Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11061
Peer-review started: June 19, 2021
First decision: July 16, 2021
Revised: July 28, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 16, 2021
Processing time: 173 Days and 11.4 Hours
Esophageal schwannomas originating from Schwann cells are extremely rare esophageal tumors. They commonly occur in the upper and middle esophagus but less frequently in the lower esophagus. Herein, we report a rare case of a large lower esophageal schwannoma misdiagnosed as a leiomyoma. We also present a brief literature review on lower esophageal schwannomas.
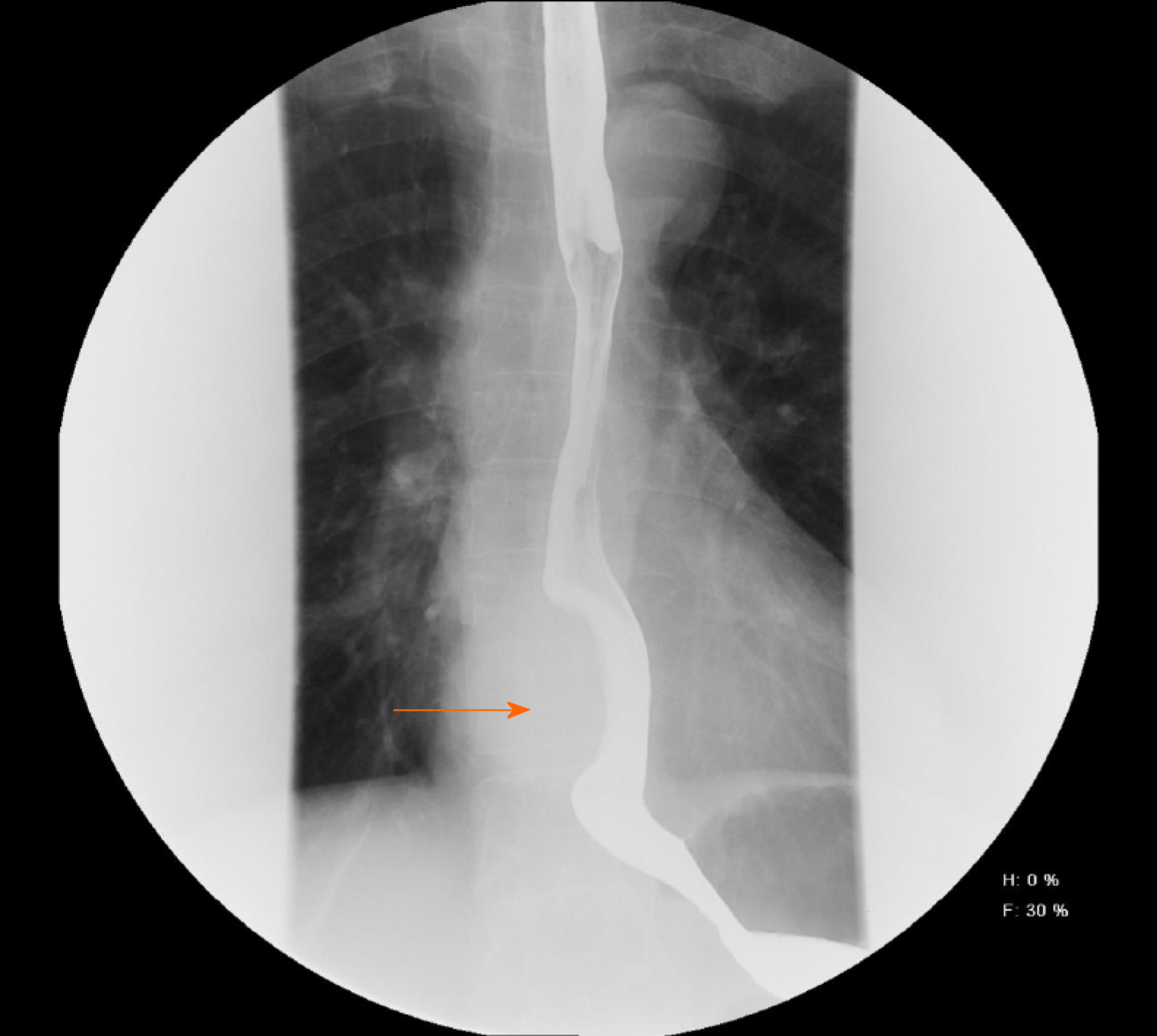
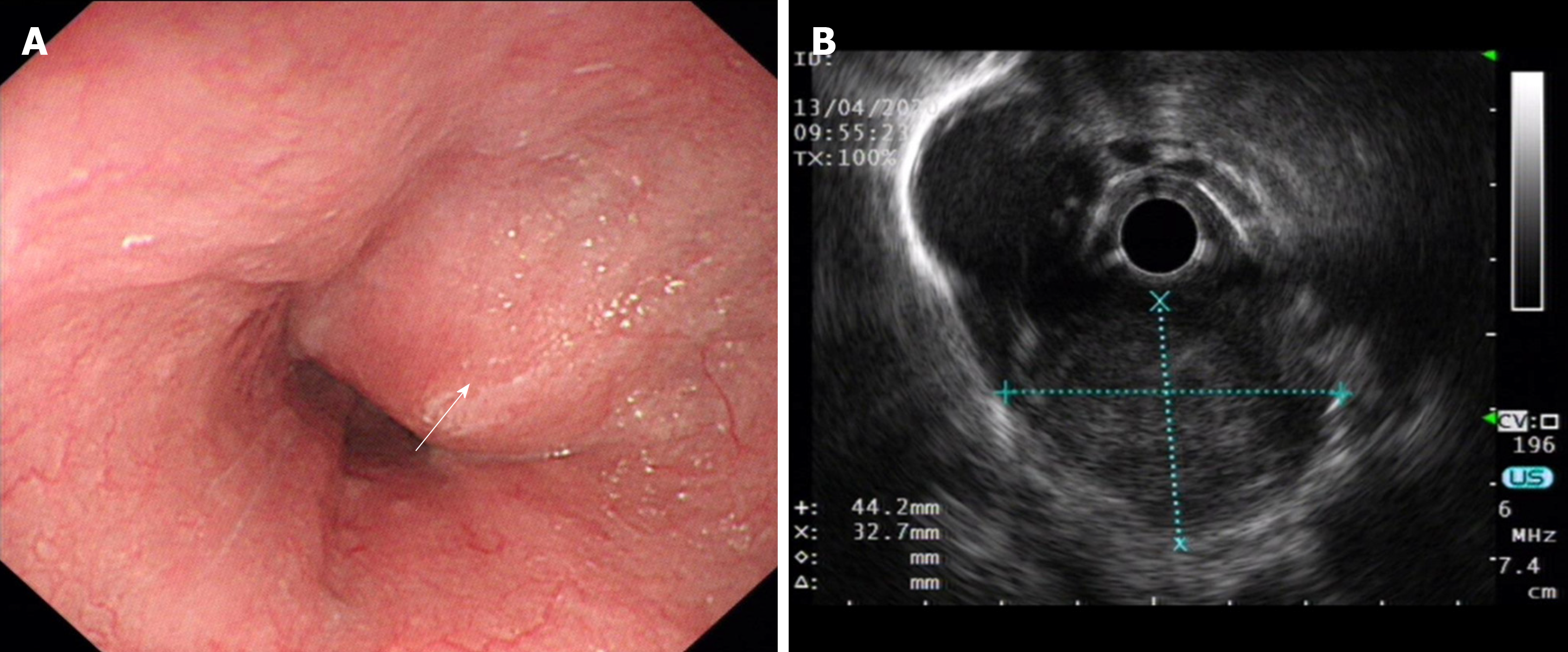
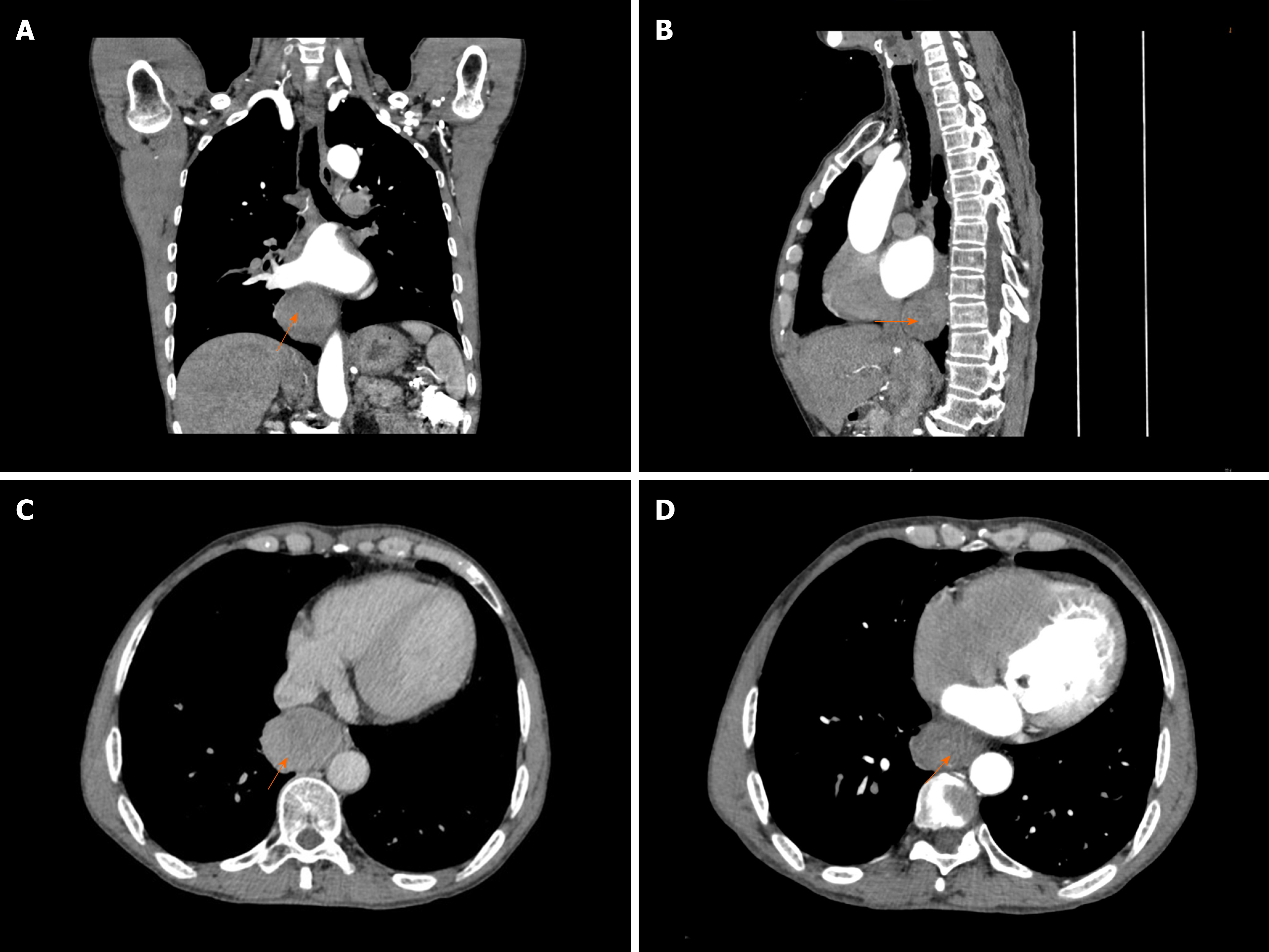
A 62-year-old man presented with severe dysphagia lasting 6 mo. A barium esophagogram showed that the lower esophagus was compressed within approximately 5.5 cm. Endoscopy revealed the presence of a large submucosal protuberant lesion in the esophagus at a distance of 32-38 cm from the incisors. Endoscopic ultrasound findings demonstrated a 4.5 cm × 5.0 cm hypoechoic lesion. Chest computed tomography revealed a mass of size approximately 53 mm × 39 mm × 50 mm. Initial tests revealed features indicative of leiomyoma. After multidisciplinary discussions, the patient underwent a video-assisted thoracoscopic partial esophagectomy. Further investigation involving immunohistochemical examination confirming palisading spindle cells as positive for S100 and Sox10 led to the final diagnosis of a lower esophageal schwannoma. There was no tumor recurrence or metastasis during follow-up.
The final diagnosis of esophageal schwannoma requires histopathological and immunohistochemical examination. The early appropriate surgery favors a remarkable prognosis.
Core Tip: Esophageal schwannomas that originate from Schwann cells are extremely rare esophageal tumors. The final diagnosis of esophageal schwannoma requires histopathological and immunohistochemical examination findings. If esophageal schwannoma is suspected, then early surgery is recommended to completely remove the tumor. The case findings revealed that Sox10 may be a potential molecular marker for esophageal schwannoma, which provides new diagnostic insights.
- Citation: Wang TY, Wang BL, Wang FR, Jing MY, Zhang LD, Zhang DK. Thoracoscopic resection of a large lower esophageal schwannoma: A case report and review of the literature. World J Clin Cases 2021; 9(35): 11061-11070
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11061.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11061
Schwannomas are neurogenic tumors that arise from proliferating Schwann cells; they grow slowly and are predominantly benign[1-3]. Schwannomas occur most commonly in the trunk, limbs, head, and neck but rarely in the gastrointestinal tract[4,5]. Most gastrointestinal schwannomas originate from the stomach or intestine; esophageal schwannomas are the rarest[3]. Esophageal schwannomas are the fewest esophageal submucosal tumors, accounting for less than 2% of all esophageal tumors[6,7]. They commonly occur in the upper and middle esophagus but less frequently in the lower esophagus[6]. This report presents the case of a 62-year-old man who had a lower esophageal schwannoma, which was misdiagnosed as leiomyoma. In addition, we performed a brief literature review of the lower esophageal schwannomas.
A 62-year-old man presented with severe dysphagia lasting 6 mo.
The patient suffered from severe dysphagia for 6 mo, with associated symptoms such as chest tightness, shortness of breath, chest pain, palpitations, and back pain.
The patient had an unremarkable medical history.
The patient had no special personal or family history.
The patient’s temperature was 36.3 °C, heart rate was 83 beats per min, respiratory rate was 23 breaths per min, blood pressure was 118/81 mmHg, and oxygen saturation in room air was 95%. A heart murmur was heard in the apical area. Findings of his lungs and abdominal examinations were normal. Neurological examination showed no significant abnormalities. There was no edema in both lower extremities.
Routine blood test results revealed the following: white blood cells 9.2 × 109/L, hemoglobin 133 g/L, and blood platelets 87 × 109/L. Tumor marker analysis showed a slight increase in CY211 (6.46 ng/mL) and carcinoembryonic antigen (3.76 ng/mL). Routine urine tests and blood biochemistries were within normal limits. His feces showed normal findings in the routine test, and parasitological examination and occult blood tests yielded negative results.
A barium esophagogram (Figure 1) showed that the lower esophagus was compressed to a size of approximately 5.5 cm, with the mucosa appearing regular. Endoscopy (Figure 2A) revealed the presence of a large submucosal protuberant lesion in the esophagus at a distance of 32-38 cm from the incisors, with a smooth surface and normal color. Endoscopic ultrasound (EUS) (Figure 2B) findings demonstrated a 4.5 cm × 5.0 cm hypoechoic lesion with interior calcified areas, possibly originating from the muscularis propria. Subsequently, chest computed tomography (Figure 3) revealed a mass measuring approximately 53 mm × 39 mm × 50 mm, protruding from the lower esophagus; its features were suggestive of leiomyoma or gastrointestinal stromal tumor. Thus, for this patient, esophageal leiomyoma was suspected during the initial diagnosis.
The patient complained of dysphagia and palpitation. Computed tomography indicated that the tumor oppressed the esophagus and left atrium. His cardiac ultrasound findings indicated rheumatic valvular heart disease. Considering that tumor compression had a serious impact on diet and cardiac function, resection surgery was recommended as the initial treatment, with strict limitations on the volume of liquid intake. The patient was recommended to wait 3 mo after this operation to undergo cardiac valve surgery.
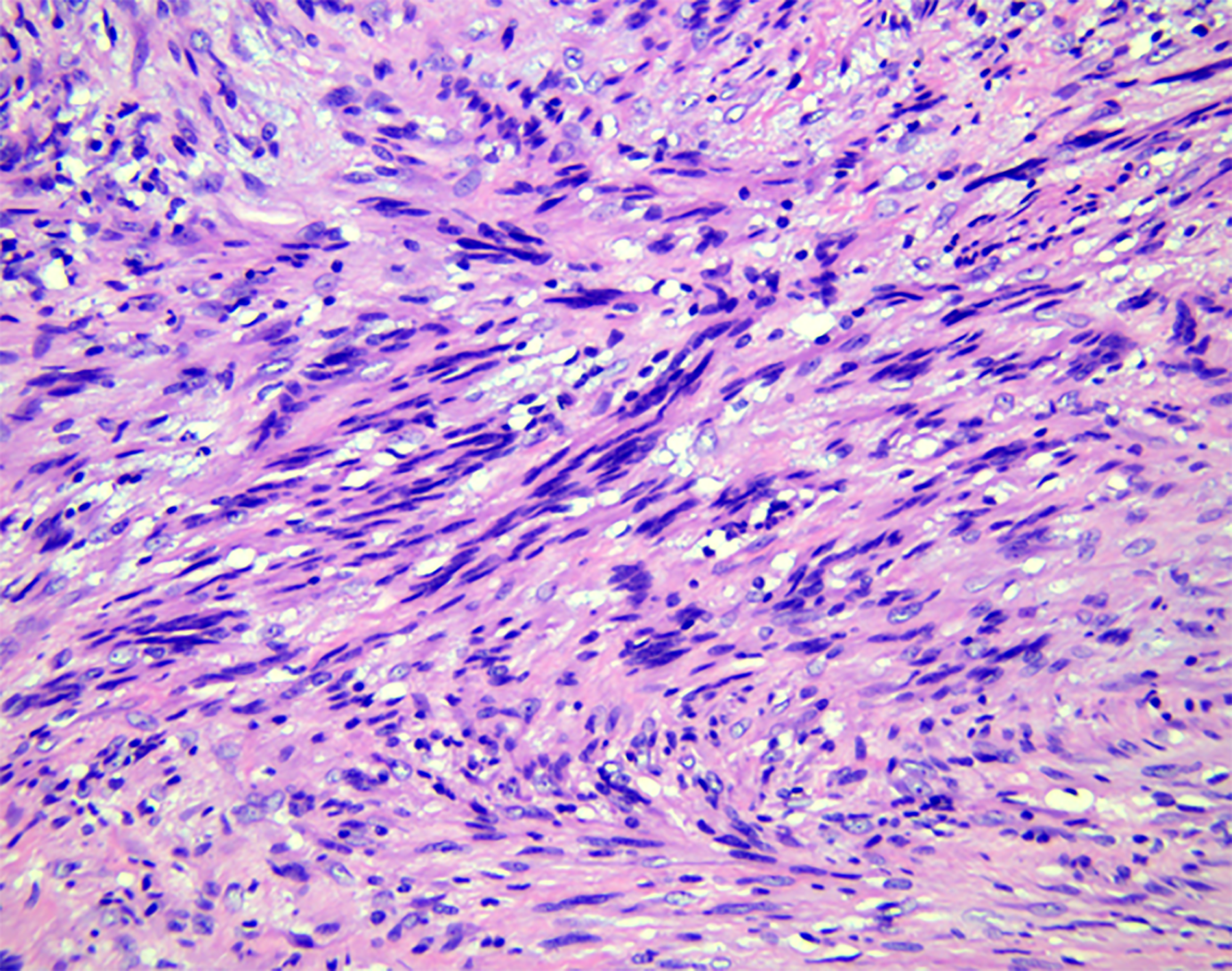
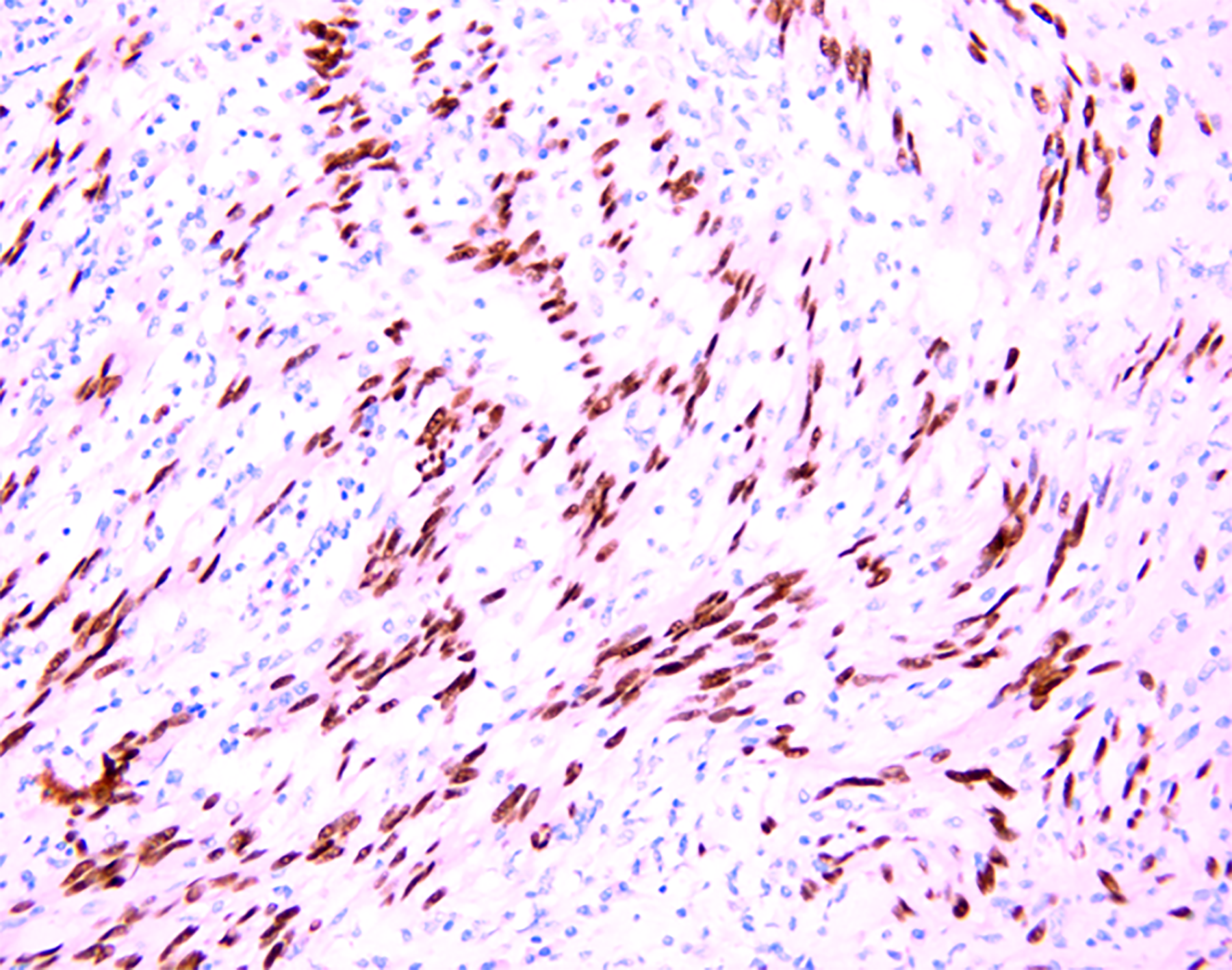
Histopathological examination (Figure 4) findings revealed a fence-like structure formed by spindle-shaped cells, and immunohistochemical examination showed positivity for S100, Sox10 (Figure 5), vimentin, and TLE1 but negativity for CD34, desmin, CD117, actin, GFAP, h-caldesmon, STAT6, DOG-1, CD21, and SMA. Hence, a final diagnosis of esophageal schwannoma was made.
The patient underwent a video-assisted thoracoscopic partial esophagectomy. During the operation, a 5.0 cm × 5.0 cm hard mass was noted, which had poor mobility and did not break through the adventitia, extending from the lower edge of the inferior pulmonary vein to the esophageal hiatus.
After surgery, the dysphagia disappeared, and the patient was relieved from other symptoms. Follow-up did not reveal local tumor recurrence or metastasis.
The most common submucosal tumors of the gastrointestinal tract are myogenic tumors such as leiomyomas and gastrointestinal stromal tumors[8]. Neurogenic tumors such as esophageal schwannomas are rare[9]. This case had several peculiarities. First, esophageal schwannomas commonly occur in middle-aged women[10]; however, the present case occurred in a male patient. Second, instead of the usual occurrence in the upper to mid-esophagus[6], this case involved the lower esophagus, which was easily ignored. Finally, the patient experienced esophageal schwannoma with rheumatic valvular heart disease, and the tumor was so large that it compressed the left atrium (Figure 3), leading to aggravated cardiac insufficiency.
We performed a statistical analysis of published cases about esophageal schwannomas and found that there were fewer cases of lower esophageal schwannomas (13) than upper to middle esophageal schwannomas (57) (Table 1), which is similar to previous reports. However, the reason why lower esophageal schwannomas are rare has not been clearly reported. Anatomically, the innervation of the striated muscle in the pharynx and the upper esophagus originates from the brain stem, but the nerve of the distal esophagus originates from the dorsal motor nucleus of the vagus nerve and ends at the ganglion of the myenteric plexus[11]. It has been shown that there are two peak areas of innervation in the cervical and thoracic regions of the esophagus in canines[12]. We speculate that the incidence of esophageal schwannoma may be related to the origin and distribution of the nerve plexus; however, no study has confirmed it clearly at present. This makes it a significant study worthy of further exploration in the future.
| Characteristics | Lower schwannoma, n (%) | Upper/middle schwannoma, n (%) | |
| Total | 13 | 57 | |
| Sex | |||
| Male | 9 (69.2%) | 14 (24.6%) | |
| Female | 4 (30.8%) | 43 (75.4%) | |
| Age | |||
| 20–29 yr | 2 (15.4%) | 2 (3.5%) | |
| 30–39 yr | 1 (7.7%) | 7 (12.3%) | |
| 40–49 yr | 2 (15.4%) | 12 (21.1%) | |
| 50–59 yr | 5 (38.5%) | 13 (22.8%) | |
| 60–69 yr | 2 (15.4%) | 17 (29.8%) | |
| 70–79 yr | 1 (7.7%) | 6 (10.5%) | |
To our knowledge, we are the first to conduct a statistical analysis of lower esophageal schwannoma data, hoping to contribute to clinical diagnosis and treatment. We will discuss the characteristics of the clinical data, symptoms, diagnosis, differential diagnosis, and treatment of lower esophageal schwannoma and compare them with those of upper/middle esophageal schwannomas.
From the statistics (Table 2) on lower esophageal schwannoma, we observed that the ages (years) with the highest prevalence were at 50-59 (38.5%)[8,13-16], followed by 20-29 (15.4%)[17,18], 40-49 (15.4%)[8,19], 60-69 (15.4%)[20], 30-39 (7.7%)[21], and 70-79 (7.7%)[22]. The mean age was 50.85 years, and the median age was 54 years, with a standard deviation of 14.536 years. These data are similar to those of total esophageal schwannoma. We also analyzed schwannomas in the upper to mid-esophagus, noting a mean age of 53.17 years and a median age of 55 years, with a standard deviation of 13.089 years. There was no significant difference in age when comparing the cases of schwannoma found in the lower esophagus and in the upper/middle esophagus.
| Case | Ref. | Age (yr)/Sex | Presenting symptom | Immunohistochemical studies | Location | Tumor size | Benign or malignant | Treatment | Postoperative complications |
| 1 | Matteo et al[17] | 22/Male | Dysphagia, chest pain, esophageal reflux-like symptom | Reactive with S100 protein and negative for desmin, DOG1, CD117, EMA, HMB45, Melan A, synaptophysin and neurofilaments | 34 to 41 cm | 75 mm | Benign but locally advanced | A subtotal esophagectomy via a muscle sparing lateral thoracotomy | No |
| 2 | Mishra et al[18] | 27/Female | Dysphagia, palpitations, weight loss, loss of appetite | Immunopositive for S100 and negative for DOG-1, CD117, CD34, and SMA | 30 cm | 120 mm × 100 mm × 100 mm | Low-grade malignant | Esophagectomy through a left thoraco-abdominal incision | Right recurrent laryngeal nerve palsy |
| 3 | Naus et al[21] | 39/Male | Burning epigastric pain | Positive for S100 protein | 34 cm | 15 mm | Benign | Endoscopic removal | No |
| 4 | Zhang et al[19] | 48/Female | Dysphagia | Positive for S100 | 30 cm | 70 mm × 60 mm × 40 mm | Benign | Robot-assisted thoracoscopic excision | No |
| 5 | Li et al[8] | 49/Male | Dysphagia | Positive staining of the tumor cells for S100, Lea-7, and PG9.5 protein, and negative staining for CD117, CD34, DOG-1, DES, and smooth muscle actin | 35 cm | 28 mm × 22 mm | Benign | STER: Submucosal tunneling endoscopic resection. The lesion was resected in a piecemeal fashion | No |
| 6 | Hsu et al[13] | 54/Male | Dysphagia | Positive for S100 protein and negative staining for actin | 35 cm | 25 mm × 20 mm × 15 mm | Benign | Submucosal tumor enucleation via left thoracotomy | No |
| 7 | Sánchez et al[14] | 54/Male | Dysphagia, weight loss | Positive for S100 and vimentin and negative for CD117 | 34 to 40 cm | 60 mm | Malignant | Ivor-Lewis esophagectomy with gastric-tube reconstruction | No |
| 8 | Trindade et al[15] | 54/Male | Esophageal reflux-like symptom | Positive for S100 and negative for smooth muscle markers | In the distal third of the esophagus | 6 mm | Benign | Endoscopic mucosal resection | No |
| 9 | Shimamura et al[16] | 56/Male | Esophageal reflux-like symptom | Strongly positive for S100 and not stain for CD117, SMA, CD68 and inhibin S100 | Distal esophagus | 5 mm | Benign | Endoscopic mucosal resection | No |
| 10 | Li et al[8] | 59/Male | Upper abdominal distension, esophageal reflux-like symptom | Positive staining of the tumor cells for S100, Lea-7, and PG9.5 protein, and negative staining for CD117, CD34, DOG-1, DES, and smooth muscle actin | 35 cm | 14 mm × 5 mm | Benign | ESE: Endoscopic submucosal excision | No |
| 11 | Shichinohe et al[20] | 61/Female | Dysphagia | positive staining of S100, and negative staining of c-kit and α-SMA | In the lower thoracic esophagus | 45 mm × 30 mm | Benign | Thoracoscopic esophageal submucosal tumor enucleation | No |
| 12 | Our case | 62/Male | Dysphagia, chest pain, palpitations, chest tightness, shortness of breath, back pain | Positivity for S100, Sox10, vimentin, and TLE1, but negativity for CD34, desmin, CD117, actin, GFAP, h-caldesmon, STAT6, DOG-1, CD21 and SMA | 32 to 38 cm | 53 mm × 39 mm × 50 mm | Benign | Video-assisted thoracoscopic partial esophagectomy | No |
| 13 | Brown et al[22] | 76/Female | Dysphagia, weight loss | Positive for S100, HMB45, and Melan A and negative for CD34, epithelial membrane antigen, smooth muscle antigen, and desmin | In the lower third of the esophagus | 50 mm × 40 mm × 20 mm | Benign | Subtotal esophagectomy | No |
With this disease, some patients show corresponding clinical symptoms. The occurrence of symptoms is related to the location and size of the tumor. We evaluated 13 patients on the lower esophageal schwannoma and found that the patients mainly showed dysphagia and esophageal reflux-like symptoms, which may be caused by the compression injury of the tumor to the esophagus-gastric junction. In addition, some patients had symptoms such as chest pain, palpitations, burning pain in the upper abdomen, back pain, chest tightness, shortness of breath, abdominal distension, loss of appetite, and so on. Three patients[14,18,22] had weight loss, including two malignant esophageal schwannomas[14,18] and one esophageal melanocytic schwannoma[22]. In upper to middle esophageal schwannomas, dyspnea was the most common symptom after dysphagia, which was caused by the proximity of the upper/middle esophagus to the trachea that is being compressed by the tumor[6,23]. It was reported that the tumor severely obstructed the trachea, resulting in dyspnea and disturbance of consciousness[24]. After ineffective endotracheal intubation, emergency subtotal esophagectomy was performed in order to save the patient[24].
Esophageal schwannoma is easily misdiagnosed as esophageal leiomyomas before the biopsy. There is no specific abnormality in laboratory examination. Computed tomography, positron emission tomography, and magnetic resonance imaging examinations can be used for auxiliary diagnosis, but schwannoma shows no notable differences from other submucosal tumors. Simultaneously, it is difficult to diagnose esophageal schwannoma through endoscopic mucosal biopsy. Deep tissue biopsy may improve the accuracy of pathology, but it increases bleeding risk[25]. Esophageal schwannoma is a hypoechoic mass with uneven echo and clear boundaries under EUS[26]. Standard EUS aids in determining the exact location and origin of lesions in different esophageal layers, and the accuracy ranges from 30% to 66%[27,28]. Rong et al[29] reported that the diagnostic accuracy of EUS-guided fine needle aspiration for submucosal tumors is 85.2%; however, it may be misdiagnosed or missed due to insufficient sampling[30]. Therefore, the final diagnosis often requires immunohistochemical examination after surgical removal of the lesion.
The resected tumor surface is gray-white and translucent. The histopathological features include that the tumor cells are arranged as fusiform to a fence-like structure or in a network to form a loose structure[30]. The immunohistochemical staining is currently the only reliable method for diagnosis. Among the 13 patients with lower esophageal schwannomas, it was found that the S100 protein had strong immunological activity and characteristics and was stained positively in all the schwannomas. CD34, CD117, desmin, actin, DOG-1, SMA, DES, and AE1/AE3 were not found in all schwannomas analyzed. HMB45 and Melan A were positive in esophageal melanocytic schwannoma[22], and their expression was not found in other patients. Esophageal schwannomas are easily misdiagnosed as gastrointestinal stromal tumors or esophageal leiomyomas. The immunohistochemical examination is the most accurate method for the differential diagnosis of esophageal tumors. The gastrointestinal stromal tumor is positive for CD34 and CD117, and the leiomyoma is positive for desmin and actin[6]. Furthermore, the upper schwannoma can be misdiagnosed as a thyroid tumor on ultrasonography[31]. Surgical exploration is recommended to confirm the diagnosis and to avoid unnecessary thyroidectomy.
However, Sox10 (Figure 5) was strongly positive in our case. To the best of our knowledge, we are the first to use Sox10 to detect esophageal schwannomas. It is worth noting that the Sox10 factor is expressed in Schwann cells and melanocyte lineages and is important for their development[32]. Recent studies have suggested that Sox10 is a potential molecular biological marker and can be used to diagnose and differentiate some tumors of the nervous system. Sox10 is consistently expressed in gastrointestinal schwannomas and can distinguish them from stromal tumors that are occasionally S100 protein positive[32]. As a molecular marker, Sox10 is superior to S100 in terms of sensitivity and specificity for the differential diagnosis of schwannoma and fibrous meningioma[33]. Studies also have shown that Sox10 has a higher specificity for tumors of neural crest origin than S100: Sox10 (specificity, 99%) and S100 (specificity, 91%)[34]. The combined use of Sox10 and S100 aids in improving the sensitivity and specificity for the diagnosis of schwannoma.
Esophageal schwannoma is insensitive to medical treatments such as radiotherapy and chemotherapy; hence, surgery is the only effective treatment, including endoscopic surgery and surgical resection. When the lesion is small, endoscopic treatment is a great choice. In the published literature on lower esophageal schwannomas, the tumor size in 6 patients was less than 3 cm; of these, 5 patients (83.3%) underwent endoscopic surgery. For larger lesions (≥ 3 cm) with suspicious features, endoscopic treatment may not be suitable[8], and surgical operation is required. The tumor size in 5 patients was between 3 cm and 7 cm, including 3 cases (60%) of thoracoscopic surgery. For tumors of size > 7 cm, thoracotomy is often the treatment of choice[35]. Statistically, 2 patients with tumors measuring > 7 cm underwent thoracotomy. For malignant esophageal schwannoma, surgical resection and lymph node dissection are necessary[18]. Thoracoscopy and thoracotomy incisions are related to the tumor location and anatomical rationality.
There are some differences in the choice of surgical incision between lower schwannoma and upper to middle schwannoma. Since the upper esophagus is partially posterior to the left subclavian artery and the middle thoracic esophagus is below the aortic arch, the left-side approach is generally not chosen for upper to middle esophageal schwannomas to avoid vascular damage[6,20]. Due to the esophageal hiatus position on the upper left of the aortic hiatus, surgery for lower schwannoma, which is located above the diaphragm, can be performed through the left chest wall without any anatomical obstacles[20]. Therefore, the surgeon chooses the left-side approach for the schwannoma on the left side of the lower thoracic esophagus[20]. The azygos vein arch is the only structure of the right thoracic mediastinum above the esophagus, and the vein is separable without any complications[20]. Due to this, both lower and upper to middle esophageal schwannoma can be approached from the right side. In the present case, the patient’s esophageal schwannoma was located on the right side of the lower esophagus, prompting the use of the right-side approach. At the same time, the patient was also experiencing cardiac insufficiency. Early appropriate resection surgery improved cardiac function and favored a great prognosis.
Briefly, esophageal schwannoma is an extremely rare disease that can be easily misdiagnosed. There is no differential laboratory examination for the disease. Initial imaging test findings are similar to those of leiomyoma cases before surgery. The definitive diagnosis of esophageal schwannoma requires histopathological and immunohistochemical examination of postoperative specimens. Studies revealed that Sox10 may be a potential molecular marker for esophageal schwannoma, but further studies with large samples for an in-depth investigation on Sox10 are required. Furthermore, if esophageal schwannoma is suspected, then early surgery is recommended to completely resect the tumor. When the patient is identified to have comorbidities, it is necessary to comprehensively evaluate the patient’s condition and choose the appropriate treatment method.
Thanks to the Pathology Department for helping us with histopathological images.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kamimura K, Tanabe H S-Editor: Chang KL L-Editor: Filipodia P-Editor: Xing YX
| 1. | Jeon HW, Kim KS, Hyun KY, Park JK. Enucleation of giant esophageal schwannoma of the upper thoracic esophagus: reports of two cases. World J Surg Oncol. 2014;12:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Sanchez-Garcia Ramos E, Cortes R, de Leon AR, Contreras-Jimenez E, Rodríguez-Quintero JH, Morales-Maza J, Aguilar-Frasco J, Irigoyen A, Reyes F, Alfaro-Goldaracena A. Esophageal schwannomas: A rarity beneath benign esophageal tumors a case report. Int J Surg Case Rep. 2019;58:220-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Souza LCA, Pinto TDA, Cavalcanti HOF, Rezende AR, Nicoletti ALA, Leão CM, Cunha VC. Esophageal schwannoma: Case report and epidemiological, clinical, surgical and immunopathological analysis. Int J Surg Case Rep. 2019;55:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Liu C, Yan L, Liu Q, Li J, Jin H, Wang J, Deng Y. Lumbar intraspinal microcystic/reticular schwannoma: Case report and literature review. Medicine (Baltimore). 2018;97:e12474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wang S, Zheng J, Ruan Z, Huang H, Yang Z. Long-term survival in a rare case of malignant esophageal schwannoma cured by surgical excision. Ann Thorac Surg. 2011;92:357-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Wu CX, Yu QQ, Shou WZ, Zhang K, Zhang ZQ, Bao Q. Benign esophageal schwannoma: A case report and brief overview. Medicine (Baltimore). 2020;99:e21527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Morales-Maza J, Pastor-Sifuentes FU, Sánchez-Morales GE, Ramos ES, Santes O, Clemente-Gutiérrez U, Pimienta-Ibarra AS, Medina-Franco H. Clinical characteristics and surgical treatment of schwannomas of the esophagus and stomach: A case series and systematic review. World J Gastrointest Oncol. 2019;11:750-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 8. | Li B, Wang X, Zou WL, Yu SX, Chen Y, Xu HW. Endoscopic resection of benign esophageal schwannoma: Three case reports and review of literature. World J Clin Cases. 2020;8:5690-5700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Zhang W, Xue X, Zhou Q. Benign esophageal schwannoma. South Med J. 2008;101:450-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Chen HC, Huang HJ, Wu CY, Lin TS, Fang HY. Esophageal schwannoma with tracheal compression. Thorac Cardiovasc Surg. 2006;54:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Patti MG, Gantert W, Way LW. Surgery of the esophagus. Anatomy and physiology. Surg Clin North Am. 1997;77:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Khurana RK, Petras JM. Sensory innervation of the canine esophagus, stomach, and duodenum. Am J Anat. 1991;192:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Hsu SD, Cheng YL, Chen A, Lee SC. Esophageal schwannoma. J Formos Med Assoc. 2003;102:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Sánchez A, Mariángel P, Carrasco C, Venturelli A, Vera G. [Malignant nerve sheath tumor of the esophagus (malignant esophageal schwannoma)]. Gastroenterol Hepatol. 2004;27:467-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Trindade AJ, Fan C, Cheung M, Greenberg RE. Endoscopic resection of an esophageal schwannoma. Dig Liver Dis. 2018;50:309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Shimamura Y, Winer S, Marcon N. A Schwannoma of the Distal Esophagus. Clin Gastroenterol Hepatol. 2016;14:A19-A20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Matteo MV, Sassorossi C, Lococo F, Ricci R, Margaritora S, Gasbarrini A, Zileri Dal Verme L. A huge esophageal Schwannoma occurring in a Caucasian young male: a case report. Eur Rev Med Pharmacol Sci. 2020;24:10703-10707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Mishra B, Madhusudhan KS, Kilambi R, Das P, Pal S, Srivastava DN. Malignant Schwannoma of the Esophagus: A Rare Case Report. Korean J Thorac Cardiovasc Surg. 2016;49:63-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Han Y, Xiang J, Li H. Robot-assisted enucleation of large dumbbell-shaped esophageal schwannoma: a case report. BMC Surg. 2018;18:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Shichinohe T, Kato K, Ebihara Y, Kurashima Y, Tsuchikawa T, Matsumoto J, Nakamura T, Tanaka E, Hirano S. Thoracoscopic enucleation of esophageal submucosal tumor by prone position under artificial pneumothorax by CO2 insufflation. Surg Laparosc Endosc Percutan Tech. 2014;24:e55-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Naus PJ, Tio FO, Gross GW. Esophageal schwannoma: first report of successful management by endoscopic removal. Gastrointest Endosc. 2001;54:520-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Brown RM, Darnton SJ, Papadaki L, Antonakopoulos GN, Newman J. A primary tumour of the oesophagus with both melanocytic and schwannian differentiation. Melanocytic schwannoma or malignant melanoma? J Clin Pathol. 2002;55:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Liu T, Liu H, Yang C, Zhang X, Xu S, Liu B. Benign esophageal schwannoma compressing the trachea requiring esophagectomy: a case report. Thorac Cardiovasc Surg. 2013;61:505-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 24. | Tomono A, Nakamura T, Otowa Y, Imanishi T, Tanaka Y, Maniwa Y, Kakeji Y. A Case of Benign Esophageal Schwannoma Causing Life-threatening Tracheal Obstruction. Ann Thorac Cardiovasc Surg. 2015;21:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Vinhais SN, Cabrera RA, Nobre-Leitão C, Cunha TM. Schwannoma of the esophagus: computed tomography and endosonographic findings of a special type of schwannoma. Acta Radiol. 2004;45:718-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Reddymasu SC, Oropeza-Vail M, Pakseresht K, Moloney B, Esfandyari T, Grisolano S, Buckles D, Olyaee M. Are endoscopic ultrasonography imaging characteristics reliable for the diagnosis of small upper gastrointestinal subepithelial lesions? J Clin Gastroenterol. 2012;46:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Rong L, Kida M, Yamauchi H, Okuwaki K, Miyazawa S, Iwai T, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc. 2012;24:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Gao ZY, Liu XB, Pandey S, Gao B, Liu P, Zhang QH, Gao YJ, Li SB. Clinicopathological features of esophageal schwannomas in mainland China: systematic review of the literature. Int J Clin Oncol. 2021;26:284-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Ahn D, Sohn JH, Kim H, Yeo CK. Large esophageal schwannoma mimicking thyroid tumor with egg-shell calcification on preoperative ultrasonography. Asian J Surg. 2017;40:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, Thompson LD, Lasota J, Wang Z, Fetsch JF. Sox10--a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol. 2015;39:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Ng J, Celebre A, Munoz DG, Keith JL, Karamchandani JR. Sox10 is superior to S100 in the diagnosis of meningioma. Appl Immunohistochem Mol Morphol. 2015;23:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Kent M, d'Amato T, Nordman C, Schuchert M, Landreneau R, Alvelo-Rivera M, Luketich J. Minimally invasive resection of benign esophageal tumors. J Thorac Cardiovasc Surg. 2007;134:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |