimmunity. Graves’ disease is an autoimmune disease that is a rare etiology of acute myocarditis. Accelerated junctional rhythm is also a rare manifestation of acute myocarditis in adults.
A rare case of new-onset Graves’ disease combined with acute myocarditis and thyrotoxic periodic paralysis is reported. The patient was a 25-year-old young man who suddenly became paralyzed and felt palpitations and dyspnea. He was then sent to our emergency department (ED). Upon arrival, electrocardiography revealed an accelerated junctional rhythm and ST-segment depression in all leads, and laboratory findings showed extreme hypokalemia and elevated troponin I, with the troponin I level being 0.32 ng/mL (reference range, 0-0.06 ng/mL). Coronary computer tomography angiography was performed, and there were no abnormal findings in the coronary arteries. Subsequently, the patient was admitted to the ED ward, where further testing revealed Graves’ disease, along with continued elevated cardiac enzyme levels and B-type natriuretic peptide (BNP) levels. The troponin I level was 0.24 ng/mL after admission. All of the echocardiography results were normal: Left atrium 35 mm, left ventricle 48 mm, end-diastolic volume 102 mL, right atrium 39 mm × 47 mm, right ventricle 25 mm, and ejection fraction 60%. Cardiac magnetic resonance was performed on the fifth day of admission, revealing myocardial edema in the lateral wall and intramyocardial and subepicardial late gadolinium enhancement in the lateral apex, anterior lateral, and inferior lateral segments of the ventricle. The patient refused to undergo an endomyocardial biopsy. After 6 d, the patient’s cardiac enzymes, BNP, potassium, and electrocardiography returned to normal. After the patient’s symptoms were relieved, he was discharged from the hospital. During a 6-mo follow-up, the patient was asymptomatic and subjected to thyroid function, liver function, kidney function, troponin I, and electrocardiograph routine tests for medicine adjustments. The hyperthyroid state was controlled.
Acute myocarditis is a rare manifestation of Graves’ disease. Accelerated junctional rhythm is also a rare manifestation of acute myocarditis in adults. When the reason for hypokalemia and elevated cardiac enzymes in patients is unknown, cardiologists should consider Graves’ disease and also pay attention to accelerated junctional rhythm.
Core Tip: Junctional rhythm is a significantly rare occurrence in patients and is a manifestation of acute myocarditis. The etiology of junctional rhythm may be attributed to autoimmunity, and physicians should not ignore such arrhythmia. In addition to viruses, autoimmune diseases like Graves’ disease can also cause acute myocarditis. The present case highlights that those endocrine diseases should not be disregarded in patients who present with cardiovascular symptoms.
- Citation: Li MM, Liu WS, Shan RC, Teng J, Wang Y. Acute myocarditis presenting as accelerated junctional rhythm in Graves’ disease: A case report. World J Clin Cases 2021; 9(35): 11085-11094
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11085.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11085
Graves’ disease is an autoimmune disorder that affects the thyroid gland[1]. Hyperthyroidism affects 0.5%-2% of females[2] in geographical areas not featuring iodine deficiency. Males show a 10-fold lower prevalence. Graves’ disease is the most frequent cause and is more likely to occur in female populations[2]. Graves’ disease would seem to be more frequent in Asian populations and less frequent in sub-Saharan Africans[2]. Thyroid hormone (TH) receptors are present in the myocardium and vascular endothelial tissues, thereby allowing changes in circulating TH concentration to modulate end-organ activity[3]. Thus, Graves’ disease can present with cardiovascular manifestations. Usually misdiagnosed as myocardial infarction, Graves’ disease combined with acute myocarditis is a rare manifestation, and the etiology is due to an autoimmune process.
When the electrical activity of the sinoatrial node is blocked or is less than the automaticity of the atrioventricular node/His bundle, a junctional rhythm originates[4]. Numerous conditions can cause a junctional rhythm, among which myocarditis is a rare etiology[4]. Acute myocarditis should be diagnosed when several differential diagnoses are excluded, such as tachycardiomyopathy (TCMP), stress cardiomyopathy, and pericardial diseases. Acute myocarditis presents with junctional arrhythmia is reported in children and seldomly reported in adults. There have been a few reports about Graves’ disease combined with acute myocarditis[5-7]. However, the patient’s manifestations differ in these cases. None of these cases presents with junctional arrhythmia. In this case, the patient presented with an accelerated junctional rhythm and myocarditis, which is unique compared with other reported cases, so that clinicians can have a new understanding of the cardiovascular complications of Graves’ disease.
Sudden paralysis, dyspnea, and vomiting for 1 d.
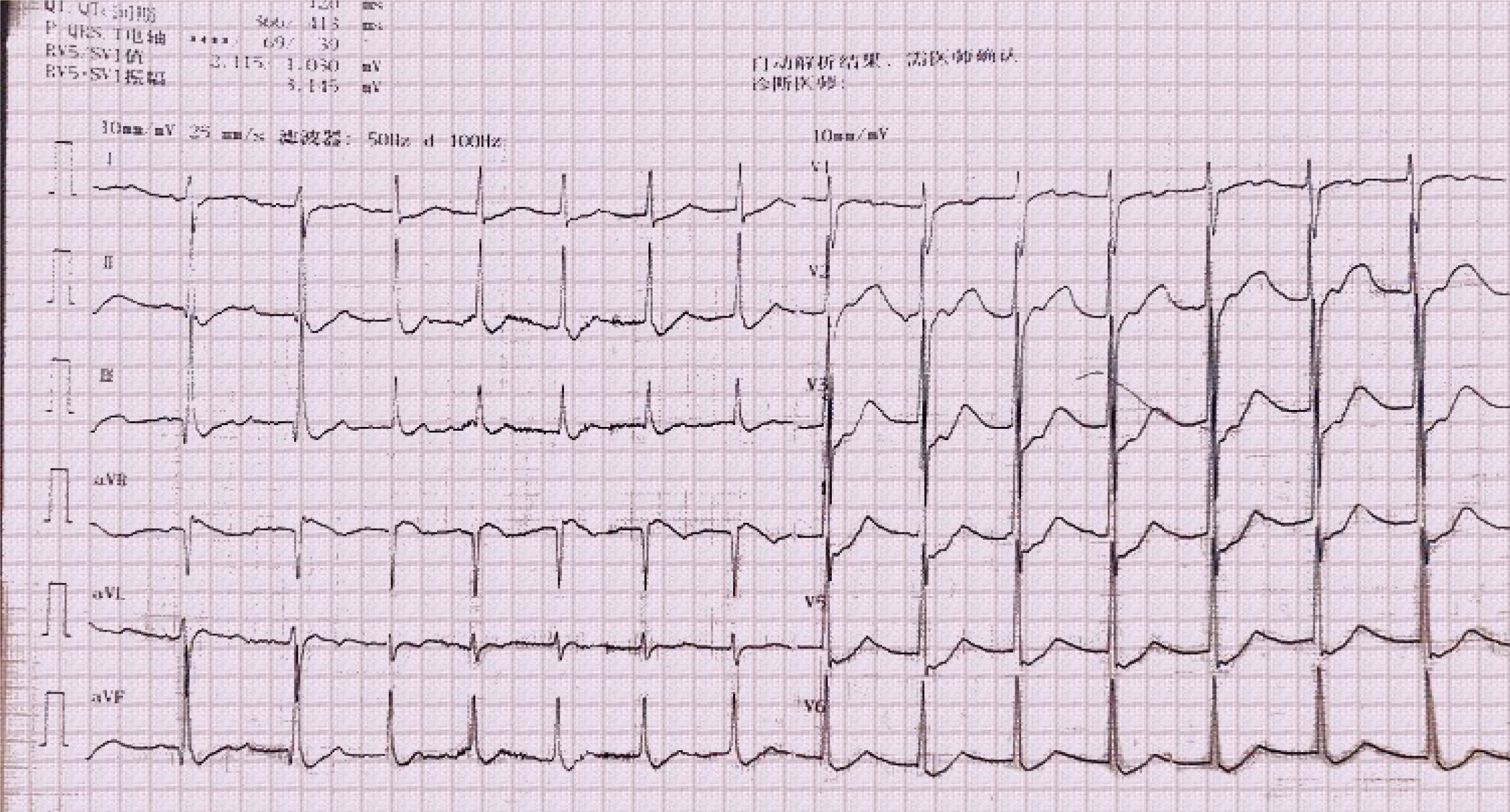
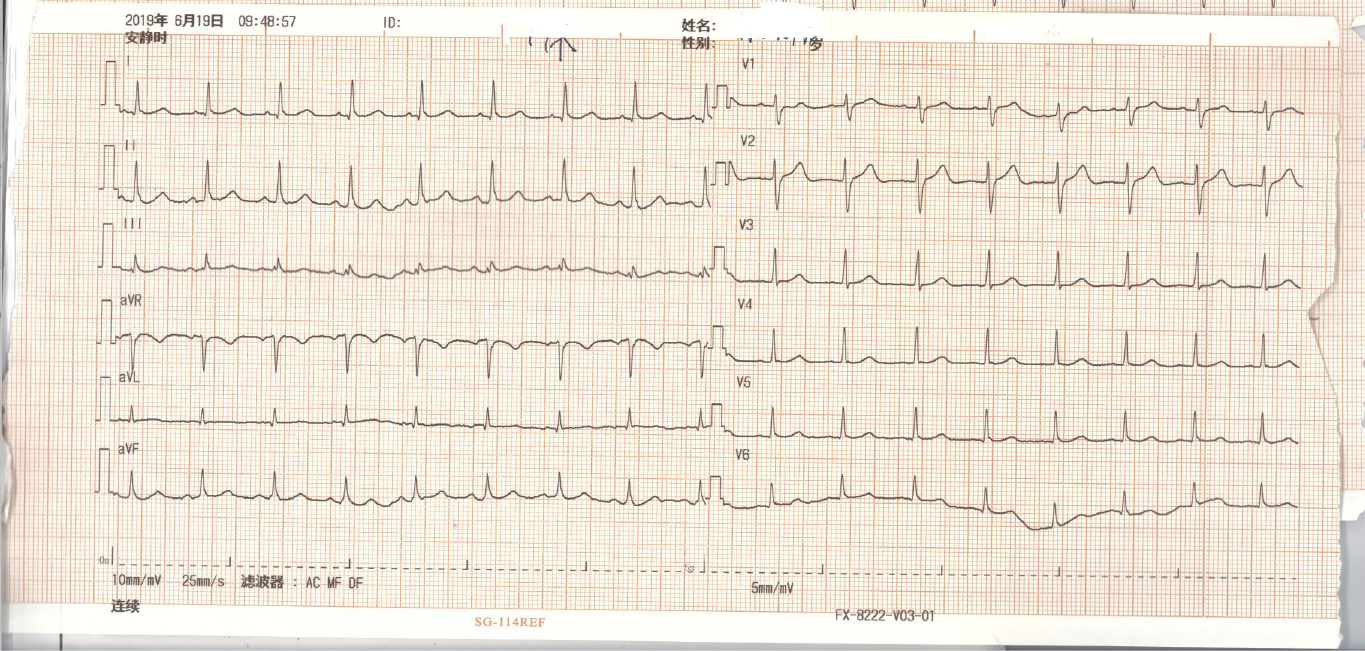
A 25-year-old young male realized that he was paralyzed when he woke in the morning. At the same time, the patient felt palpitations, dyspnea, and nausea, with one instance of vomiting gastric contents. The patient was then brought to the emergency department (ED) by ambulance. Upon arrival, electrocardiography revealed an accelerated junctional rhythm (heart rate 91 beats per minute, Figure 1) and ST-segment depression in all leads. The laboratory results showed potassium 1.7 mmol/L and troponin I 0.32 ng/mL (reference range, 0-0.06 ng/mL). Acute myocardial infarction or acute myocarditis and hypokalemic periodic paralysis were considered, and thus, the ED administered potassium supplements orally and intravenously, oxygen inspiration, aspirin, and clopidogrel. Metoprolol was administered to control the heart rate. Due to the young age of the patient and no risk factors contributing to acute myocardial infarction, the ED department suggested an emergent coronary computer tomography (CT) angiography and a brain computer tomography to rule out more dangerous diseases. The results showed no abnormal findings of the coronary artery and the brain. Accordingly, the patient was diagnosed with acute myocarditis. The patient was then admitted to the ED ward, in which he was diagnosed with suspected acute myocarditis and hypokalemic periodic paralysis (reason unknown). The next step was to determine the primary disease.
The patient had no previous health issues.
The patient’s family history did not reveal anything significant to the present condition. The patient was healthy and had not taken any drugs previously. He also reported no recent changes in weight.
The patient was conscious and afebrile, and his blood pressure was 110/65 mmHg. He was agitated and sweating profusely. Muscle strength was grade 2. According to the patient’s high metabolic condition, hyperthyroidism was considered the most common cause of hypokalemic periodic paralysis in young males. We especially checked the thyroid gland. There was no exophthalmos of the patient’s eyes, and no restriction of eye movements. There were no hand tremors. Palpation of the thyroid showed II degree of swelling of the thyroid gland with no abnormal findings on the isthmus. There was no tenderness. On auscultation of the thyroid, a bruit could be heard. The lungs, heart, and abdomen were subsequently examined, all of which were normal.
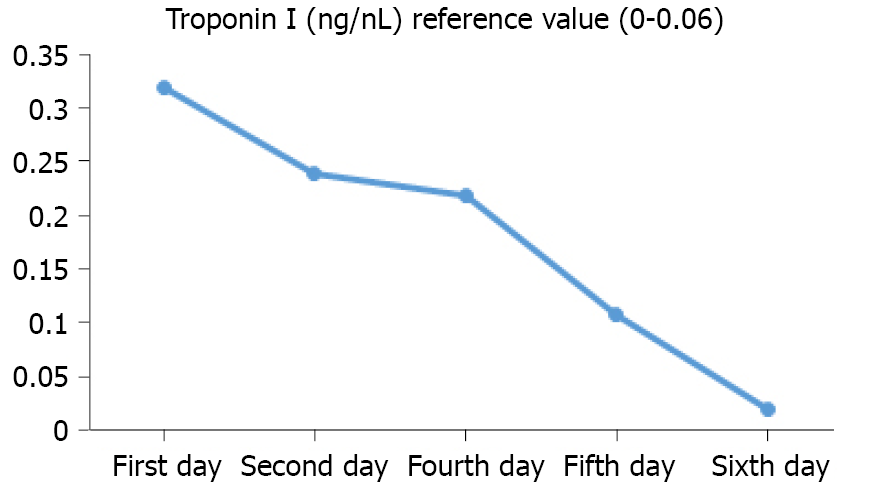
Thyroid function tests revealed a hyperthyroid state, and thus, Graves’ disease was considered: T3 17.51 pmol/L (3.1-6.8 pmol/L), T4 39.68 pmol/L (12-22 pmol/L), thyroid-stimulating hormone (TSH) 0.005 μIU/mL (0.27-4.2 μIU/mL), thyroglobulin 94.77 ng/mL, anti-thyroglobulin antibodies 18.35 IU/mL (normal), TSH receptor antibody (TSHR-AB) 13.76 IU/L (0-1.5 IU/L), and thyroid peroxidase antibody 77.67 IU/mL (0-34 IU/mL). Other significant laboratory findings revealed elevated troponin I and elevated B-type natriuretic peptide (BNP) [troponin I 0.24 ng/mL (reference range, 0-0.06 ng/mL) and BNP 196.24 pg/mL]. The troponin I level measurement was performed five times, and the trend is shown in Figure 2. The inflammatory markers C-reactive protein and erythrocyte sedimentation rate were also measured, which were elevated to 12.6 mg/L and 50.3 mm/h, respectively (the references were within 0.5 mg/L and 20 mm/h, respectively). Initially, viral myocarditis was considered. The nucleic acids of 13 common virus types were checked in throat swabs and no positive results were found. The 13 virus types were as follows: Adenovirus, influenza-a, influenza-b, parainfluenza virus, respiratory syncytial virus, Bocavirus, rhinovirus, influenza H1N1, chlamydia, metapneumovirus, influenza H3N2, coronavirus, and Mycoplasma pneumoniae. Since the belief was that autoimmunity might be the etiology, cardiac magnetic resonance (CMR) and endocardial myocardial biopsy (EMB) were suggested.
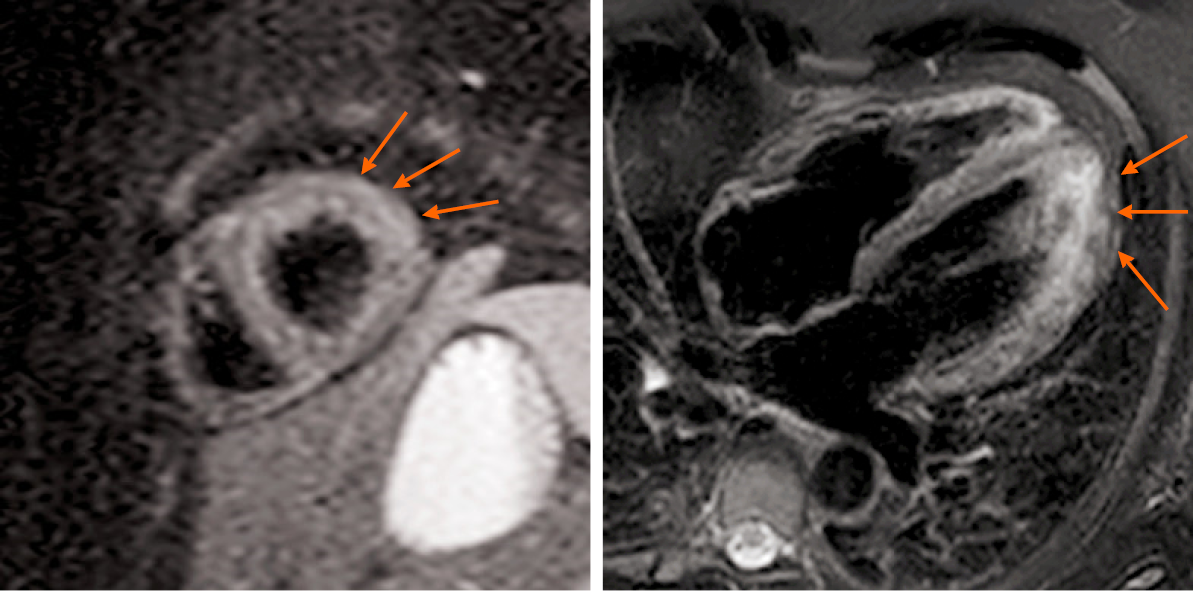
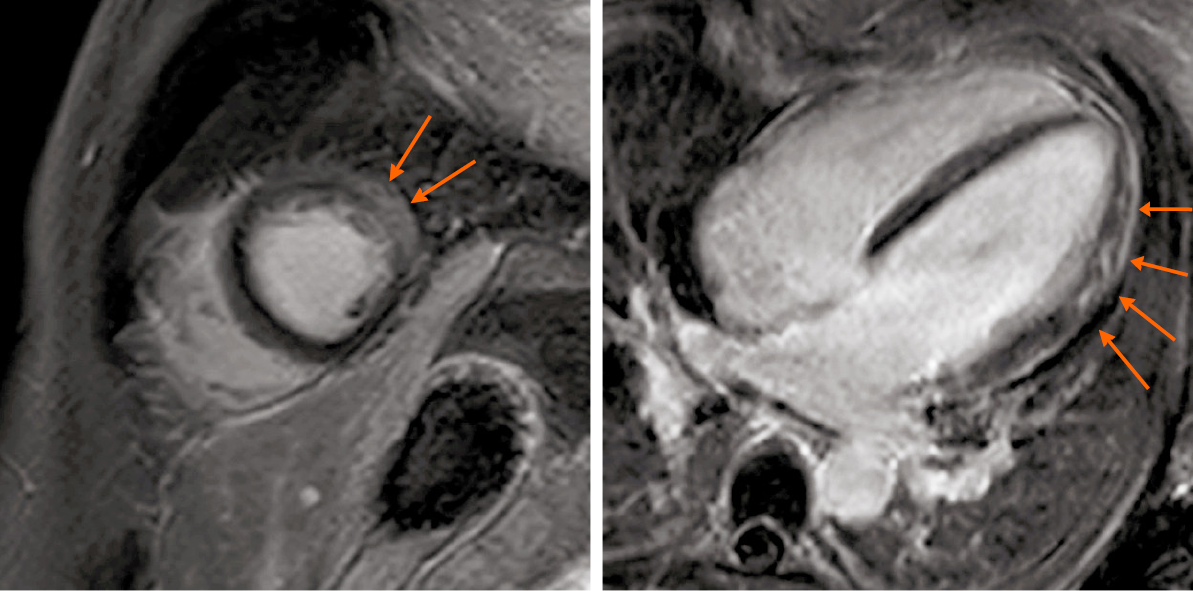
CMR was performed on the fifth day of admission. The results showed myocardial edema in the lateral wall and intramyocardial and subepicardial late gadolinium enhancement in the lateral apex, anterolateral, and inferior lateral segments of the ventricle (Figure 3 and 4). Said results suggested acute myocarditis. The patient refused to undergo an EMB examination, but echocardiography was performed, with the results being normal: Left atrium 35 mm, left ventricle (LV) 48 mm, end-diastolic volume 102 mL, right atrium 39 mm × 47 mm, right ventricle 25 mm, and ejection fraction (EF) 60%. Thyroid ultrasonography was performed to confirm the diagnosis of Graves’ disease, which showed an enlarged thyroid gland and rich blood flow signal, and no tumor was found. Thyroid static imaging was then performed to exclude subacute thyroiditis, which showed bilateral lobe swelling and increased function. Such examinations confirmed the diagnosis of Graves’ disease.
Acute myocarditis presenting as an accelerated junctional rhythm in Graves’ disease.
An endocrinologist was consulted, who suggested that the patient should undergo radioactive iodine therapy. However, the patient expressed a preference for taking medicine. Thus, according to recommendations, methimazole 20 mg/d was administered to treat hyperthyroidism, while trimetazidine 60 mg/d, metoprolol 50 mg/d, and calcium dibutyryl adenosine cyclophosphate 40 mg/d were administered for myocarditis.
The patient’s symptoms were relieved within 6 d, and troponin I, BNP, and electrocardiography tests were performed. All tests showed normal results (Figure 5). The patient was discharged from the hospital and was instructed to continue taking methimazole, trimetazidine, and metoprolol.
A 6-mo follow-up process was performed in the emergency clinic and by phone calls, and the patient continued taking metoprolol, trimetazidine, and thiamazole. The patient was asymptomatic aside from several symptoms of thyrotoxicosis, and subjected to thyroid function, liver function, kidney function, troponin I, and electrocardiograph routine tests for medicine adjustments. After 45 d, all of the patient’s symptoms disappeared and thyroid function improved: T3 12.26 pmol/L (3.1-6.8 pmol/L), T4 28.37 pmol/L (12-22 pmol/L), and TSH 0.07 μIU/mL (0.27-4.2 μIU/mL). After 80 d, the euthyroid state was restored, and the patient’s liver and kidney functions were in good condition. Electrocardiography and troponin I levels were also normal. Methimazole was adjusted to 5 mg/d and metoprolol was adjusted to 23.75 mg/d.
Acute myocarditis is an acute injury of the myocardium that manifests as arrhythmia, dyspnea, and elevated cardiac enzymes. Acute myocarditis is usually caused by a viral infection but can sometimes be caused by autoimmunity. An autoimmune state is always triggered in patients with acute autoimmune myocarditis, such as systemic lupus erythematosus, rheumatoid arthritis, and others[8]. Graves’ disease is also an autoimmune disease and can manifest as acute autoimmune myocarditis. However, acute autoimmune myocarditis is rarely observed in patients with Graves’ disease. Despite a previous case report in which acute autoimmune myocarditis could have been a manifestation of Graves’ disease[5], the patient did not manifest with junctional arrhythmia and was not suffering from new-onset Graves’ disease. Thus, there are several significant differences in comparison with the present case report. The rarity and diagnosis of this case are further clarified in Tables 1 and 2[8].
| Ref. | Setting | Main findings | Correlation and difference compared with this case |
| [6] | A 29-year-old male presents with hyperthyroidism and chest pain | The patient is diagnosed with new-onset of Graves’ disease combined with myocarditis | The manifestation in the study is similar to this case report. However, that patient has already known that he had hyperthyroidism, which reduces the difficulty of the diagnosis. That patient does not present with hypokalemic periodic paralysis. Withal, the patient presents with sinus tachycardia on the electrocardiograph instead of a junctional rhythm |
| [5] | A 40-year-old male presents with refractory hyperthyroidism and chest pain | The patient is diagnosed with Graves’ disease combined with myocarditis | The study is similar to the above research. The patient is finally diagnosed with refractory Graves’ disease combined with myocarditis. No other manifestations are observed |
| [7] | A 31-year-old woman with 2-mo pregnancy with hyperthyroidism complained of palpitation and excessive sweating | The patient is diagnosed with Graves’ disease combined with myocarditis | The patient has reduced ejection fraction in the study. Besides, the patient does not present with other combinations except for myocarditis, which is different from this case |
| Examinations and presentations | Features |
| ECG/Holter/stress test | Newly abnormal 12 lead ECG and/or Holter and/or stress testing, any of the following: I to III degree atrioventricular block, or bundle branch block, ST/T wave change (ST elevation or non-ST elevation, T wave inversion), sinus arrest, ventricular tachycardia or fibrillation and asystole, atrial fibrillation, reduced R wave height, intraventricular conduction delay (widened QRS complex), abnormal Q waves, low voltage, frequent premature beats, supraventricular tachycardia |
| Myocardiocytolysis markers | Elevated TnT/TnI |
| Functional and structural abnormalities on cardiac imaging (echo/angio/CMR) | New, otherwise unexplained LV and/or RV structure and function abnormality (including incidental finding in apparently asymptomatic subjects): regional wall motion or global systolic or diastolic function abnormality, with or without ventricular dilatation, with or without increased wall thickness, with or without pericardial effusion, with or without endocavitary thrombi |
| Tissue characterization by CMR | Oedema and/or LGE of classical myocarditic pattern |
| Clinical presentationsa | Acute chest pain, pericarditic, or pseudo-ischaemic (1) New-onset (days up to 3 mo) or worsening of: Dyspnea at rest or exercise, and/or fatigue, with or without left and/or right heart failure signs; (2) Subacute/chronic (> 3 mo) or worsening of: dyspnea at rest or exercise, and/or fatigue, with or without left and/or right heart failure signs; (3) Palpitation, and/or unexplained arrhythmia symptoms and/or syncope, and/or aborted sudden cardiac death; and (4) Unexplained cardiogenic shock |
The present patient’s electrocardiograph, elevated troponin I, normal coronary arteries, symptoms, and CMR results were consistent with acute myocarditis[8]. However, several differential diagnoses, such as TCMP, stress cardiomyopathy, and pericardial diseases, had to be excluded. If there is evidence of persistent or frequently occurring tachycardia or frequent premature ventricular complexes, the possibility of TCMP should be considered when eliciting a history of any new diagnosis of LV dysfunction. The traditional clinical presentation includes symptoms and signs of congestive heart failure and dilated cardiomyopathy. Other factors that point to a diagnosis of TCMP include: (1) Evidence of a previously normal EF and a degree of LV dysfunction out of proportion to other comorbidities; (2) no other cause of non-ischemic cardiomyopathy (e.g., hypertension, alcohol or drug use, and stress (3) absence of left ventricular hypertrophy; (4) relatively normal LV dimensions (LV end-diastolic dimension below 5.5 cm); (5) recovery of LV function after control of tachycardia (by rate control, cardioversion or radiofrequency ablation within 1-6 mo); and (6) rapid decline in LV ejection fraction following the recurrence of tachycardia in a patient with recovered LV function after previous control of tachycardia[9]. The patient had no previous health issues and had no history of tachycardia. Moreover, the patient’s heart rate was 91 bpm initially, which could not be defined as tachycardia. Hence, there was no evidence of persistent tachycardia. Echocardiography and CMR did not reveal any LV dysfunction. The ejection fraction was normal, and there were no significant abnormalities in the cardiac structure. No dilation of the atrium and ventricles was observed, and no hypertrophy was observed. The results above could exclude the possibility of TCMP[9]. The patient did not meet the criteria for stress cardiomyopathy listed in the guidelines of the Heart Failure Association-European Society of Cardiology Criteria and the Revised Mayo Clinic Criteria[10]. The patient did not have left ventricular dysfunction, wall motion abnormalities, or emotional disorders, and echocardiography was normal. The patient’s CMR confirmed the diagnosis of myocarditis, which excluded the probability of stress cardiomyopathy[10]. According to the latest diagnostic criteria[11], acute pericarditis could be excluded in the patient. The patient did not have chest pain, and a pericardial friction rub was not heard. There was no new ST-segment elevation or PR segment depression in the patient, and CMR results did not suggest pericardial involvement. Since myopericarditis has myocardial involvement, the clinical presentation thereof is considerably similar to that of myocarditis. Myopericarditis was diagnosed when the patient had both acute pericarditis and elevated myocardial injury biomarkers. As aforementioned, acute pericarditis was excluded in the patient, and CMR did not show pericardial involvement. As the primary disease in this patient was myocarditis, myopericarditis could also be excluded[11]. According to the latest diagnostic criteria, EMB should be performed, but the patient refused this procedure. The patient’s myocarditis was deduced to be attributed to autoimmunity. Treatment of primary diseases is of vital importance. The differential diagnostic process of this case is further clarified in Table 3.
| Ref. | Findings | Correlation with this study |
| [10] | The revised Mayo Clinic Criteria: (1) Transient hypokinesis, akinesis, or dyskinesis of the left ventricular midsegments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often, but not always present; (2) absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) absence of pheochromocytoma or myocarditis | CMR is a useful tool to confirm the diagnosis, and the pattern of DGE at CMR is useful to distinguish myocarditis from stress cardiomyopathy. The patient’s clinical presentation and CMR did not meet these criteria. Thus, stress cardiomyopathy was excluded in this patient |
| [11] | The clinical diagnosis of pericarditis can be made with two of the following criteria: (1) Chest pain (> 85%–90% of cases)—typically sharp and pleuritic, improved by sitting up and leaning forward; (2) pericardial friction rub (≤ 33% of cases)—a superficial scratchy or squeaking sound best heard with the diaphragm of the stethoscope over the left sternal border; (3) electrocardiogram changes (up to 60% of cases)—with new widespread ST elevation or PR depression in the acute phase; and (4) pericardial effusion (up to 60% of cases, generally mild). Additional signs and symptoms may be present according to the underlying etiology or systemic disease (i.e., signs and symptoms of systemic infection such as fever and leukocytosis, or systemic inflammatory disease or cancer). Diagnosis of predominant pericarditis with myocardial involvement, or “myopericarditis”, can be clinically established if patients with definite criteria for acute pericarditis show elevated biomarkers of myocardial injury (troponin I or T, CK-MB fraction) without newly developed focal or diffuse impairment of left ventricular function in echocardiography or CMR | The patient did not present with the manifestations in the diagnostic criteria. Thus, acute pericarditis and myopericarditis were excluded |
| [4] | The electrocardiography of a junctional rhythm shows a narrow complex QRS wave, along with retrograde P waves, sometimes are overlapped in the QRS waves. The RP interval is lower than 200 ms. Treatment of a junctional rhythm primarily depends on the underlying cause of the rhythm. If the heart rate is within 60 to 100 beats per min, accelerated junctional rhythm is considered. Aetiology-based treatment is recommended | The patient’s electrocardiography was consistent with accelerated junctional rhythm. Treatment of Graves’ disease is fundamental |
| [14] | Diagnosis of Graves’ disease is now usually based on anti-TSH-receptor antibody assays and thyroid ultrasonography. | |
| [17] | TSHR-Ab is a specific biomarker for Graves’ disease. In addition to thyroid function and TSHR-Ab determination, most clinicians would request thyroid ultrasound and less often isotope scanning. A color-flow or power Doppler examination characterizes vascular patterns and quantifies thyroid vascularity. Beta-adrenergic blockade is recommended in all suitable patients with Graves’ hyperthyroidism | The patient’s positive TSHR-Ab and ultrasound examination results were consistent with Graves’ disease. Moreover, the patient’s thyroid static imaging further proved the diagnosis of Graves’ disease |
| [17] | Patients with newly diagnosed Graves’ hyperthyroidism should be treated with ATD. RAI therapy or thyroidectomy may be considered in patients who prefer this approach. Patients with side effects or recurrence after a course of ATD, cardiac arrhythmias, and thyrotoxic periodic paralysis are candidates for RAI | The patient was diagnosed with new-onset Graves’ disease. The ATD must be initiated. Thus, methimazole 20 mg/d was administered. The patient was combined with thyrotoxic periodic paralysis, which was suitable for RAI. However, he refused this treatment method. The patient was combined with acute myocarditis, beta-blocker was administered |
| [18] | Hypokalemia is present in most patients. Abnormal thyroid hormones like elevated T4, or elevated T3 and low TSH might be present. The thyroid uptake scan might show increased uptake. The goal for treatment is to supplement potassium quickly along with the reduction of thyroid hormones. Non-selective beta-blockers have been shown to improve neuromuscular symptoms by reducing the intracellular shift of phosphate and potassium | The patient was administered with potassium supplements, ATD and a beta-blocker, all of which met the treatment criteria |
| [8] | The core principles of treatment in myocarditis are optimal care of arrhythmia and heart failure and, where supported by evidence, aetiology-targeted therapy. For patients with autoimmune diseases, treatment of primary disease is of vital importance | The patient did not have heart failure, and a beta-blocker was administered to treat his arrhythmia. The treatment of Graves’ disease is significant for his myocarditis |
Junctional arrhythmia, including accelerated junctional rhythm and junctional tachycardia, is rarely seen in patients with myocarditis. If the patient’s heart rate does not exceed 100 bpm, such conditions can be referred to as an accelerated junctional rhythm. No related reports on acute myocarditis and accelerated junctional rhythm were found, but there were reports on junctional tachycardia, usually seen in infants and children. Junctional tachycardia is also known as junctional ectopic tachycardia (JET), and the mechanism thereof is the same as accelerated junctional rhythm. Junctional tachycardia is thought to arise from the atrioventricular node and the His bundle area[12]. The incessant form of junctional ectopic tachycardia with 1:1 ventriculoatrial conduction, is a regular, short RP, narrow complex tachycardia and similar to typical Atrial Ventricular Nodal Reentry Tachycardia[12]. The patient’s electrocardiography findings were consistent with an accelerated junctional rhythm, which is rarely seen in children with acute viral myocarditis and even rarer in adults. There has been one report of junctional tachycardia in a child[13]. The etiology of accelerated junctional rhythm in the present patient could be attributed to autoimmunity (Table 3).
Graves’ disease manifests as a hyperthyroid state but is also an autoimmune process. Based on the patient’s thyroid function tests, hyperthyroidism was diagnosed. Measurements of the serum levels of TRAb and thyroid ultrasonography are the most important diagnostic tests for Graves’ disease. Following the latest guidelines[14], the patient had high TSHR-AB, and thyroid static imaging further confirmed the diagnosis of Graves’ disease. Graves’ disease treatment includes radioactive iodine (RAI), antithyroid drugs, and thyroidectomy[15,16]. For the present patient, thyroidectomy was not suitable. The patient had acute myocarditis and thyrotoxic periodic paralysis, and RAI was more suitable for rapidly controlling the patient’s hyperthyroid state. Attempts were made to persuade the patient to accept RAI, but he and his family opted for treatment by medicine. Thus, in accordance with the guidelines and the endocrinologist’s suggestions, methimazole was administered, and the thyroid function was routinely checked. The patient was advised to accept radioactive iodine therapy if methimazole could not control his hyperthyroid state. The diagnosis and treatment of the diseases are further clarified in Table 3.
A limitation of the present case is that EMB was not performed. Current guidelines recommend EMB only in a limited number of clinical scenarios that do not include some common presentations of myocarditis, particularly pseudo-infarction[8]. The guidelines give the highest levels of recommendations for EMB in life-threatening clinical manifestations[8]. The patient’s symptoms of myocarditis were atypical. Therefore, he met the indications for EMB according to the guidelines. Although the patient was advised to accept EMB, the patient still refused. EMB could have provided a definite diagnosis for the patient and been especially beneficial in defining the type of myocarditis. According to the latest guidelines[8], since CMR has a good correlation with EMB, the patient could be diagnosed with myocarditis according to CMR, and other diseases could be excluded. The main issue is that EMB can be beneficial in defining the type of myocarditis, in terms of being autoimmune or viral. However, the treatment of the patient was not primarily affected. According to the latest guidelines of myocarditis, conventional therapy is the same in all types of myocarditis. New treatment methods include anti-viral therapy or immunosuppressive therapy, but the patient did not show any sign of viral infection. Hence, anti-viral treatment was not necessary. The patient was diagnosed with Graves’ disease, and autoimmune myocarditis could not be excluded. Thus, the treatment of primary disease was of vital importance. The patient recovered quickly after his symptoms of hyperthyroidism were controlled. EMB was beneficial for the patient, but the patient did not accept this procedure. EMB would be better for diagnosis but would not have primarily affected this case. As such, the decision of the patient was ultimately accepted after failing to persuade him.
In the present case, the patient had an accelerated junctional rhythm, which is significantly rare in adults and is a manifestation of acute myocarditis. The etiology may be attributed to autoimmunity, and cardiologists should not ignore such arrhythmia. From the present patient, autoimmune diseases such as Graves’ disease can also cause acute myocarditis in addition to viruses. Thus, cardiologists should not ignore such endocrine diseases.
Usually seen in young males, Graves’ disease can manifest as thyrotoxic periodic paralysis, in which sudden paralysis and extreme hypokalemia will be experienced. The correction of hypokalemia and hyperthyroidism will relieve the symptoms. The electrocardiograph of an accelerated junctional rhythm usually shows an absence of P waves and a heart rate within 60-100 rates per minute. Accelerated junctional rhythm is a manifestation of acute myocarditis. Clinicians should not ignore endocrine diseases when facing patients with cardiac manifestations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Cardiac and Cardiovascular Systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Longchamp G, Son TQ S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Subekti I, Pramono LA. Current Diagnosis and Management of Graves’ Disease. Acta Med Indones 2018; 50: 177-182 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Wémeau JL, Klein M, Sadoul JL, Briet C, Vélayoudom-Céphise FL. Graves’ disease: Introduction, epidemiology, endogenous and environmental pathogenic factors. Ann Endocrinol (Paris). 2018;79:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, Peeters R, Zaman A, Iervasi G. Thyroid Hormones and Cardiovascular Function and Diseases. J Am Coll Cardiol. 2018;71:1781-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 4. | Yamama H, Shamai AG. Junctional Rhythm. In: StatPearls [Internet]. Treasure Island (FL): StatPearls 2021. [PubMed] |
| 5. | Demoulin R, Poyet R, Parsai C, Capilla E, Rohel G, Pons F, Jego C, Cellarier GR. [Acute autoimmune myocarditis secondary to Graves’ disease: a case report]. Rev Med Interne. 2020;41:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lancaster ST, Koons KL, Lee YJ, Mazimba S, Kwon Y. Acute autoimmune myocarditis as a manifestation of Graves’ disease: A case report and review of the literature. Clin Case Rep. 2019;7:1489-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Wu L, Wang W, Leng Q, Tang N, Zhou N, Wang Y, Wang DW. Focus on Autoimmune Myocarditis in Graves’ Disease: A Case-Based Review. Front Cardiovasc Med. 2021;8:678645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636-2648, 2648a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2274] [Article Influence: 189.5] [Reference Citation Analysis (0)] |
| 9. | Martin CA, Lambiase PD. Pathophysiology, diagnosis and treatment of tachycardiomyopathy. Heart. 2017;103:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1955-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 382] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 11. | Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921-2964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1457] [Cited by in RCA: 1593] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 12. | Alasti M, Mirzaee S, Machado C, Healy S, Bittinger L, Adam D, Kotschet E, Krafchek J, Alison J. Junctional ectopic tachycardia (JET). J Arrhythm. 2020;36:837-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Cunningham MEA, Doroshow R, Olivieri L, Moak JP. Junctional ectopic tachycardia secondary to myocarditis associated with sudden cardiac arrest. HeartRhythm Case Rep. 2017;3:124-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Bartalena L. Diagnosis and management of Graves’ disease: a global overview. Nat Rev Endocrinol. 2013;9:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 15. | Kotwal A, Stan M. Current and Future Treatments for Graves’ Disease and Graves’ Ophthalmopathy. Horm Metab Res. 2018;50:871-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur Thyroid J. 2018;7:167-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 515] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 17. | Siddamreddy S, Dandu VH. Thyrotoxic Periodic Paralysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls 2021. [PubMed] |
| 18. | Correia M, Darocki M, Hirashima ET. Changing Management Guidelines in Thyrotoxic Hypokalemic Periodic Paralysis. J Emerg Med. 2018;55:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |