Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11036
Peer-review started: July 1, 2021
First decision: July 26, 2021
Revised: July 27, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: December 16, 2021
Processing time: 161 Days and 21 Hours
Androgen insensitivity syndrome is an X-linked recessive genetic disease caused by mutations in the androgen receptor gene (AR). However, the underlying molecular mechanisms for the majority of AR variants remain unclear. In this study, we identified a point variant in three patients with complete androgen insensitivity syndrome (CAIS), summarized the correlation analysis, and performed a literature review.
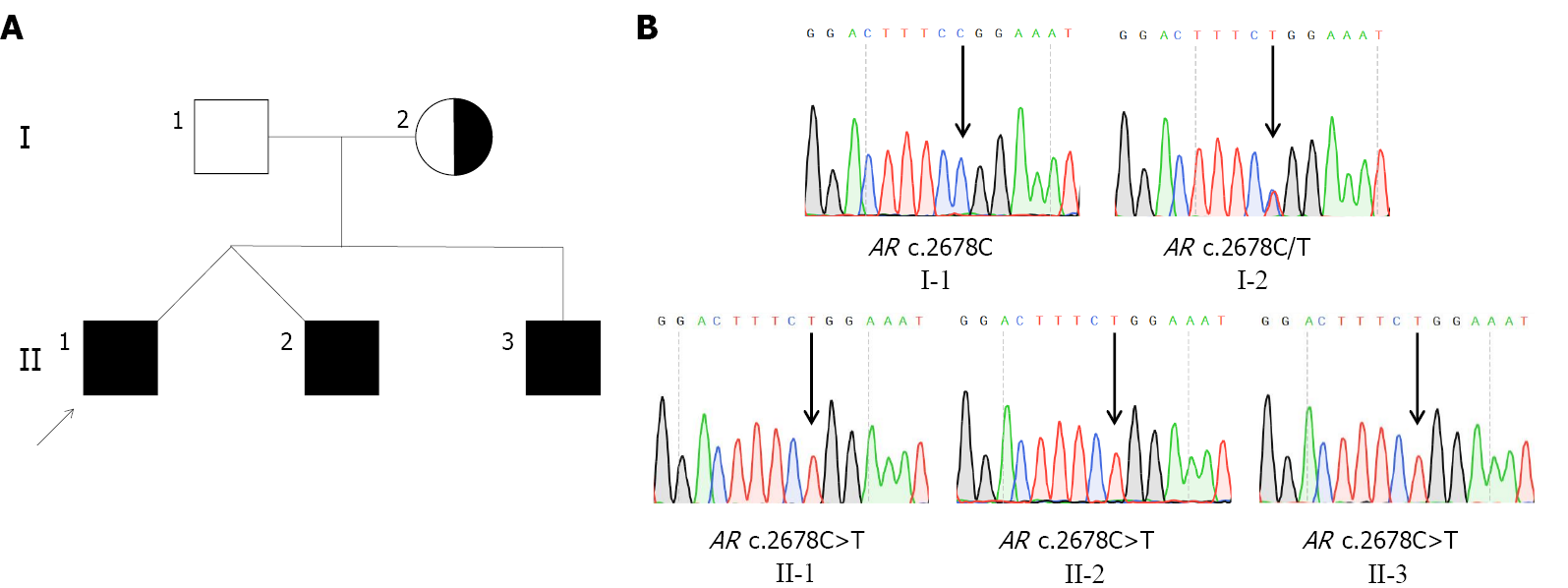
The proband was raised as a girl. In infancy, she was first referred to hospital with a right inguinal hernia. Ultrasonography revealed the absence of a uterus and ovaries, and a testis-like structure located at the inguinal canal. Further diagnostic workup detected a 46, XY karyotype, and fluorescence in situ hybridization analysis showed the presence of the SRY gene. Histological analysis revealed the excised tissue to be testicular. Twelve years later, she was admitted to our hospital with a lack of breast development. Her pubic hair and breasts were Tanner stage I. She had normal female external genitalia. Blood hormone tests showed normal testosterone levels, low estradiol levels, and high gonadotropin levels. Her two siblings underwent similar examinations, and all three had a rare hemizygous missense mutation in AR: c.2678C>T. In vitro functional analyses revealed decreased nuclear translocation in AR-c.2678C>T mutation cells.
This case of CAIS was caused by an AR variant (c.2678C>T). Functional studies showed impaired nuclear translocation ability of the mutant protein.
Core Tip: A hemizygous variant c.2678C>T (p.P893L) was found in the Ligand-binding domain of the AR gene in a Chinese family affected with complete androgen insensitivity syndrome (CAIS). Online prediction tools were used to predict the disease-causing potential of this variant. Structural analysis revealed that the amino acid substitution affected protein properties, and in vitro functional studies showed the nuclear translocation ability of the mutant protein to be impaired. CAIS in this family was concluded to be caused by the c.2678C>T variant, whose pathogenesis resulting in an androgen insensitivity syndrome phenotype may be related to decreased nuclear translocation.
- Citation: Wang KN, Chen QQ, Zhu YL, Wang CL. Complete androgen insensitivity syndrome caused by the c.2678C>T mutation in the androgen receptor gene: A case report. World J Clin Cases 2021; 9(35): 11036-11042
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11036
As a hereditary condition, androgen insensitivity syndrome (AIS, OMIM: 300068) is characterized by complete or partial resistance to the biological actions of androgen in male karyotype individuals[1], which is the most common cause of 46, XY disorders of sex development (46, XY DSD). Clinical manifestations range from phenotypic females (complete type) to mild hypovirilization (partial type), or men with mild manifestations of gynecomastia and/or infertility[2]. Characteristic features of complete androgen insensitivity syndrome (CAIS) include a female phenotype, breast development, absent or sparse pubic and axillary hair, a short blind-ending vagina, and an absence of the uterus and ovaries. In 46 XY males, the prevalence of CAIS is estimated to range from 1:20400 to 1:99100[3].
As an X-linked recessive genetic condition, AIS is caused by mutations in the androgen receptor gene (AR; OMIM: 313700). AR encodes a 110 kDa AR protein[4], known as the DHT receptor or NR3C4, which belongs to a family of nuclear receptors typically located in the cytoplasm. The AR normally forms a multimeric complex with heat shock proteins (HSPs). When androgen hormone reaches the cytoplasm, it causes a dissociation between the AR and HSPs, then binds to the AR itself and causes the migration of this new complex inside the nucleus. The AR then dimerizes and enhances the transcription of androgen-responsive genes by binding hormone response elements[5]. AR protein is composed of three major functional domains: an N-terminal domain (NTD), a DNA-binding domain (DBD), and a Ligand-binding domain (LBD).
The LBD first promotes the interaction between the receptor and HSPs in the cytoplasm, then with the androgen hormone it causes AR migration to the nucleus. The LBD is encoded by exons 4–8, and contains 11 α-helices associated with two anti-parallel β-sheets in a sandwich-like conformation with a central ligand binding pocket in which the ligand can bind[6].
To date, more than 1000 variants of AR have been recorded in the human gene mutation database (http://www.hgmd.cf.ac.uk/). Most mutations are in the AR-LBD, while mutations in the AR-DBD are less frequent, and AR-NTD mutations are very rare. Polymorphic mutations associated with AIS have been observed in the LBD domain. However, it is not clear how these mutations affect androgen sensitivities for AR through impaired physiology.
In this present study, one Chinese family of a proband and her siblings with CAIS was investigated. AR sequencing identified the same hemizygous missense mutation, p.P893L, in the LBD of AR in all three siblings. Moreover, computational analysis and functional study were performed to research the pathogenesis of this variant.
The proband (II-1) was admitted to our hospital because of a lack of breast deve
The proband (II-1)’s main symptom is the lack of breast development as a 12-year-old girl.
In the past, the proband (II-1) was once referred to hospital with a right inguinal hernia in 2006, as a 3-mo-old girl. Based on the clinical evaluations, the patient was diagnosed with 46, XY DSD. The male gonads were surgically removed because of the risk of malignant tumors.
The proband (II-1) was the first child of Han Chinese nonconsanguineous parents. She was born full term with a birth weight of 2600 g and a length of 50 cm.
The proband (II-1) has two sisters (II-2, II-3). As the twin of the proband, the girl (II-2) underwent a similar physical examination, laboratory examination, karyotype analysis, imaging, surgery, and pathological examination. The third girl (II-3, a 4-year-old girl) was the younger sibling of the twins. Following a genetic diagnosis, laparoscopic surgery was performed to remove the gonads located in the pelvis because of the risk of malignant tumors. As expected, histological analysis of the excised gonads showed them to be testicular tissue. Both parents were healthy.
The proband (II-1)’s pubic hair and breasts were at Tanner stage I. She had normal female external genitalia without clitoromegaly. Her labia were normal, and the vagina and urethra had separate openings.
In 2006, proband (II-1)’s chromosome karyotype was 46, XY karyotype, and fluorescent in situ hybridization analysis showed that the SRY gene was positive. Histological analysis revealed the excised tissue to be testicular. In 2018, blood hormone tests showed normal testosterone levels, low estradiol levels, and high gonadotropin levels (Table 1).
| Items | Patient | Normal value | ||
| II-1 | II-2 | II-3 | ||
| Age | 12-yr-old | 12-yr-old | 4-yr-old | |
| LH (mIU/mL) | 8.03 | 12.35 | 1.05 | < 1-4 IU/L |
| FSH (mIU/mL) | 61.7 | 72.3 | 41 | < 1-3 IU/L |
| Testosterone (ng/dL) | 10.8 | 13.4 | 11.3 | 0.5-20 ng/dL |
| Estradiol (pg/mL) | < 11.8 | < 11.8 | < 11.8 | 50-110 pg/mL |
In 2006, the proband (II-1)’s ultrasound examination showed no uterus and ovaries, but revealed the presence of a testis-like structure located near the right hernia sac, and a testis-like structure at the lower part of the left inguinal canal.
AR sequencing was performed to provide a definitive diagnosis. Peripheral blood samples were obtained from the patients and their parents. DNA was extracted using the TaKaRa blood genome DNA extraction kit (TaKaRa Bio, Mountain View, CA, United States) following the manufacturer’s instructions. Sanger sequencing was performed and results were analyzed using Chromas Lite v2.01 software (Technelysium Pty Ltd., Tewantin, Australia). Pathogenicity was predicted using the bioinformatics tools Mutation Taster (www.mutationtaster.org), polymorphism phenotyping-2 (PolyPhen-2, http://genetics.bwh.harvard.edu/pph2), and Sorting Intolerant from Tolerant (SIFT, https://sift.bii.a-star.edu.sg/) programs.
A structural representation of the AR mutant was generated using the molecular visualization system in the open-source foundation PyMOL 2.4 (https://pymol.org/2/). The PDB ID (4OEA) of wild-type (WT) human AR-LBD was retrieved from the RCSB database (http://www.rcsb.org).
The following plasmids were constructed using wild-type AR expression plasmids as templates, which were obtained from Hanbio Biotechnology Co. Ltd. (Shanghai, China). And Human full-length AR cDNA was amplified from the AR expression plasmid using previously described primer pairs[7]. Amplicons were double digested by EcoRI and BamH1, and then subcloned into the pEGFP-N1 vector to generate the fusion protein expression plasmid pEGFP-AR WT. The mutant fusion protein expression plasmid pEGFP-AR P893L was introduced by a two-step PCR. Mutant amplicons were subcloned into the pEGFP-N1 vector, forming the mutant plasmid. The integrity of all inserts and their cloning borders had been verified by Sanger sequencing.
Human embryo kidney 293T cells (HEK-293T) and monkey kidney COS-7 cells were cultured in 24-well plates until 70%-80% confluence and transfected with 0.5 ug pEGFP- AR-WT, or pEGFP-AR P893L, as well as 0.5ug pEGFP-N1 control plasmid using Lipofectamine™ 2000 reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol.
Twenty-four hours after transfection, cells were treated with 100 nM Testosterone (T, Sigma). 40 min after hormone stimulation, cells were rinsed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde. They were permeabilized with 0.5% Triton X-100 in PBS, blocked with 3% bovine serum albumin at room temperature for 1 h, then incubated overnight with a mouse anti-DDDDK-Tag mAb (AE005, ABclonal, Wuhan, China) at 4°C. After rinsing with PBS, cells were incubated with CyTM 3 AffiniPure Goat Anti-Mouse lgG (H +L) secondary antibody (Jackson ImmunoResearch, West Grove, PA, United States) at room temperature for 1 h. Then, nuclei were stained with 4, 6-diamidino-2-phenyl indole (Beyotime, Haimen, China). Coverslips were mounted in 50% glycerol, and cells were observed and photographed under a laser confocal microscope (Fluoview FV1000, Olympus, Tokyo, Japan).
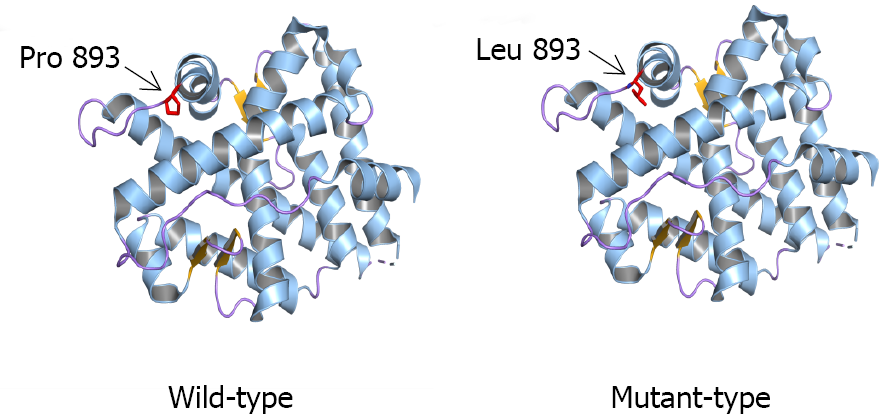
Genetic analysis revealed that all three siblings and their mother had a rare hemizygous mutation c.2678C>T (p. P893L) in exon 8 of AR. The father of the siblings had a WT sequence at this site, indicating that the variant showed maternal inheritance (Figure 1). Bioinformatics analysis using MutationTaster, SIFT, and Polyphen-2 predicted that the variant would be disease-causing, deleterious, and probably damaging, respectively, confirming it to have a very high pathogenic potential. Three-dimensional structural modeling indicated that the missense variant altered the LBD domain of the mutant AR protein relative to WT AR (Figure 2).
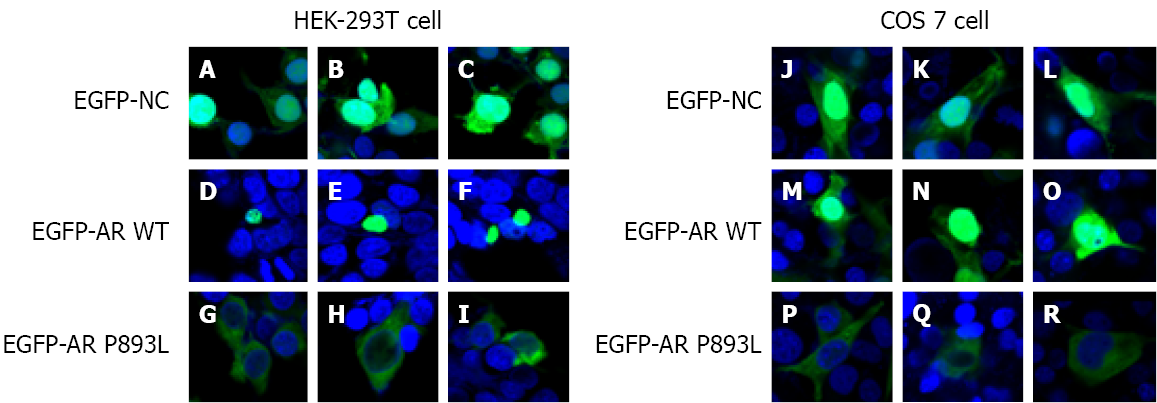
Subcellular localization results showed that EGFP-AR WT fusion proteins were translocated into the nucleus in vehicle-treated cells (Figure 3 D–F, M–O). EGFP-AR P893L fusion proteins were unable to enter the nucleus and showed a uniform distribution in the cytoplasm (Figure 3 G–I, P–R). These findings suggest that the p.P893L mutation affects the AR intracellular transport of AR by impairing nuclear translocation of the protein.
The final diagnosis of the proband and siblings is CAIS.
Both older patients started to receive estrogen replacement therapy with oral estradiol valerate since the age of 12.5 years.
During long-term follow-up, blood hormone tests showed normal testosterone levels, low estradiol levels, and high gonadotropin levels. Both older patients (aged 15 years at the time of this study) showed pubic hair at Tanner stage IV, and breasts at Tanner stage III. The younger sibling (aged 7 years at the time of this study) had pubic hair and breasts that were still at Tanner stage I.
In this study, the hemizygous variant c.2678C>T (p.P893L) in the LBD of AR was found to be causative of CAIS phenotypes for the three patients. Moreover, our in vitro functional study showed that nuclear translocation was decreased in AR-c.2678C>T mutation cells.
This variant (p.P893L) has been reported twice as a causative factor of CAIS[8,9]. However, few structural and functional studies[10,11] have been undertaken until now. Our in vitro work showed that the c.2678C>T missense variant affected the intracellular transport of AR by weakening its translocation from the cytoplasm to the nucleus. Subsequently, this may lead to the loss of AR biological function which could explain the pathogenicity of this variant.
The LBD (amino acids 646-920) contains specific binding sites for androgens, various transcricption factors of coactivation and the activation function-2 (AF-2) region[12]. The LBD region is fundamental for specific hormone receptor binding, nuclear translocation, and androgen-induced transcription. LBD variants with low to intermediate transcriptional activation displayed aberrant Kd values for hormone binding and decreased nuclear translocation[13]. In the previously reported study[8] about this mutation (p.P893L), cotransfection studies with an androgen-responsive reporter gene revealed a diminished transactivation property of the mutant androgen receptor. In the current variant, the amino acid substitution of proline to leucine occurs in the direct vicinity of the proposed C-terminal α-helix of the LBD containing the AF-2 transcriptional activating function core. Proline is a very rigid residue, so induces a particular backbone conformation that might be required at this position. The substitution to leucine may disturb this.
Clinically, malignant transformation of the gonads is the most feared complication in women with CAIS, timing of gonadectomy to prevent cancer is an issue of debate. Historically, individuals with CAIS were managed by the removal of gonadal tissue to avert the risk of gonadal malignancy[14]. However, clinical practice has recently changed. The oncological risk of CAIS children is relatively low and remains low until adulthood (0.02%-3%). Deans et al[15] found that the neoplastic risk for womem under the age of 30 is approximately 0.02%, while the tumor risk for women over that age is up to 22%. Chaurdy et al[16] reported a neoplastic risk ranging from 0.8% to 22%, and an overall risk for 133 patients over 20 years of age was around 1.5%[16]. Thus, gonadectomy could be postponed until after pubertal age to guarantee initial spontaneous pubertal development and avoid the need for hormonal replacement therapy[17,18]. In any case, gonadectomy after puberty is still controversial.
In summary, the c.2678C>T (p.P893L) AR variant was identified as a causative factor of CAIS in three siblings. This variant was shown by functional studies to impair nuclear translocation of the protein.
The authors would like to thank the proband and her family for providing blood samples and agreeing to participate in this research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kanda T S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Hughes IA, Werner R, Bunch T, Hiort O. Androgen insensitivity syndrome. Semin Reprod Med. 2012;30:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Boehmer AL, Brinkmann O, Brüggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, Niermeijer MF, Brunner HG, Rouwé CW, Waelkens JJ, Oostdijk W, Kleijer WJ, van der Kwast TH, de Vroede MA, Drop SL. Genotype vs phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab. 2001;86:4151-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Sakkiah S, Ng HW, Tong W, Hong H. Structures of androgen receptor bound with ligands: advancing understanding of biological functions and drug discovery. Expert Opin Ther Targets. 2016;20:1267-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Nadal M, Prekovic S, Gallastegui N, Helsen C, Abella M, Zielinska K, Gay M, Vilaseca M, Taulès M, Houtsmuller AB, van Royen ME, Claessens F, Fuentes-Prior P, Estébanez-Perpiñá E. Structure of the homodimeric androgen receptor ligand-binding domain. Nat Commun. 2017;8:14388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Liu C, Lyu Y, Li P. A hemizygous mutation in the androgen receptor gene causes different phenotypes of androgen insensitivity syndrome in two siblings by disrupting the nuclear translocation. Mol Genet Genomics. 2020;295:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Peters I, Weidemann W, Romalo G, Knorr D, Schweikert HU, Spindler KD. An androgen receptor mutation in the direct vicinity of the proposed C-terminal alpha-helix of the ligand binding domain containing the AF-2 transcriptional activating function core is associated with complete androgen insensitivity. Mol Cell Endocrinol. 1999;148:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Kanayama H, Naroda T, Inoue Y, Kurokawa Y, Kagawa S. A case of complete testicular feminization: laparoscopic orchiectomy and analysis of androgen receptor gene mutation. Int J Urol. 1999;6:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ledig S, Jakubiczka S, Neulen J, Aulepp U, Burck-Lehmann U, Mohnike K, Thiele H, Zierler H, Brewer C, Wieacker P. Novel and recurrent mutations in patients with androgen insensitivity syndromes. Horm Res. 2005;63:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yuan SM, Zhang YN, Du J, Li W, Tu CF, Meng LL, Lin G, Lu GX, Tan YQ. Phenotypic and molecular characteristics of androgen insensitivity syndrome patients. Asian J Androl. 2018;20:473-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Tyutyusheva N, Mancini I, Baroncelli GI, D'Elios S, Peroni D, Meriggiola MC, Bertelloni S. Complete Androgen Insensitivity Syndrome: From Bench to Bed. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Elfferich P, van Royen ME, van de Wijngaart DJ, Trapman J, Drop SL, van den Akker EL, Lusher SJ, Bosch R, Bunch T, Hughes IA, Houtsmuller AB, Cools M, Faradz SM, Bisschop PH, Bunck MC, Oostdijk W, Brüggenwirth HT, Brinkmann AO. Variable loss of functional activities of androgen receptor mutants in patients with androgen insensitivity syndrome. Sex Dev. 2013;7:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Weidler EM, Linnaus ME, Baratz AB, Goncalves LF, Bailey S, Hernandez SJ, Gomez-Lobo V, van Leeuwen K. A Management Protocol for Gonad Preservation in Patients with Androgen Insensitivity Syndrome. J Pediatr Adolesc Gynecol. 2019;32:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Deans R, Creighton SM, Liao LM, Conway GS. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin Endocrinol (Oxf). 2012;76:894-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Chaudhry S, Tadokoro-Cuccaro R, Hannema SE, Acerini CL, Hughes IA. Frequency of gonadal tumours in complete androgen insensitivity syndrome (CAIS): A retrospective case-series analysis. J Pediatr Urol. 2017;13:498.e1-498.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Lanciotti L, Cofini M, Leonardi A, Bertozzi M, Penta L, Esposito S. Different Clinical Presentations and Management in Complete Androgen Insensitivity Syndrome (CAIS). Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Döhnert U, Wünsch L, Hiort O. Gonadectomy in Complete Androgen Insensitivity Syndrome: Why and When? Sex Dev. 2017;11:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |