Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11007
Peer-review started: May 9, 2021
First decision: June 24, 2021
Revised: August 4, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: December 16, 2021
Processing time: 214 Days and 19.4 Hours
As a congenital metabolic bone disease caused by defective osteoclastic resorption of immature bone, osteopetrosis is characterized by diffused sclerosis of bones, brittle bones, easy fracturing, narrow medullary canals, and a weak fracture healing ability. At present, clear standards and principles for the treatment of fractures in patients with osteopetrosis are lacking. Non-operative treatment can prevent fracture hematoma and preserve the blood supply to the bone fragments, while being associated with frequent failures and higher mortality rates. Meanwhile, closed reduction and internal fixation with intramedullary nail (CRIF + IMN) approaches can also protect blood supply to the fracture site. However, IMN cannot be used for the vast majority of patients with osteopetrosis due to the narrowing of medullary canals. Thus, open reduction and internal fixation with plate remains the most appropriate surgical method for treating fractures in patients with osteopetrosis, but this approach is complicated by the lack of intramedullary hematopoiesis in such patients. Fracture healing primarily depends on the blood supply to the external periosteum. Open reduction can also easily destroy the periosteum and cause delayed fracture healing or even nonunion; however, CRIF may be the most practical approach. As a result, it would be prudent to solve the difficulty of drilling during the operation and the problem of postoperative nonunion.
In 2018, we treated an adult patient with osteopetrosis presenting with a subtrochanteric fracture. The fracture was fixed using a femoral locking compression plate. Because of delayed consolidation, at 12 mo postoperatively the patient was further treated with platelet-rich plasma (PRP) combined with radial extracorporeal shock wave therapy (rESWT). Antero-posterior and lateral radiographs obtained at the latest follow-up (10 mo) showed that the callus had grown at the original fracture site, and the medial fracture line almost disappeared.
Osteosynthesis remains the first choice of treatment approach for fractures in patients with osteopetrosis, especially peritrochanteric fractures. Preoperative preparation is necessary to avoid risks such as drill bit breakage and iatrogenic fracture during the operation. Moreover, fractures in a patient with osteopetrosis present with a high risk of delayed union and nonunion, which can be potentially cured with PRP + rESWT.
Core Tip: Osteopetrosis is a rare clinical disease which heightens the risk of fractures, but it is significantly difficult to perform surgical treatments in patients with osteopetrosis. Numerous risks such as drill bit breakage and iatrogenic fracture exist during the operation, which can lead to the failure of treatment. We present herein, a case of a subtrochanteric fracture in an adult patient with osteopetrosis that was fixed using a femoral locking compression plate. The postoperative consolidation was delayed, and the patient was subsequently treated with platelet-rich plasma (PRP) combined with radial extracorporeal shock wave therapy (rESWT). This case highlights the ultimate importance of preoperative preparation to avoid the potential risks of surgical failure. Furthermore, fractures in a patient with osteopetrosis have a high risk of delayed union and nonunion, which might be cured by PRP combined with rESWT.
- Citation: Yang H, Shao GX, Du ZW, Li ZW. Treatment for subtrochanteric fracture and subsequent nonunion in an adult patient with osteopetrosis: A case report and review of the literature. World J Clin Cases 2021; 9(35): 11007-11015
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11007.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11007
Osteopetrosis is a group of rare genetic diseases characterized by overly dense bones throughout the body[1,2]. Among the three types of osteopetrosis, autosomal recessive osteopetrosis (ARO) exhibits the most serious clinical symptoms, which generally manifest in infancy in the form of developmental malformations and abnormalities of multiple organs and systems, truly being a life-threatening disease[3].
Meanwhile, another form of osteopetrosis, autosomal dominant osteopetrosis (ADO), presents with mild symptoms[3,4], emerging in adulthood in most patients. The characteristic clinical manifestations of ADO include systemic bone sclerosis and narrowing, or even occlusion, of the medullary canal[5]. Moreover, the increased bone density actually weakens the bone, leading to a heightened risk of fractures[6].
Currently, there is no clear consensus on whether conservative or surgical treatments should be adopted for fractures in patients with osteopetrosis [7]. It is generally believed that non-operative treatment regimens should be selected for children, adolescents, and most of the patients presenting with upper limb fractures[8]. However, conservative treatment of femoral intertrochanteric, subtrochanteric, and femoral neck fractures can easily cause coxa vara deformities, delayed bone union, or nonunion[9]. In regard to these fractures, surgical treatment has been previously associated with more favorable prognoses[8]; however, surgical methods come with their own set of technical challenges. First, fixation with plate or intramedullary nail is particularly difficult due to increased bone density[10]. Second, there is an increased risk of iatrogenic fractures because of the brittle nature of the bones[11,12]. Finally, there is also a postoperative risk of delayed consolidation and nonunion because of impaired bone remodeling[13].
The current paper reports a case of a subtrochanteric fracture in an adult patient with osteopetrosis that was fixed using a femoral locking compression plate. The postoperative consolidation was delayed, and the patient was subsequently treated with platelet-rich plasma (PRP) combined with radial extracorporeal shock wave therapy (rESWT). Herein, we report the diagnosis and treatment process and the results of a 10 mo follow-up.
A 38-year-old male patient was admitted to our emergency department at The Second Hospital of Jilin University with complaints of hip pain, swelling, and limitation of motion due to a fall.
The patient suffered from swelling, pain, and limited movements of his left hip due to an accidental fall. He was subsequently rushed to a local hospital and diagnosed with "osteopetrosis and pathological fracture of the proximal femur" by X-ray examination. He came to our hospital for further diagnosis and treatment 9 h after injury.
The patient had no relevant medical history prior to the injury, and no history of surgery or blood transfusion.
The patient denied any history of genetic disorders or inbreeding in his family, and stated that his childhood development was normal.
The patient’s vital signs were as follows: Temperature of 36.4 °C, heart rate of 86 bpm, respiratory rate of 20 breaths per minute, blood pressure of 134/84 mmHg, and 99% oxygen saturation in room air. Subsequent physical examination on admission revealed slight swelling of the left hip and left thigh, local tenderness and percussion pain, and limited movement of the left hip, with good flexion and extension of the left knee, left ankle, and left toe. The skin sensation of the left lower limb was slightly decreased. Meanwhile, the pulse of the left dorsal pedis artery and peripheral perfusion were good.
Main abnormal indicators were as follows: Parathyroid hormone 111.90 pg/mL, urine specific gravity 1.010, uric acid 520 µmol/L, D-dimer 1.39 µg/mL, fibrinogen degradation product 6.8 µg/mL, blood phosphorus 1.61 mmol/L, white blood cell count 10.6 × 109/L, neutrophil percentage 84.1%, and lymphocyte count 11.3%.
Meanwhile, the main normal indicators were as follows: Hb 152g/L, PLT 144.5 × 109/L, PT 11.0 s, APTT 25.6 s, TT 13.6 s, U-BIL(-), UBG(-), KET(-), BLD(+-), Calcitonin 3.86 pg/mL, TPOAb 36 U/mL, TG-Ab < 15.0 U/mL, FT3 4.45 pmol/L, and FT4 13.34 pmol/L.
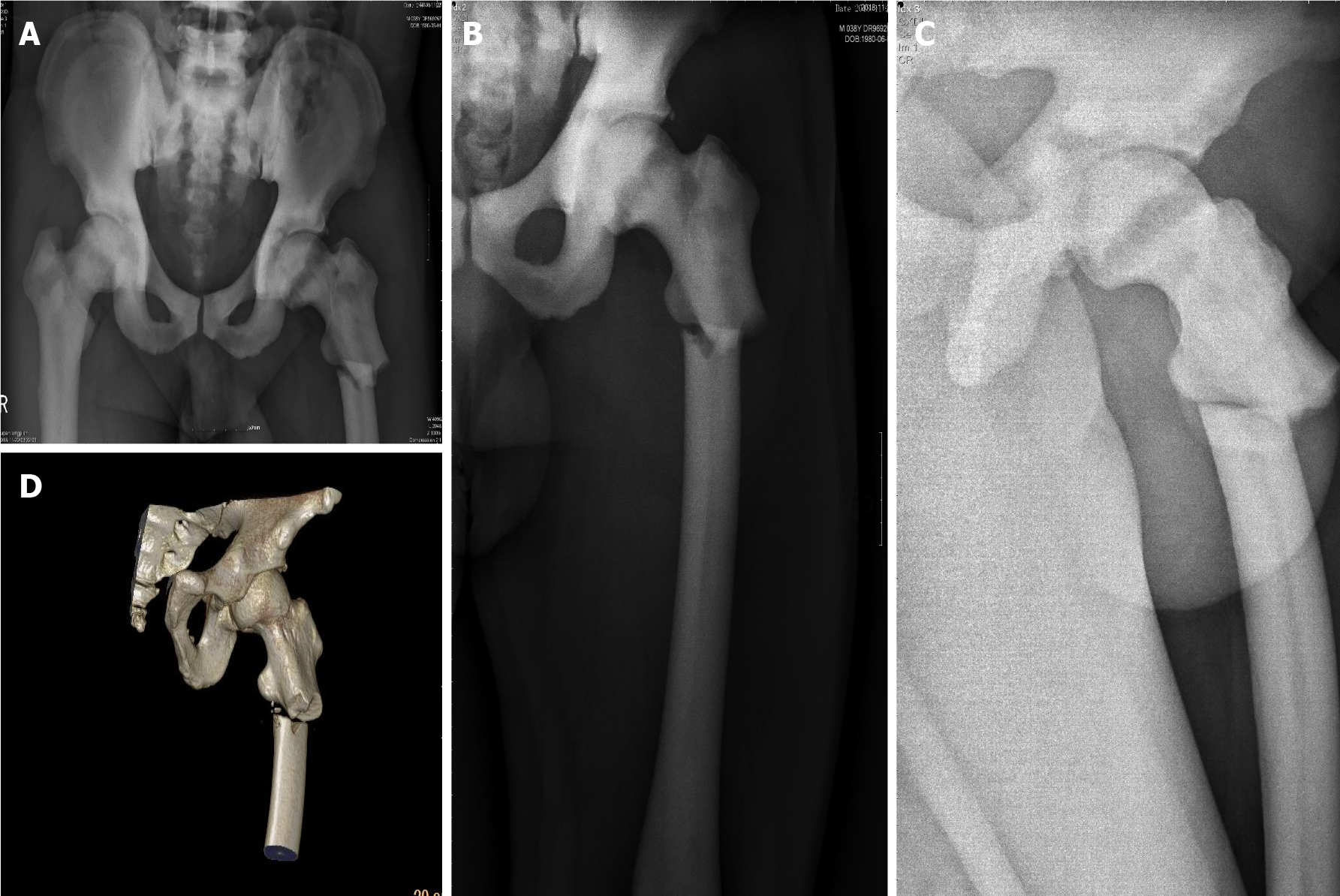
Plain X-ray imaging illustrated discontinuous cortical bone of the left proximal femur, separation and displacement of the bone fragments, increased bone density of pelvic bones and bilateral femurs, thickened cortical bone, and narrowed bone marrow canal (Figure 1).
Subtrochanteric fracture and osteopetrosis.
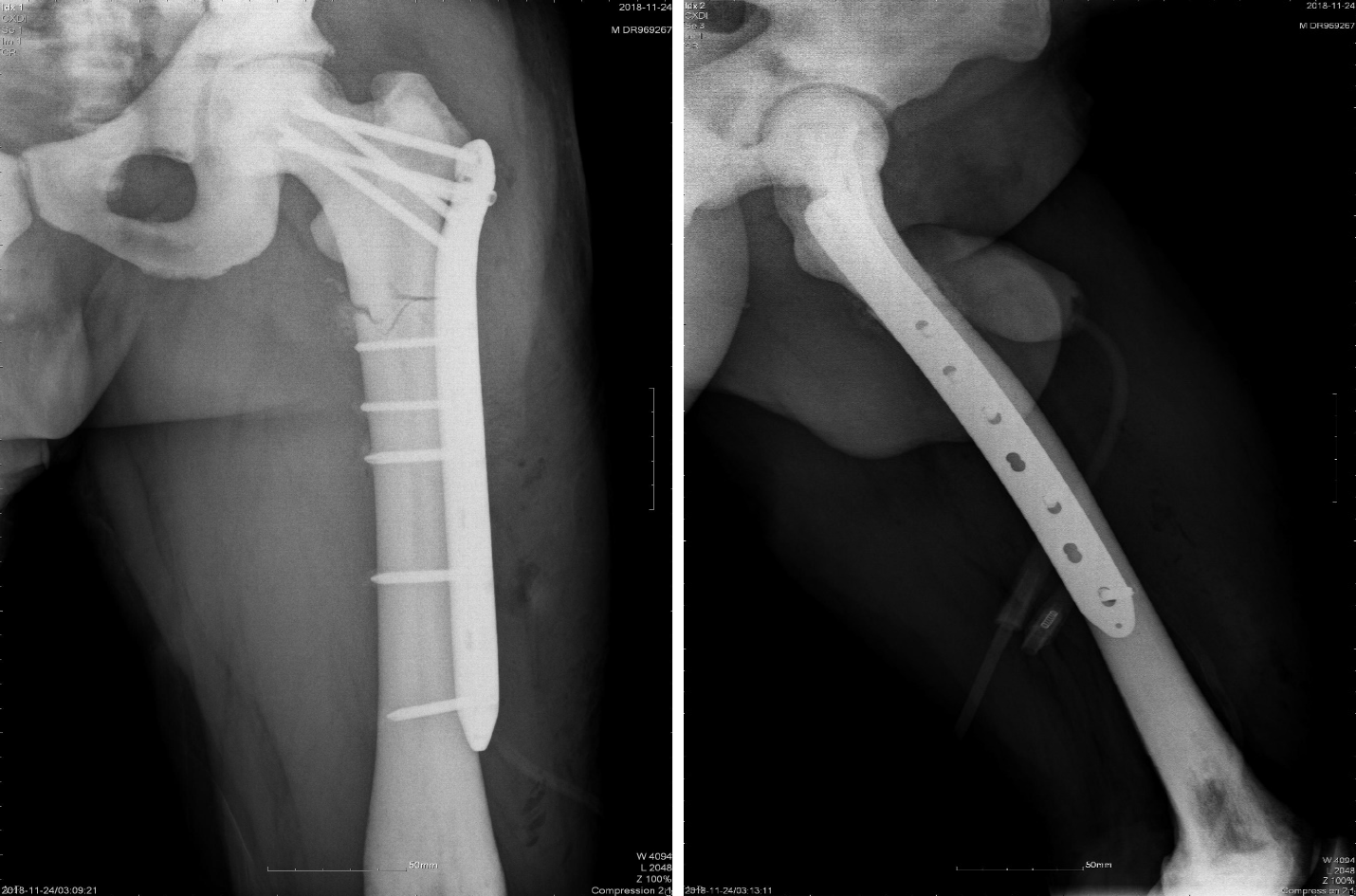
Since the results of physical, imaging, and laboratory examinations indicated no contraindications, an operation was performed on the second day after admission. We chose the open reduction method and internal fixation with femoral locking compression plate to treat the fracture. First, general anesthesia was administered during the operation. We employed a bone holding device and Kirschner wire for temporary fixation. Facing the difficulty of drilling during the operation, we opted to use a low-speed and high torque electric drill, changing the drill bit several times and assisting with physiological saline cooling. Although the operation time was prolonged, the surgery was completed successfully (Figure 2).
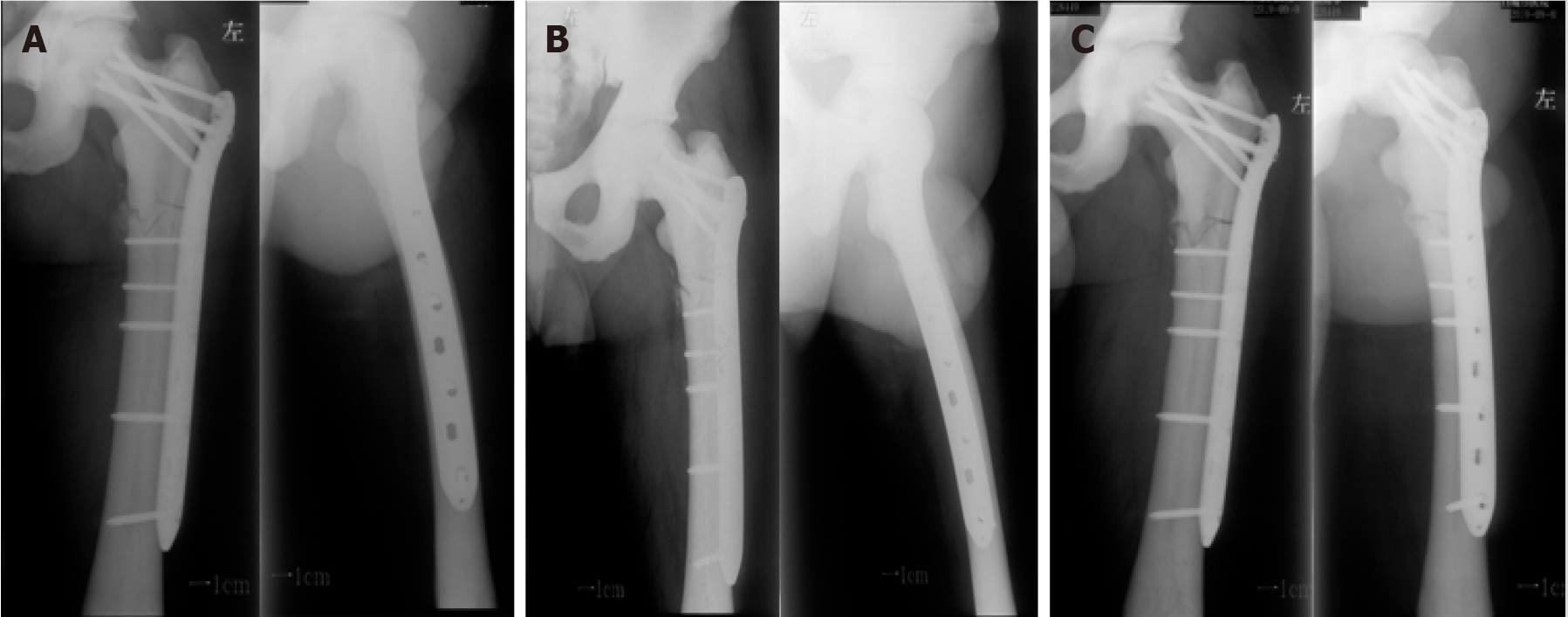
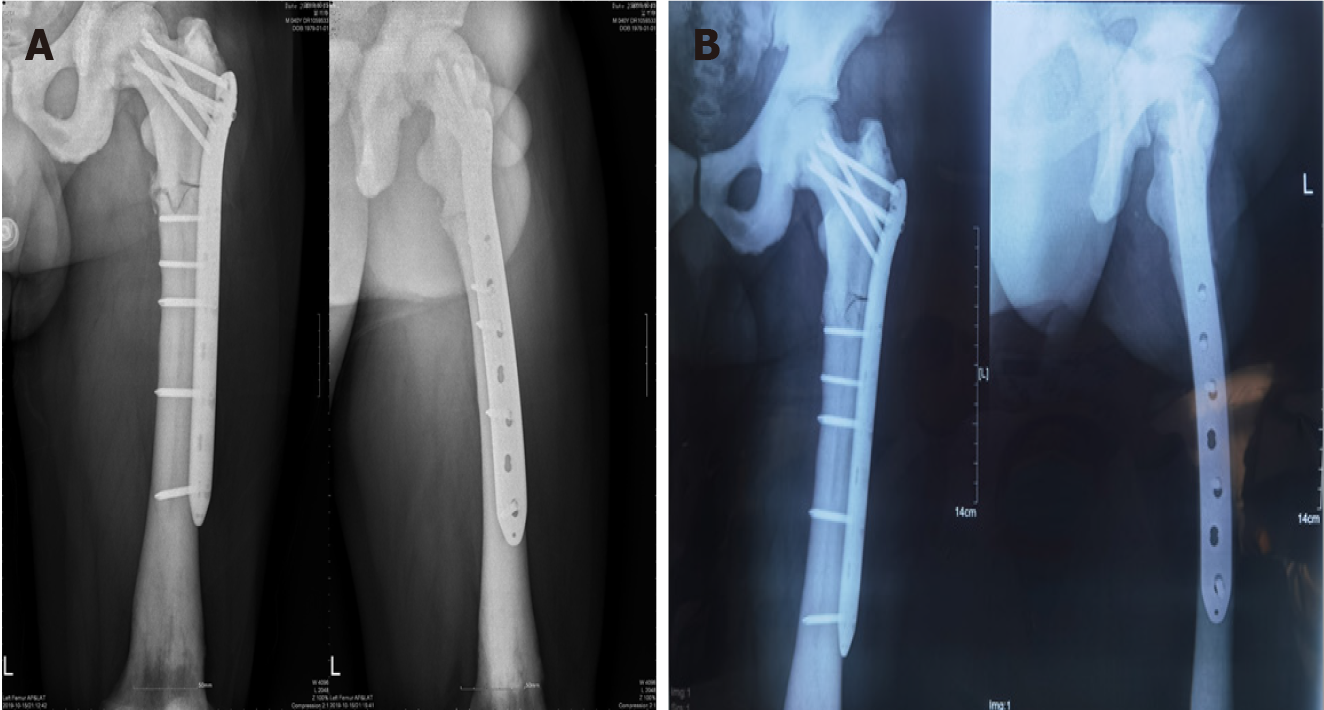
After the operation, the patient received supportive treatment for prevention of infection and anticoagulants, and there were no perioperative or postoperative complications. The patient was transferred to a local hospital for follow-up treatment on the 7th day after operation. At 2, 3, and 10 mo postoperatively, plain radiography was performed, which showed that the fracture line was still clearly visible, with no obvious signs of fracture healing (Figure 3). Unfortunately, the follow-up demonstrated nonunion of the bone. After consultations with the treating physicians, the patient accepted treatment with three PRP + rESWT sessions. Antero-posterior and lateral radiographs obtained at the latest follow-up (10 mo after the last treatment) illustrated that the callus had grown at the original fracture site, and the medial fracture line almost disappeared (Figure 4).
The patient also walked and participated in many daily activities without any unusual complaints. However, there was a risk of plate fracture and refracture, since the lateral cortex had not achieved bone healing, even though the medial side healed. In this regard, the patient and his family were informed of the risk, and an iliac bone graft was recommended (pelvic CT examination revealed the presence of a certain amount of cancellous bone in the iliac bone). The patient and his family members expressed their understanding, but refused to accept the surgical treatment. It remains to be seen how this situation will be resolved in the long term.
Osteopetrosis, first reported by Albers-Schönberg in 1904, is precipitated by genetic mutations that result in the failure of osteoclast differentiation or functions and lead to reduced bone resorption. Consequently, all body’s bones become hard and brittle like marble due to inefficient resorption, so the condition is also known as “marble bone disease”, as well as “Albers-Schönberg disease”[14]. In addition, since the number and activity of osteoclasts are decreased, the patients with osteopetrosis present with an increased risk of developing hypocalcemia, which can further cause epilepsy and hyperparathyroidism. Meanwhile, in infants, the disease not only impairs the bone remodeling system, but also causes myelopoiesis disorder due to narrowing of the medullary cavity, and secondary hepatosplenomegaly due to excessive extramedullary hemopoiesis[15]. Skeletal deformities of the head and face can also lead to hydrocephalus and stenosis of nerve foramen, which may damage optic and facial nerves[16]. A large proportion of children afflicted with osteopetrosis do not survive till adulthood, which represents a serious problem to parents all over the world[14]. In the case of a mild adult type of osteopetrosis, only the whole-body bone mass is increased and the medullary canals are narrowed, which do not result in developmental malformation and other abnormalities.
Further adding to the plight, the standards and principles for the treatment of fractures in patients with osteopetrosis are not yet clearly formulated[7]. Armstrong et al[8] conducted a survey of the membership of the Pediatric Orthopedic Society of North America, which incorporated the experience of 57 surgeons who treated 79 fractures in patients with osteopetrosis, with their findings illustrating that the majority of patients with osteopetrosis were successfully treated using the conservative approach. On the other hand, they also observed that non-operative treatment of femoral intertrochanteric, subtrochanteric, and femoral neck fractures could precipitate coxa vara deformities and delayed bone union or nonunion. In another study, Birmingham and McHale[9] treated a patient presenting with autosomal-dominant osteopetrosis, a subtrochanteric fracture, and an ipsilateral femoral neck fracture with a hip spica cast after 6 wk in traction. During the follow-up, they found that 2.5 years after the injury, the subtrochanteric fracture was united and in slight varus and external rotation. Furthermore, Kim et al[13] employed the intramedullary nailing (IMN) approach to treat two cases of adult osteopetrosis with subtrochanteric fractures. In the first case, IMN left a gap at the fracture site because the distal fragment was not effectively reamed, and the patient showed delayed union and subsequently underwent a dynamization procedure 6 mo postoperatively, which resulted in bone healing 10 mo after the dynamization. The second patient presented with a bilateral subtrochanteric fracture. The left femur fracture healed 8 mo after IMN, while on the right side, the patient underwent open reduction and internal fixation with a locking plate, as the medullary canal was too short and narrow. Intramedullary fixation was excluded, but the fracture line still did not disappear 20 mo after the operation. Moreover, Amit et al[17] treated subtrochanteric fractures in two patients with osteopetrosis by means of open reduction and internal fixation with a locking plate, and both the patients achieved bone healing 21 and 23 wk after operation, respectively.
Additionally, surgical treatment of patients with osteopetrosis presents with heightened difficulty compared to ordinary fracture patients. There is a certain risk of drill bit breakage and iatrogenic fracture during the operation, which can lead to failure of treatment[9-12]. However, as long as preoperative preparations and corresponding surgical strategies are well formulated in a timely manner, surgical treatment is still heralded as the first-choice treatment for fractures in patients with osteopetrosis[7,18,19]. Unfortunately, regardless of whether the operation is successful or not, the risk of delayed union or even nonunion still persists[18].
The hard-done work of our peers has highlighted that the risk of the aforementioned delayed postoperative union and the nonunion associated with impaired bone remodeling[13,20]. To elaborate, Matsuo et al[21] reported the case of an osteopetrosis patient presenting with a femoral shaft fracture below a plate who underwent open reduction and internal fixation with locking plate and wire cerclage. During follow-up, they observed that fracture healing was delayed postoperatively, and the plate fractured 14 mo after operation. The occurred fracture healed after treatment with a double locking plate. In the case of our patient, we did not rely on steel wire fixation, as we believe that plate fixation is accurate and provides enough stability. Steel plate fixation was performed following the AO principles, which indicate the use of a plate length greater than three times the fracture in comminuted fractures, and plate length greater than eight to ten times the fracture length in simple fractures. The principles further suggest a screw/plate ratio of less than 0.5 to create a long lever arm and decrease the bending loads on the distal screws. Lastly, a span of at least two or three screw holes should be left open over the fracture to decrease stress concentration[22,23]. We speculate that the reasons for poor healing may be linked to insufficient blood supply and osteogenetic factors. There is a wide variety of methods to promote bone healing for clinicians. Although autologous bone grafting is deemed as the “gold-standard” approach, it is associated with complications and additional treatment costs[24]. Nevertheless, there is no doubt that autogenous bone graft can augment fracture healing, but for patients with no obvious defects after fracture reduction[25], the usage of autogenous bone graft or even reamed nail + bone graft at the first operation remains to be further discussed. In our patient, the limb function was respectable, and accordingly he did not want to proceed with treatment associated with potential trauma. As a result, we tried to employ some minimally invasive methods to improve the probability of fracture healing, such as haemopoietic stem cell transplant (HSCT), PRP, and extracorporeal shock wave therapy (ESWT).
Various authors have highlighted the ability of HSCT to effectively restore normal bone resorption and hematopoiesis in severe autosomal recessive osteopetrosis[26], while also being associated with many potential adverse effects, including acute rejection, graft vs host disease, and veno-occlusive events[27]. Meanwhile, PRP is a well-known plasma product obtained by centrifugation and fractionation of autologous venous blood. The concentrated platelets can be used to produce various growth factors, such as PDGF, factor-β, VEGF, FGF, and others[28]. In addition, several in vitro studies have indicated that PRP can promote neovascularization[29] and enhance osteoprogenitor cell proliferation and differentiation[30,31]. However, many scholars still doubt the clinical effectiveness of PRP[32,33]. Lastly, ESWT is a physical stimulation therapy, which includes focused (fESWT) and radial (rESWT) therapy types[34,35]. In our case, we employed the rESWT type, which uses compressed air or a magnetic field to emit projectiles. The projectiles strike a metal applicator placed on the patient’s skin to generate stress waves[35], and these waves get transmitted into tissues and stimulate the process of bone fracture healing[36-39]. Furthermore, Kertzman et al[38] employed the rESWT approach to treat 22 patients with bone non-union, 16 of whom achieved bone union after 6 mo. ESWT is widely used in the treatment of delayed union and nonunion of fracture and appears to be an effective treatment with no obvious complications[39].
Osteosynthesis is regarded as the first-choice treatment approach for fractures in patients with osteopetrosis, especially peritrochanteric fractures. Meanwhile, if the condition of medullary canal permits, the preferred choice is closed reduction and intramedullary nailing. However, for most patients with osteopetrosis, only open reduction and plate fixation are allowed, and there is a significantly higher risk of delayed union and nonunion in patients with osteopetrosis compared to normal patients. In order to solve this problem, low trauma intervention such as PRP and rESWT can be tried.
We are grateful to the patient who has given his informed consent for the publication of this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu CC S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Huang J, Pan J, Xu M, Xu S. Successful open reduction and internal fixation for displaced femoral fracture in a patient with osteopetrosis: Case report and lessons learned. Medicine (Baltimore). 2017;96:e7777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Shapiro F. Osteopetrosis. Current clinical considerations. Clin Orthop Relat Res. 1993;34-44. [PubMed] |
| 3. | Bollerslev J, Mosekilde L. Autosomal dominant osteopetrosis. Clin Orthop Relat Res. 1993;45-51. [PubMed] |
| 4. | Scaramuzzo L, Messuti L, Manicone PF, Raffaelli L, Rossi B, Gallenzi P, Berardi D, Maccauro G. Clinical and histological modifications in osteopetrotic bone: a review. J Biol Regul Homeost Agents. 2009;23:59-63. [PubMed] |
| 5. | Ihde LL, Forrester DM, Gottsegen CJ, Masih S, Patel DB, Vachon LA, White EA, Matcuk GR Jr. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31:1865-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Dahl N, Holmgren G, Holmberg S, Ersmark H. Fracture patterns in malignant osteopetrosis (Albers-Schönberg disease). Arch Orthop Trauma Surg. 1992;111:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hasan O, Pathan AZ, Naqi H, Aqueel T, Hashmi P, Lakdawala RH. Inheritance patterns, challenges, and outcomes of fracture management in osteopetrosis patients. CASE series and review of pertinent literature. Ann Med Surg (Lond). 2018;36:191-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Armstrong DG, Newfield JT, Gillespie R. Orthopedic management of osteopetrosis: results of a survey and review of the literature. J Pediatr Orthop. 1999;19:122-132. [PubMed] |
| 9. | Birmingham P, McHale KA. Case reports: treatment of subtrochanteric and ipsilateral femoral neck fractures in an adult with osteopetrosis. Clin Orthop Relat Res. 2008;466:2002-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Chhabra A, Westerlund LE, Kline AJ, McLaughlin R. Management of proximal femoral shaft fractures in osteopetrosis: a case series using internal fixation. Orthopedics. 2005;28:587-592. [PubMed] |
| 11. | Rolauffs B, Bernhardt TM, von Eiff C, Hart ML, Bettin D. Osteopetrosis, femoral fracture, and chronic osteomyelitis caused by Staphylococcus aureus small colony variants (SCV) treated by girdlestone resection--6-year follow-up. Arch Orthop Trauma Surg. 2002;122:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Su YJ, Chiang WK, Chang KS. Chalk bones and pathological fractures: case report and review of the literature. J Emerg Med. 2003;25:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kim J, Park YC, Moon HS, Do WS, Yang KH. Intramedullary nailing for subtrochanteric fracture in autosomal dominant Type II osteopetrosis: Case report of 2 patients. Medicine (Baltimore). 2020;99:e21648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Dozier TS, Duncan IM, Klein AJ, Lambert PR, Key LL Jr. Otologic manifestations of malignant osteopetrosis. Otol Neurotol. 2005;26:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Al-Tamimi YZ, Tyagi AK, Chumas PD, Crimmins DW. Patients with autosomal-recessive osteopetrosis presenting with hydrocephalus and hindbrain posterior fossa crowding. J Neurosurg Pediatr. 2008;1:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Amit S, Shehkar A, Vivek M, Shekhar S, Biren N. Fixation of Subtrochanteric Fractures in Two Patients with Osteopetrosis Using a Distal Femoral Locking Compression Plate of the Contralateral Side. Eur J Trauma Emerg Surg. 2010;36:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Aslan A, Baykal YB, Uysal E, Atay T, Kirdemir V, Baydar ML, Aydoğan NH. Surgical treatment of osteopetrosis-related femoral fractures: two case reports and literature review. Case Rep Orthop. 2014;2014:891963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Zhang ZF, Wang D, Wu LD, Dai XS. Case report: A 10 years follow-up of periprosthetic femoral fracture after total hip arthroplasty in osteopetrosis. Chin J Traumatol. 2017;20:173-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Bhargava A, Vagela M, Lennox CM. "Challenges in the management of fractures in osteopetrosis"! Injury. 2009;40:1167-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Matsuo T, Lee SY, Iwakura T, Fukui T, Oe K, Matsumoto T, Matsushita T, Nishida K, Kuroda R, Niikura T. Locking plate osteosynthesis for a femoral fracture and subsequent nonunion in a patient with osteopetrosis. Int J Surg Case Rep. 2018;51:395-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Wagner M, Frigg R. Internal Fixators-Concepts and Cases Using LCP and LISS. New York: Thieme; 2006. |
| 23. | Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34 Suppl 2:B63-B76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 332] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93:2227-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 25. | Azi ML, Aprato A, Santi I, Kfuri M Jr, Masse A, Joeris A. Autologous bone graft in the treatment of post-traumatic bone defects: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975;190:784-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 223] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Bacon S, Crowley R. Developments in rare bone diseases and mineral disorders. Ther Adv Chronic Dis. 2018;9:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Buza JA 3rd, Einhorn T. Bone healing in 2016. Clin Cases Miner Bone Metab. 2016;13:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G, Simopoulos C, Kokka A, Patsouris E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Kasten P, Vogel J, Luginbühl R, Niemeyer P, Weiss S, Schneider S, Kramer M, Leo A, Richter W. Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics. Cells Tissues Organs. 2006;183:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 32. | Roffi A, Di Matteo B, Krishnakumar GS, Kon E, Filardo G. Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review. Int Orthop. 2017;41:221-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, Ayeni OR, Bhandari M. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Speed C. A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med. 2014;48:1538-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Schmitz C, Császár NB, Milz S, Schieker M, Maffulli N, Rompe JD, Furia JP. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br Med Bull. 2015;116:115-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Ibrahim MI, Donatelli RA, Schmitz C, Hellman MA, Buxbaum F. Chronic plantar fasciitis treated with two sessions of radial extracorporeal shock wave therapy. Foot Ankle Int. 2010;31:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Gollwitzer H, Gloeck T, Roessner M, Langer R, Horn C, Gerdesmeyer L, Diehl P. Radial extracorporeal shock wave therapy (rESWT) induces new bone formation in vivo: results of an animal study in rabbits. Ultrasound Med Biol. 2013;39:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Kertzman P, Császár NBM, Furia JP, Schmitz C. Radial extracorporeal shock wave therapy is efficient and safe in the treatment of fracture nonunions of superficial bones: a retrospective case series. J Orthop Surg Res. 2017;12:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Schaden W, Mittermayr R, Haffner N, Smolen D, Gerdesmeyer L, Wang CJ. Extracorporeal shockwave therapy (ESWT)--First choice treatment of fracture non-unions? Int J Surg. 2015;24:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |