Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10999
Peer-review started: June 20, 2021
First decision: July 16, 2021
Revised: July 25, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 16, 2021
Processing time: 172 Days and 16 Hours
Immunoglobulin (Ig) G4-associated diseases are a group of systemic diseases involving multiple organs and are also known as IgG4-associated sclerosing diseases. IgG4-associated lymphadenopathy occurring in the lymph nodes is characterized by a lack of specificity due to its clinicopathological characteristics and must be differentiated from a variety of lesions, such as Castleman disease, lymphatic follicular reactive hyperplasia, and lymphoma.
A 65-year-old male patient, with Guillain-Barre syndrome for 5 years, presented to our hospital complaining of bilateral orbital mass for 2 years. After hospitalization, the results of the patient’s laboratory tests showed that immunoglobulin subgroup IgG4 was 33.90 g/L and IgG was 30.30 g/L, but serum interleukin-6 was normal. The pathological morphology of orbital mass and cervical lymph node were consistent, which showed that a large number of plasma cells and eosinophils were observed in the lymphatic follicles, and the interstitial fibrous tissue was proliferative. Immunohistochemistry showed that CD20 (B cells) (+), CD3 (T cells) (+), CD38 (+), IgG (+), IgG4 positive cells > 100/high powered field, and IgG4/IgG > 40%. Combined with clinical and immunohistochemical results, lymphadenopathy was consistent with Castleman disease-like IgG4-associated sclerosing disease. Prednisone acetate treatment was given at 40 mg/d. After 2 wk, the superficial lymph nodes and orbital masses shrank, and the IgG4 level decreased. As prednisone acetate was regularly used at a reduced dosage, no recurrence of the disease has been observed.
This case suggested that it is necessary to proceed cautiously in clinical practice with such patients, and immunoglobulin, complement, interleukin-6, C-reactive protein, and other examinations should be performed to confirm the diagnosis.
Core Tip: Immunoglobulin (Ig) G4-related disease is a group of systemic immune diseases. There is no unified standard for the differentiation of IgG4-related disease from plasma cell Castleman disease. These diseases are sometimes difficult to distinguish from one another. We reported a case of IgG4-related lymph node disease with an orbital mass mimicking Castleman disease. The pathological morphology was similar to Castleman disease, which may lead to misdiagnosis. This case suggested that it is necessary to proceed cautiously in clinical practice with such patients, and immunoglobulin, complement, interleukin-6, C-reactive protein, and other examinations should be performed to confirm the diagnosis.
- Citation: Hao FY, Yang FX, Bian HY, Zhao X. Immunoglobulin G4-related lymph node disease with an orbital mass mimicking Castleman disease: A case report. World J Clin Cases 2021; 9(35): 10999-11006
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10999.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10999
Immunoglobulin (Ig) G4-related disease (IgG4-RD) is a group of systemic immune diseases characterized by elevated serum IgG4, a large amount of IgG4+ plasma cell infiltration in tissues, occasional eosinophilic granulocytes, interstitial fibroplasia, and small phlebitis obliterans[1-3]. Castleman disease is a clinically rare lymphoproliferative disorder. Castleman disease and IgG4-RD may present some common clinical manifestations, such as enlarged lymph nodes and elevated serum IgG4 levels, which make the clinical diagnosis and differential diagnosis more difficult and challenging[4]. Here, we report a case of IgG4-related lymph node disease with an orbital mass mimicking Castleman disease and review the relevant literature.
A 65-year-old male patient was admitted to the hospital on January 21, 2020 due to a “bilateral orbital mass for 2 years.”
Two years prior, the patient developed binocular swelling, exophthalmos, and decreased vision accompanied by hand tremor without any other accompanying symptoms, and the above symptoms became progressively worse.
He suffered from Guillain-Barre syndrome 5 years ago and left hand tremor after recovery.
The patient denied alcohol consumption and allergies to food or medicines.
Right orbital mass and bilateral cervical lymph node enlargement was observed. The large one was about 1.6 cm × 0.9 cm. No other obvious positive signs were found.
After hospitalization, the results of the patient’s laboratory tests showed that the erythrocyte sedimentation rate was 47.0 mm/h, D-dimer was 1450.00 ng/mL, immunoglobulin subgroup IgG4 was 33.90 g/L, IgG was 30.30 g/L, total protein was 64.7 g/L, and albumin was 26.1 g/L. Antinuclear antibody was weakly positive (titer 1:100), complement C3 was 0.79 g/L, complement C4 was 0.10 g/L, and complement C1q was 102.20 mg/L. Urinary protein was 4+, but serum interleukin (IL)-6 was normal. Thyroid function, thyroid stimulating hormone receptor antibody, routine blood tests, C-reactive protein (CRP), brain natriuretic peptide (BNP)/pro-brain natriuretic peptide (PBNP), and rheumatoid factors were not significantly abnormal, and extractable nuclear antigen antibody spectrum, anti-cyclic citrate peptide antibody, anti-neutrophilic cytoplasmic antibody, and anti-phospholipid antibody were negative.
On January 9, 2021, orbital computed tomography (CT) performed in the outpatient department showed bilateral external eye muscle and periocular changes, exophthalmos, swelling of the right eyelid, and multiple bone resorption changes in the medial orbital wall on both sides.
On January 21, 2021, ultrasound examination showed nodular goiter (thyroid imaging reporting and data system 3 type) and right cervical lymph node enlargement.
On February 2, 2021, ultrasonography showed bilateral lymph node enlargement. The large one on the right was 1.6 cm × 0.9 cm, and the large one on the left was 1.9 cm × 1.1 cm.
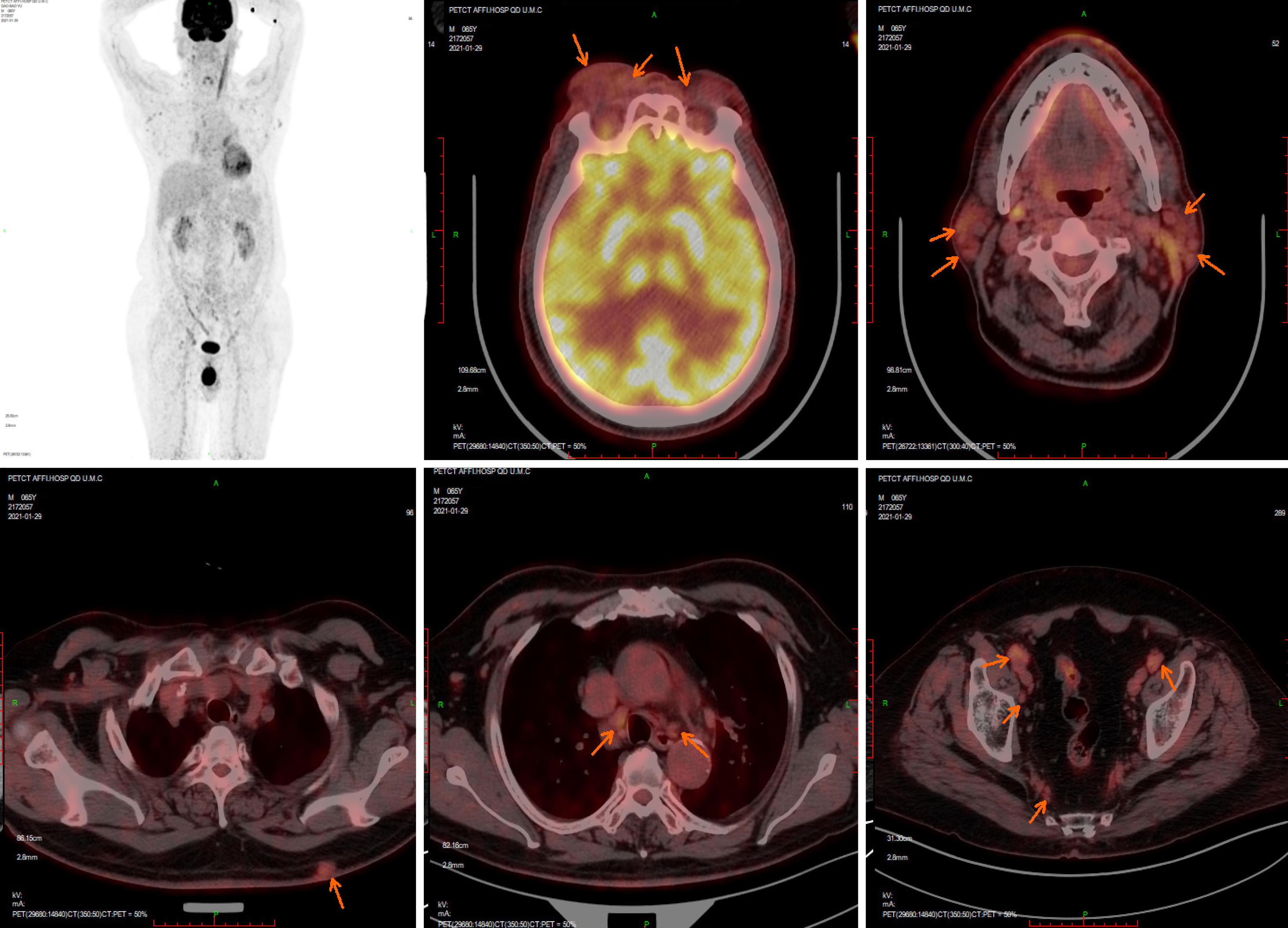
On January 29, 2021, positron emission tomography/CT results showed that the bilateral ophthalmic muscles, lacrimal glands, intraorbital soft tissue, subcutaneous soft tissue nodules in the back, bilateral mediastinal pleura, and several superficial and deep lymph nodes all showed increased metabolism, accompanied by retroperitoneal fibrosis (Figure 1).
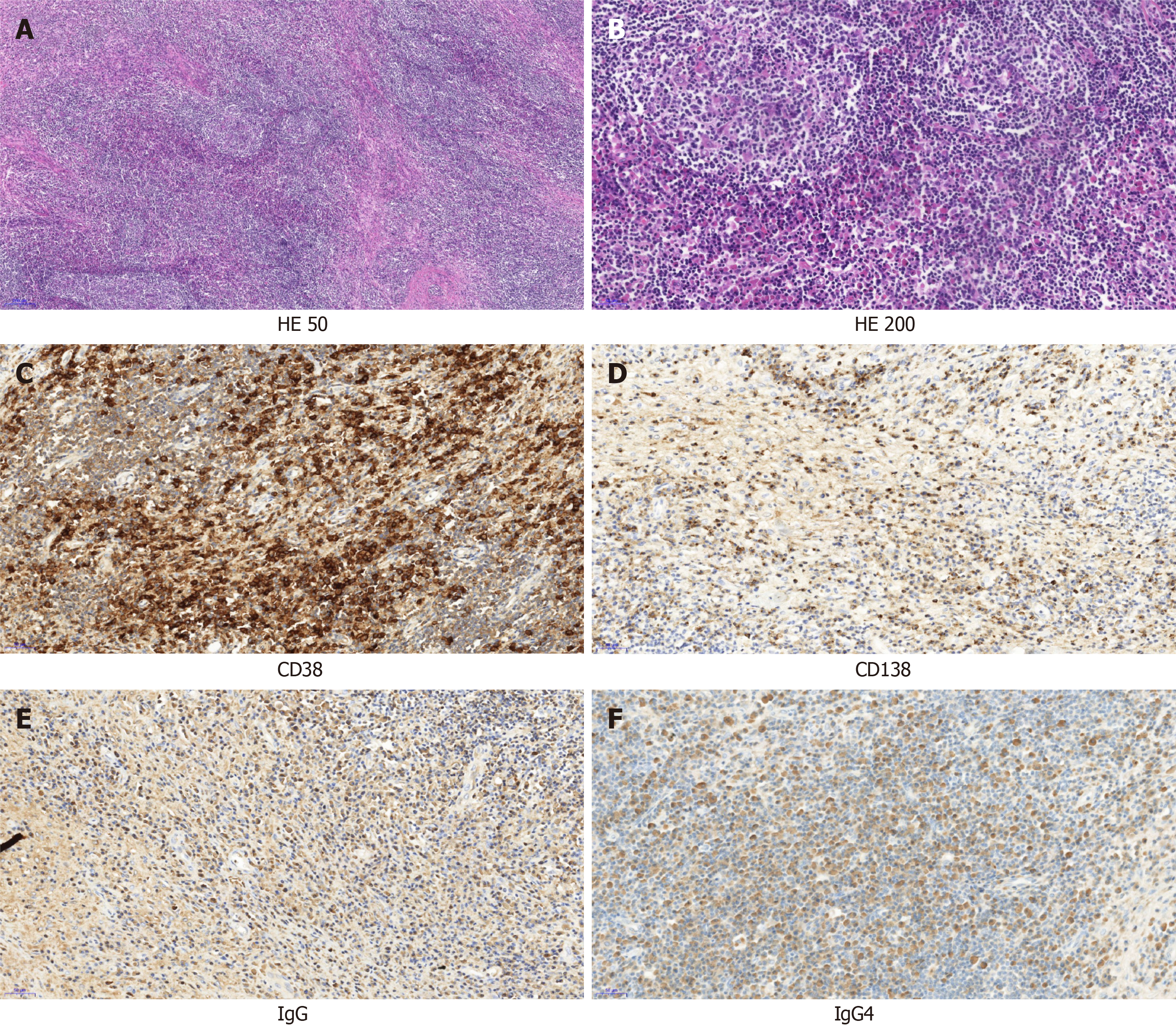
On January 25, 2021, right orbital mass resection was performed, and postoperative pathological diagnosis showed that a large number of plasma cells and eosinophils were observed in the lymphatic follicles. The interstitial fibrous tissue was proliferative (Figure 2A and B). Immunohistochemistry showed CD20 (B cells) (+), CD3, CD4, and CD8 (T cells) (+), CD38 and CD138 (plasma cells) (+), S100 was scattered (+), CD1α was scattered (+), Langerin (-), Epstein-Barr encoding region (-), Ki-67 (+, approximately 40%), IgG (+), IgG4 positive cells > 100/high powered field, and IgG4/IgG > 40% (Figure 2C-F). Combined with clinical and immunohistochemical results, these results were consistent with IgG4-associated sclerosing disease.
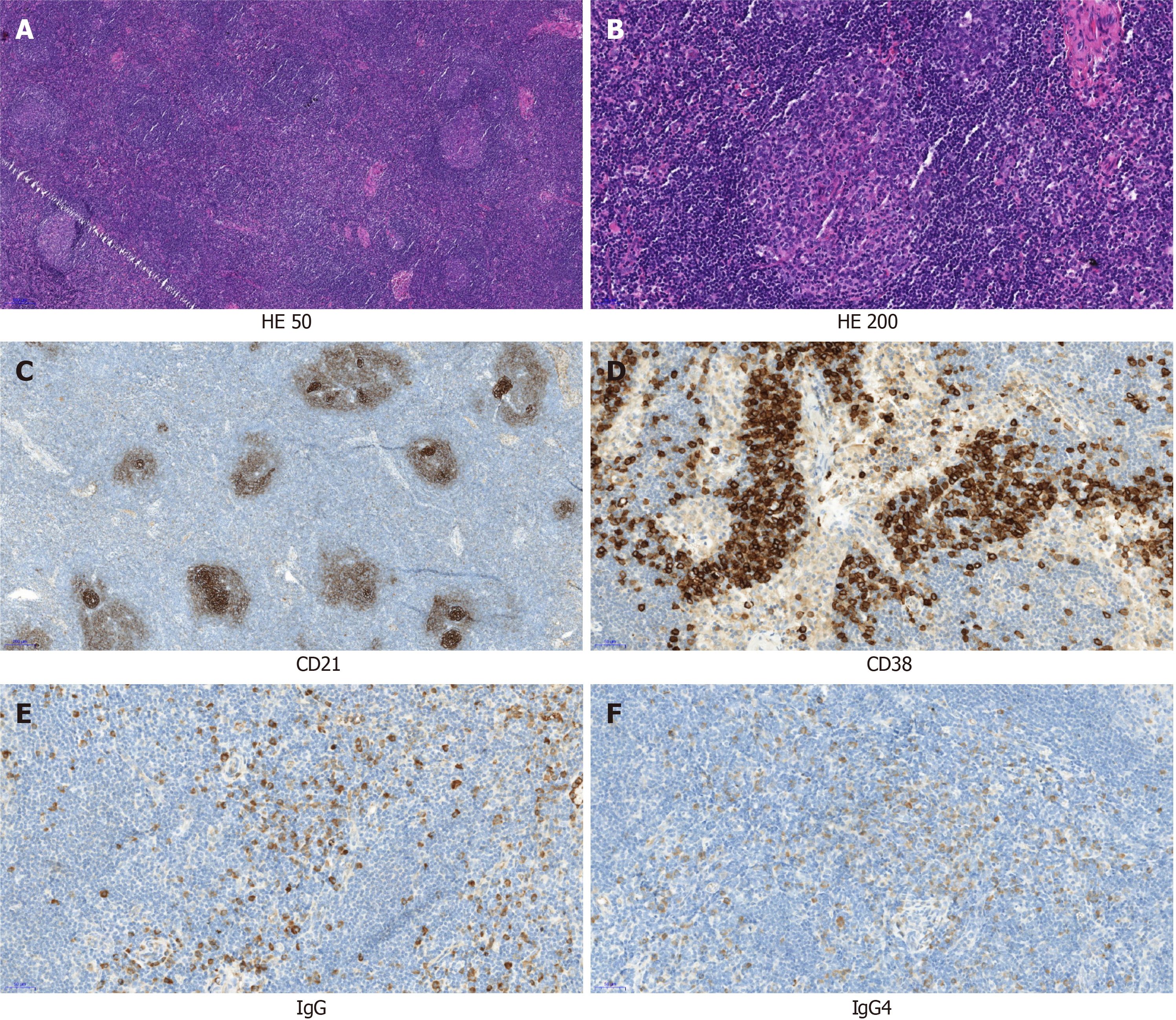
On February 4, 2021, the pathological results of the left neck lymph node biopsy showed that the lymph node structure was still present, mainly exhibiting follicular hyperplasia, small blood vessels in the follicle were extended, and a large number of plasma cells were observed in the interfollicular area (Figure 3A and B). Immunohistochemical results showed that CD20 (B cells) (+), CD3 (T cells) (+), CD38 plasma cells (+), IgG (+), IgG4 (+), CD21 (follicular dendritic cell network) (+), Ki-67 (+); (approximately 90% in follicles and approximately 20% in the interfollicular area), and IgG4/IgG > 40% (Figure 3C-F). Combined with clinical and immunohistochemical results, lymphadenopathy was consistent with Castleman disease-like IgG4-associated sclerosing disease.
The final diagnosis of the presented case was Castleman disease-like IgG4-associated sclerosing disease.
Prednisone acetate treatment was given at 40 mg/d. After 2 wk, the superficial lymph nodes and orbital masses shrank, and the IgG4 level decreased upon re-examination.
At present, prednisone acetate was regularly used at a reduced dosage, and no recurrence of the disease has been observed.
IgG4-associated diseases are a group of systemic diseases involving multiple organs and are also known as IgG4-associated sclerosing diseases. Clinically involved organs include the pancreas, bile duct, retroperitoneum, lung interstitium, breast, kidney, salivary gland, liver, lymph node, and other tissues and organs, and the affected organs are different at different ages[5]. The typical histological features of IgG4-RD include dense lymphoplasmacytic infiltrates, storiform-type fibrosis, and obliterative phlebitis[6]. The diagnosis of at least two of the above three criteria is needed, usually diffuse lymphoplasmic cell infiltration and storiform-type fibrosis[7,8]. However, lymph nodes, the lung, and other organs and tissues often do not have the characteristic manifestations of storiform-type fibrosis and phlebitis obliterans. IgG4-correlated disease in the lymph nodes is easily misdiagnosed due to the lack of specificity of its clinical pathological features, which have been identified in a variety of pathological conditions, such as Castleman disease, inflammatory pseudotumor, and lymphoid follicle hyperplasia of reactivity (such as lymphoma), and final diagnosis should combine medical history, physical examination, serological examination, imaging, pathology, and immunohistochemistry[9-11].
Studies have shown that FDG positron emission tomography/CT has a sensitivity of 85.7% and a specificity of 66.1% in the diagnosis of IgG4-RD and may have a potential differential ability for patients with clinically suspected IgG4-RD[12]. We presented a case of IgG4-RD with Castleman disease-like alterations that included positron emission tomography/CT. IgG4 immunostaining was necessary for the diagnosis of IgG4-RD, and a proportion of IgG4+/IgG+ plasma cells greater than 40% and the IgG4+ cell count are important parameters[13,14]. This case met the criteria. Effective initial treatment with glucocorticoids is one of the characteristics of IgG4-RD, and rituximab therapy should be considered for patients for whom glucocorticoids are ineffective or who are dependent on glucocorticoids[15].
Castleman disease, first reported in 1956, is a clinically rare lymphoproliferative disorder. According to the different scope of involvement, it was divided into unicentric Castleman disease and multicentric Castleman disease (MCD). Histologically, the disease was divided into a clear vascular type, plasma cell type, and mixed type. MCD usually manifests as multiple lymph node enlargement, hepatosplenomegaly, kidney injury, pulmonary symptoms and signs, and ascites and can be accompanied by high fever, night sweats, and other systemic symptoms[16]. Because the disease is relatively rare in clinical practice, there is no unified first-line treatment plan at present, but simple glucocorticoids have poor efficacy. Combined chemotherapy, rituximab, or anti-IL-6 treatment are often necessary, and the prognosis is poor[17-20].
It has been reported that IgG4-associated lymphadenopathy may have Castleman disease-like characteristics, and some scholars believe that a subset of plasma cell Castleman disease is actually IgG4-associated lymphadenopathy[21,22]. IgG4-RD share similarities with Castleman disease, but there are also differences. MCD and IgG4-RD can be distinguished based on the following aspects: (1) Clinical manifestations: lymph node enlargement in MCD is more prominent and is often accompanied by fever, anemia, severe hypoproteinemia, and other systemic symptoms, while lymph node lesions of IgG4-RD are usually less than 2 cm in diameter and often involve the lacrimal glands, salivary glands, pancreas, and retroperitoneum; (2) Inflammation indicators: CRP, IL-6, and vascular endothelial growth factor are usually significantly increased in MCD patients; (3) In terms of immunoglobulin and complement, increased IgG in MCD patients may be accompanied by increased IgA and IgM, with normal complement levels, while the course of IgG4-RD may involve the activation process of complement, leading to decreased complement levels; (4) Pathological features: IgG4+ plasma cells may appear in MCD patients, but IgG4+/IgG+ plasma cells usually account for less than 40%; and (5) Therapeutic response of glucocorticoids: IgG4-RD patients respond well to initial treatment with glucocorticoids, while MCD patients generally respond poorly[4,23-25].
The patient in this case had lacrimal gland, lymph node, retroperitoneal, and other lesions, decreased complement C3, normal IL-6 levels, IgG4+/IgG+ plasma cells greater than 40%, and a good response to glucocorticoid treatment. All of these features were in line with IgG4-associated lymphadenopathy. However, the pathological morphology of this patient was very similar to that of Castleman disease, which may lead to misdiagnosis. Therefore, it is necessary to proceed cautiously in clinical practice with such patients, and Ig, complement, IL-6, CRP and other examinations should be performed to confirm the diagnosis.
There is no unified standard for the differentiation of IgG4-RD from plasma cell Castleman disease, and these diseases are sometimes difficult to distinguish from one another. It is necessary to proceed cautiously in clinical practice with such patients Histopathological characteristics, laboratory testing, and clinical treatment should be considered comprehensively to provide a basis for clinical treatment and prognosis evaluation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nepal SP S-Editor: Chang KL L-Editor: Filipodia P-Editor: Li JH
| 1. | Lanzillotta M, Mancuso G, Della-Torre E. Advances in the diagnosis and management of IgG4 related disease. BMJ. 2020;369:m1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 2. | Maritati F, Peyronel F, Vaglio A. IgG4-related disease: a clinical perspective. Rheumatology (Oxford). 2020;59:iii123-iii131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Satou A, Notohara K, Zen Y, Nakamura S, Yoshino T, Okazaki K, Sato Y. Clinicopathological differential diagnosis of IgG4-related disease: A historical overview and a proposal of the criteria for excluding mimickers of IgG4-related disease. Pathol Int. 2020;70:391-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Zhang P, Peng L, Fei Y, Zhang W, Feng R. Clinical characteristics of a concurrent condition of IgG4-RD and Castleman's disease. Clin Rheumatol. 2018;37:3387-3395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Lu H, Teng F, Zhang P, Fei Y, Peng L, Zhou J, Wang M, Liu X, Zhu L, Wang L, Luo X, Liu Z, Li J, Zhao Y, Zhang W, Zeng X. Differences in clinical characteristics of IgG4-related disease across age groups: a prospective study of 737 patients. Rheumatology (Oxford). 2021;60:2635-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Deshpande V. The pathology of IgG4-related disease: critical issues and challenges. Semin Diagn Pathol. 2012;29:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Martínez-Valle F, Orozco-Gálvez O, Fernández-Codina A. Update in ethiopathogeny, diagnosis and treatment of the IgG4 related disease. Med Clin (Barc). 2018;151:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Maehara T, Moriyama M, Nakamura S. Pathogenesis of IgG4-related disease: a critical review. Odontology. 2019;107:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Takeuchi M, Sato Y, Takata K, Kobayashi K, Ohno K, Iwaki N, Orita Y, Yoshino T. Cutaneous multicentric Castleman's disease mimicking IgG4-related disease. Pathol Res Pract. 2012;208:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Izumi Y, Takeshita H, Moriwaki Y, Hisatomi K, Matsuda M, Yamashita N, Kawahara C, Shigemitsu Y, Iwanaga N, Kawakami A, Kurohama H, Niino D, Ito M, Migita K. Multicentric Castleman disease mimicking IgG4-related disease: A case report. Mod Rheumatol. 2017;27:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Nowak V, Agaimy A, Kristiansen G, Gütgemann I. Increased IgG4-positive plasma cells in nodular-sclerosing Hodgkin lymphoma: a diagnostic pitfall. Histopathology. 2020;76:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Tang CYL, Chua WM, Cheng LTJ, Fong W, Zaheer S, Lam WW. 18F-FDG PET/CT Manifestations of IgG4-related Disease. Br J Radiol. 2021;94:20210105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Bledsoe JR, Della-Torre E, Rovati L, Deshpande V. IgG4-related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018;126:459-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Chen LYC, Mattman A, Seidman MA, Carruthers MN. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104:444-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | Lanzillotta M, Fernàndez-Codina A, Culver E, Ebbo M, Martinez-Valle F, Schleinitz N, Della-Torre E. Emerging therapy options for IgG4-related disease. Expert Rev Clin Immunol. 2021;17:471-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Said J. Multicentric Castleman disease: consensus at last? Blood. 2017;129:1569-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Liu AY, Nabel CS, Finkelman BS, Ruth JR, Kurzrock R, van Rhee F, Krymskaya VP, Kelleher D, Rubenstein AH, Fajgenbaum DC. Idiopathic multicentric Castleman's disease: a systematic literature review. Lancet Haematol. 2016;3:e163-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | van Rhee F, Voorhees P, Dispenzieri A, Fosså A, Srkalovic G, Ide M, Munshi N, Schey S, Streetly M, Pierson SK, Partridge HL, Mukherjee S, Shilling D, Stone K, Greenway A, Ruth J, Lechowicz MJ, Chandrakasan S, Jayanthan R, Jaffe ES, Leitch H, Pemmaraju N, Chadburn A, Lim MS, Elenitoba-Johnson KS, Krymskaya V, Goodman A, Hoffmann C, Zinzani PL, Ferrero S, Terriou L, Sato Y, Simpson D, Wong R, Rossi JF, Nasta S, Yoshizaki K, Kurzrock R, Uldrick TS, Casper C, Oksenhendler E, Fajgenbaum DC. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132:2115-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (1)] |
| 19. | Abramson JS. Diagnosis and Management of Castleman Disease. J Natl Compr Canc Netw. 2019;17:1417-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135:1353-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 21. | Mochizuki H, Kato M, Higuchi T, Koyamada R, Arai S, Okada S, Eto H. Overlap of IgG4-related Disease and Multicentric Castleman's Disease in a Patient with Skin Lesions. Intern Med. 2017;56:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Otani K, Inoue D, Fujikura K, Komori T, Abe-Suzuki S, Tajiri T, Itoh T, Zen Y. Idiopathic multicentric Castleman's disease: a clinicopathologic study in comparison with IgG4-related disease. Oncotarget. 2018;9:6691-6706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Sato Y, Kojima M, Takata K, Morito T, Asaoku H, Takeuchi T, Mizobuchi K, Fujihara M, Kuraoka K, Nakai T, Ichimura K, Tanaka T, Tamura M, Nishikawa Y, Yoshino T. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Mod Pathol. 2009;22:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Sasaki T, Akiyama M, Kaneko Y, Mori T, Yasuoka H, Suzuki K, Yamaoka K, Okamoto S, Takeuchi T. Distinct features distinguishing IgG4-related disease from multicentric Castleman's disease. RMD Open. 2017;3:e000432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Nishimura MF, Igawa T, Gion Y, Tomita S, Inoue D, Izumozaki A, Ubara Y, Nishimura Y, Yoshino T, Sato Y. Pulmonary Manifestations of Plasma Cell Type Idiopathic Multicentric Castleman Disease: A Clinicopathological Study in Comparison with IgG4-Related Disease. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |