Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10871
Peer-review started: May 30, 2021
First decision: July 14, 2021
Revised: July 14, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 16, 2021
Processing time: 193 Days and 24 Hours
Patients with end-stage liver disease usually have varying degrees of malnutri
The study aim was to investigate the value of the controlling nutritional status (CONUT) score and psoas muscle thickness per height (PMTH) in predicting prognosis in LT.
We retrospectively analyzed the clinical data of 313 patients who underwent classic orthotopic LT from January 2016 to December 2018 in Tianjin First Central Hospital affiliated with Tianjin Medical University. The CONUT score is derived from the preoperative serum albumin and total cholesterol levels, and total lymphocyte count. Patients were divided into low (≤ 4), medium (5–8), and high (9–12) CONUT score groups perioperative characteristics, Clavien-Dindo grade III/IV/V postoperative complications, graft loss and infection, and cumulative postoperative survival in the three groups were compared 3 mo after LT. PMTH was calculated as the ratio of the transverse thickness of the psoas muscle in the umbilical plane to the height of the patient. The cutoff values of receiver operating characteristic curves were determined separately for men and women. The values were 14.1 cm/m2 for women and 17.9 cm/m2 for men. The patients were then divided into low and high PMTH groups by the cutoff values. The comparison of data between the two groups was the same as above.
Patients with medium and high CONUT scores had lower preoperative serum hemoglobin, more intraoperative red blood cell (RBC) transfusions, longer postoperative intensive care unit stay and hospital stays, higher 7 and 14 preoperative-day serum bilirubin levels, and a higher incidence of postoperative grade III/IV complications and infections than patients with low CONUT scores. Differences in the 3-mo cumulative survival among the three groups were not significant. Patients with a low PMTH had higher preoperative serum urea nitrogen, more intraoperative packed RBC and frozen plasma transfusions, longer times to postoperative ventilator extubation, higher incidence of total postoperative complications, and a lower 3-mo cumulative survival than those with a high PMTH.
A CONUT score ≥ 5 and a low PMTH were both associated with poor prognosis in LT. The CONUT score had no predictive value for short-term patient survival after LT, but the PMTH was predictive of short-term patient survival after LT.
Core Tip: This retrospective study analyzed the short-term prognosis after liver transplantation (LT) using two nutrition assessment tools, the controlling nutritional status score and psoas muscle thickness per height. Both had predictive value for prognosis in LT.
- Citation: Dai X, Gao B, Zhang XX, Li J, Jiang WT. Value of the controlling nutritional status score and psoas muscle thickness per height in predicting prognosis in liver transplantation. World J Clin Cases 2021; 9(35): 10871-10883
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10871.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10871
Previous studies have demonstrated that malnutrition is an independent predictor of death in patients with end-stage liver disease[1], indicating that nutrition levels are important for the progression of disease. The incidence of malnutrition in patients undergoing liver transplantation (LT) for end-stage liver disease can be as high as 50%-90%, and malnutrition can lead to a poor prognosis[2]. At present, there are no uniform nutritional assessments for patients awaiting LT. To better evaluate the nutritional status of patients with liver diseases and explore the relationship between malnutrition and complications after LT, we analyzed the controlling nutritional status (CONUT) score and psoas muscle thickness per height (PMTH) in LT patients.
The CONUT score has been used to assess patient nutritional status by considering nutritional-related indicators, including serum albumin, serum total cholesterol, and total lymphocyte count[3]. The CONUT score has been verified and used as an independent risk factor for death to predict the prognosis of patients with rectal and stomach cancer[4,5]. The PMTH is defined as the ratio of the transverse thickness of the psoas muscle to the height of the patient at the umbilical level. It requires no additional tools and is relatively easy to calculate[6]. The PMTH has been used to diagnose sarcopenia, which is an important manifestation of malnutrition[7], and to predict mortality in patients with cirrhosis on waiting lists[8]. In this study, we explored the role of the CONUT score and PMTH in predicting prognosis in LT patients.
A total of 313 patients seen from January 2016 to December 2018 at the Tianjin First Central Hospital Affiliated with Tianjin Medical University were included. Patients eligible for inclusion were: (1) 18-65 years of age with orthotopic LT for the first time; (2) With graft livers obtained from a postmortem donation; (3) With hepatocellular carcinoma meeting the UCSF criteria[9] (i.e. solitary tumors ≤ 6.5 cm or ≤ 3 nodules with the largest lesion ≤ 4.5 cm and total tumor diameter ≤ 8 cm); (4) With cirrhosis diagnosed by liver biopsy or imaging including B-ultrasonography, liver stiffness measurement, or abdominal computed tomography (CT); and (5) With acute liver failure diagnosed by evidence of coagulation abnormality [international normalized ratio (INR) > 1.5] and any degree of mental alteration (encephalopathy) in a patient without preexisting cirrhosis and with an illness of < 26 wk duration[10].
Patients (1) undergoing retransplantation, salvage LT or multiple organ tran
Grouping was based on the CONUT score, which was calculated from the last postoperative tests, obtained within 3 d before LT, and including albumin ≥ 35.0 g/L, score 0; 30.0-34.9 g/L, score 2; 25.0-29.9 g/L, score 4, < 25.0 g/L, score 6; total cholesterol ≥ 180 mg/dL, score 0; 140-179 mg/dL, score 1; 100-139 mg/dL, score 2; < 100 mg/dL, score 3; total lymphocytes ≥ 1600/mm3, score 0; 1200-1599/mm3, score 1; 800-1199/mm3, score 2; < 800/mm3, score 3[11]. Patients were divided into low, medium, and high CONUT score groups by low scores of ≤ 4, medium scores of 5–8, and high scores of 9–12). A total of 281 patients were eligible, but 32 were excluded because of a lack of serum total cholesterol.
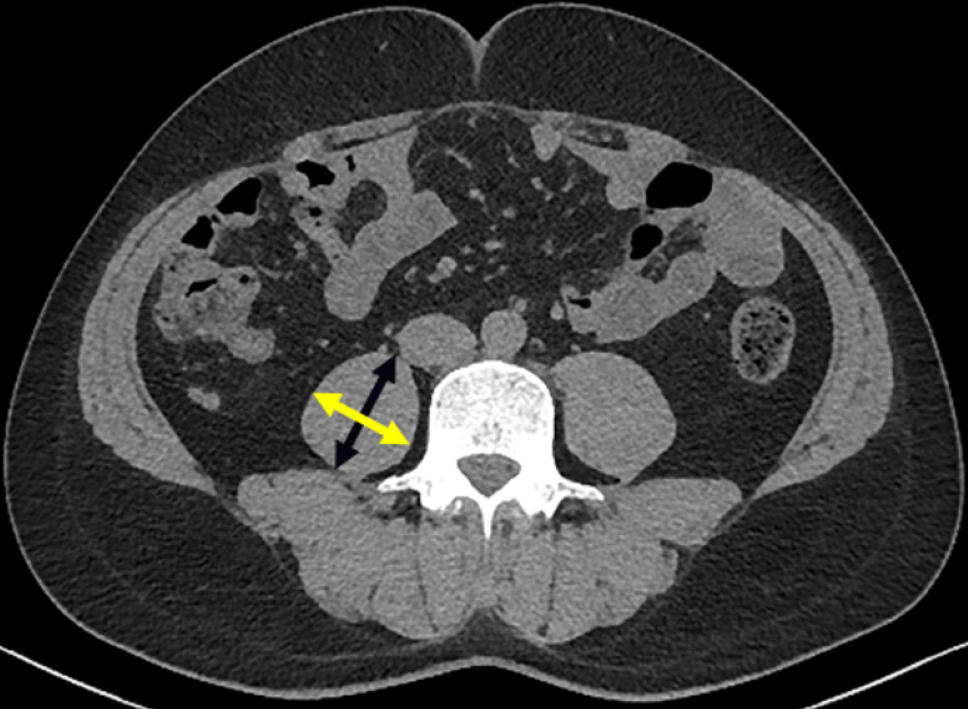
For measurement, calculation, and grouping by PMTH, patients were routinely examined by abdominal CT within 2 mo of LT with a GE revolution CT 64-slice scanner. PMTH was defined as the ratio of the transverse thickness of the psoas muscle in the umbilical plane to the height of the patient. The transverse thickness of the right psoas muscle at the umbilical level was measured on CT images perpendicular to the axial diameter, and the axial psoas thickness was the maximum diameter of the psoas in the axial view (Figure 1). Because it has been reported that the PMTH is correlated with the sex and mortality of cirrhosis patients[6], receiver operating characteristic (ROC) curves were generated for men and women according to postoperative patient mortality. PMTH cutoff values were determined by the optimal Youden index. Patients were divided into high PMTH and a low PMTH groups. A total of 254 patients were eligible, but 59 who lacked CT scans obtained within 2 mo of LT were excluded from the analysis.
Perioperative indicators included patient age, sex, body mass index (BMI), preoperative model for end-stage liver disease (MELD) score, urea nitrogen (BUN), hemoglobin, albumin, white blood cell (WBC) count, platelet (PLT) count, total cholesterol, total lymphocyte count, receipt of intraoperative packed red blood cell (RBC) or frozen plasma transfusion, intraoperative blood loss, anhepatic phase and operation time, postoperative ventilator extubation time, length of intensive care unit (ICU) and hospital stays, postoperative serum total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase, creatinine, and BUN on postoperative days 7 and 14.
Postoperative Clavien-Dindo complications of grade III and greater severity were collected[12]: Grade III complications require surgical, endoscopic, or radiological intervention, grade IV complications are life-threatening and require ICU management, including single and multiorgan dysfunction, and grade V complications are fatal. Grade III included vascular complications such as portal vein stenosis, thrombosis, hepatic artery thrombosis, splenorenal shunt, and splenic arterial steal syndrome; biliary complications such as bile leakage, bile drainage obstruction, and bile duct stenosis. Other complications include retransplantation, pleural effusion requiring thoracentesis, peritoneal effusion requiring abdominocentesis, abdominal bleeding, placement of a drainage tube at the site of T-tube outlet leakage, and nasointestinal tube insertion, etc. Grade IV complications included respiratory, heart, liver, and renal failure, requiring treatment tracheotomy, extracorporeal membrane oxygenation, artificial extracorporeal liver support and hemodialysis. Grade V complications were those causing death, including septic shock, hemorrhagic shock, heart failure, liver failure, renal failure, and intracranial hemorrhage. Graft loss and infection were observed; the diagnosis of infection was made following infection diagnosis guidelines[13], and the patients were followed up for 3 mo.
The statistical analysis were performed with SPSS 20.0. Normally distributed data were reported as means ± SD, and the independent sample t-test was used for intergroup comparisons. Data that did not have a normal distribution were reported as medians and quartiles, and the Mann-Whitney U or Kruskal-Wallis tests were used for intergroup comparisons. Classification data were reported as numbers and percentages (%), and χ2 or Fisher’s exact tests were used for intergroup comparisons. The area under the ROC curve (AUC) and 95% confidence intervals (CIs) were used to find the optimal values of the PMTH. Survival rates were estimated by the Kaplan-Meier method. P < 0.05 indicated statistical significance for all tests.
Grouping by the CONUT score resulted in 65 low-score, 173 medium-score, and 43 high-score patients (Tables 1 and 2). Comparison of the perioperative data found that patients in medium and high CONUT score groups had lower serum hemoglobin levels, more intraoperative RBC transfusions, longer ICU and hospital times and higher serum bilirubin levels on postoperative days 7 and 14 than patients in the low-score group. No differences were observed between the medium- and high-score groups (P < 0.05). There were no significant differences in sex, age, BMI, MELD score, etiology, serum BUN level, WBC and PLT counts, anhepatic phase, operation time, postoperative ventilator extubation time, or postoperative serum AST or Cr levels on the postoperative days 7 and 14 among the three groups (P > 0.05).
| Group | Low CONUT | Medium CONUT | High CONUT | P value |
| Age | 49.7 ± 9.9 | 50.2 ± 9.8 | 46.6 ± 10.1 | 0.102 |
| Sex (M:F) | 48:17 | 128:45 | 34:9 | 0.801 |
| BMI (kg/m2) | 23.5 (21.2-25.4) | 23.7 (21.6-27.0) | 24.3 (21.8-28.0) | 0.515 |
| MELD score | 14 (10-20) | 15 (10-21) | 18 (13-21) | 0.155 |
| Etiology | 0.386 | |||
| HBV (n) | 42 | 90 | 22 | |
| HCV (n) | 1 | 2 | 0 | |
| Alcohol (n) | 4 | 20 | 5 | |
| NASHE(n) | 4 | 10 | 2 | |
| Autoimmune diseases (n) | 8 | 23 | 11 | |
| Cryptogenic (n) | 1 | 2 | 1 | |
| Other (n) | 4 | 26 | 3 |
| Group | Low CONUT | Medium CONUT | High CONUT | P value |
| HB (g/L) | 122.6 ± 25.5 | 96.8 ± 26.52 | 89.2 ± 17.9 | < 0.001a; < 0.001b; 0.074c |
| WBC (cells/mm3) | 4.95 (3.24-6.54) | 4.63 (3.43-6.94) | 4.8 ± 3.3 | 0.101 |
| PLT (cells/mm3) | 113.0 (58.0-116.5) | 105.0 (46.5-166.0) | 111.1 ± 79.1 | 0.493 |
| Albumen (g/L) | 38.8 ± 4.53 | 31.8 (29.0-34.4) | 26.34 ± 2.65 | < 0.001a; < 0.001b; < 0.001c |
| Total cholesterol (mg/dL) | 148.08 (117.75-178.81) | 102.67 (81.39-113.68) | 80.7 ± 29.81 | < 0.001a; < 0.001b; < 0.001c |
| Total lymphocytes (cells/mm3) | 1190 (860-1730) | 700 (440-1170) | 530(380-770) | < 0.001a; < 0.001b; < 0.001c |
| Intraoperative data | ||||
| Packed red cell transfusion (units) | 6.0 (4.0-10.0) | 10.0 (8.0-12.0) | 10.0 (8.0-16.0) | < 0.001a; < 0.001b; 0.158c |
| Frozen plasma transfusion (mL) | 1800.0 (1000.0-2100.0) | 2000.0 (1600.0-2000.0) | 2078.6 ± 638.4 | 0.180a; 0.018b; 0.319c |
| Blood loss (mL) | 1600.0 (800.0-2000.0) | 2000.0 (1500.0-2400.0) | 2000.0 (1500.0-2400.0) | 0.069a; 0.004b; 1.00c |
| Anhepatic phase (min) | 42.0 (40.0-50.0) | 45.0 (37.0-50.0) | 45.0 ± 11.4 | 0.849 |
| Operation time (h) | 7.5 (6.7-8.3) | 7.5 (7.0-8.4) | 7.5 (6.6-9.0) | 0.542 |
| Postoperative data | ||||
| Time of ventilator extubation (h) | 6.0 (4.0-9.0) | 10.5 (5.0-31.4) | 6.5 (3.5-24.0) | 0.237 |
| ICU stay (d) | 3.0 (2.0-4.0) | 4.0 (3.0-4.9) | 3.0 (3.0-4.96) | < 0.001a; 0.049b; 1.00c |
| hospital stay (d) | 25.0 (21.0-30.0) | 35.0 (26.0-37.3) | 33.0 (27.0-38.0) | < 0.001a; < 0.001b; 0.94c |
| Day 7 TBil (μmol/L) | 30.0 (18.09-43.5) | 48.0 (27.5-75.5) | 55.6± 37.9 | 0.21a; 0.01b; 1.00c |
| Day 14 TBil (µmol/L) | 19.0 (13.25-32.0) | 33.0 (19.0-51.9) | 42.0 (22.0-72.0) | 0.01a; < 0.001b; 1.00c |
| Day 7 AST (U/L) | 27.0 (19.5-35.8) | 24.0 (15.5-39.1) | 26.3 ± 16.5 | 0.180 |
| Day 14 AST (U/L) | 25.0 (20.1-38.0) | 26.0 (18-0-39.5) | 26.0 (17.0-40.0) | 0.809 |
| Day 7 ALT (U/L) | 79.0 (56.5-104.0) | 65.0 (39.0-97.5) | 57.0 (34.0-93.0) | 0.685a; 0.019b; 0.069c |
| Day 14 ALT (U/L) | 61.0 (35.0-81.5) | 47.0 (32.5-72.3) | 43.0 (21.0-70.0) | 0.153 |
| Day 7 INR | 1.31 (1.14-1.47) | 1.30 (1.15-1.51) | 1.27 (1.15-1.34) | 0.719 |
| Day 14 INR | 1.26 (1.08-1.27) | 1.34 (1.15-1.51) | 1.34 (1.13-1.71) | 0.499 |
| Day 7 Cr (μmol/L) | 55.0 (46.2-63.0) | 62.0 (49.0-80.5) | 57.0 (40.0-71.0) | 0.097 |
| Day 14 Cr (μmol/L) | 58.0 (45.5-68.0) | 62.0 (49.0-73.5) | 59.1 ± 23.4 | 0.161 |
| Day 7 BUN (mmol/L) | 7.71 (5.04-9.45) | 8.2 (6.1-11.5) | 7.5 (5.8-10.7) | 0.71 |
| Day 14 BUN (mmol/L) | 5.28(4.30-6.75) | 7.13 (5.20-9.52) | 6.0 (4.6-8.9) | 0.13a; 0.01b; 0.796c |
Comparison of the incidence of complications in the three groups (Table 3) found that patients in the low-score group had a lower incidence of grade III/IV/V complications and infections than patients in the medium- and high-score groups (grade III, 12.3% vs 35.3% vs 44.2%; grade IV, 1.5% vs 9.2% vs 11.6%; grade V, 1.5% vs 9.8% vs 7.0%; and infections, 26.1% vs 47.4% vs 58.1%, all P < 0.05). No significant differences were observed between the medium- and high-score groups (P > 0.05). There were no significant differences in graft loss among the three groups (P = 1.000).
| Group | Low CONUT | Medium CONUT | High CONUT | P value |
| Cases | 65 | 173 | 43 | |
| Grade III complications | 8 (12.3) | 61 (35.3) | 19 (44.2) | < 0.01a; 0.01b; 0.278c |
| Grade IV complications | 1 (1.5) | 16 (9.2) | 5 (11.6) | 0.04a; 0.025b; 0.054c |
| Grade V complications | 1 (1.5) | 17 (9.8) | 3 (7.0) | 0.031a; 0.143b; 0.564c |
| Graft loss | 1 (1.5) | 4(2.3) | 1 (2.3) | 1.000 |
| Infections | 17 (26.1) | 82 (47.4) | 25 (58.1) | 0.03a; 0.01b; 0.207c |
Cumulative 3-mo survival rates at postoperative month 3 were 98.5%, 90.2%, and 93% in the low-, medium-, and high-score groups, respectively. No significant differences were observed among the three groups (P > 0.05, 95%CI: 0.831-0.873).
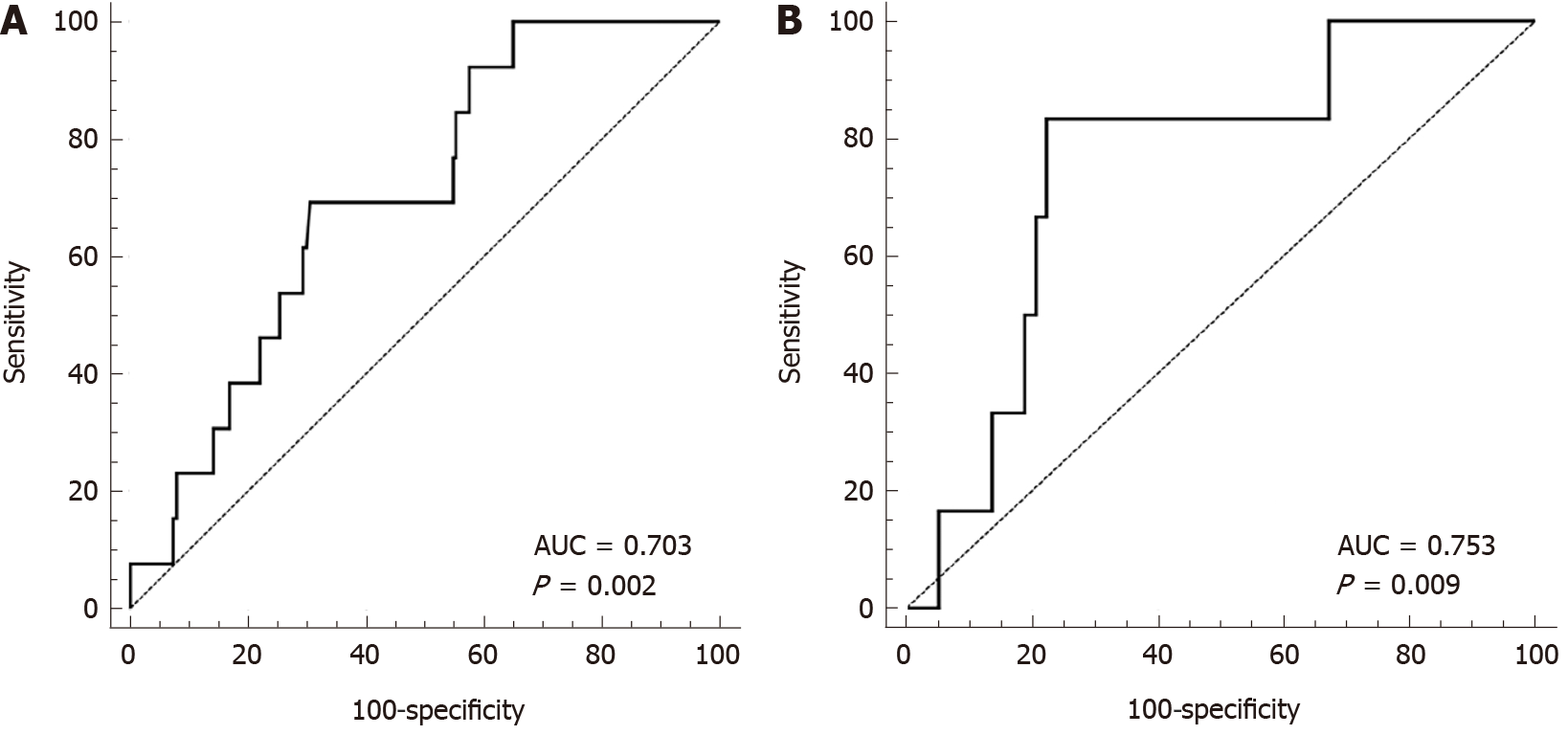
The PMTH of male patients was 20.11 ± 3.68 cm/m2, and the AUC of the ROC curve for male patients (Figure 2A) was 0.703 (P = 0.02, 95%CI: 0.633-0.767). The optimal Youden index was 0.387, and the cutoff point was 17.9 cm/m2. The male high PMTH group consisted of 127 male patients with scores higher than 17.9 cm/m2 and the male low PMTH group consisted of 63 patients with scores lower than 17.9 cm/m2. The AUC of the ROC curve for female patients was 0.753 (P = 0.009, 95%CI: 0.629-0.852), the optimal Youden index was 0.609, and the cutoff point was 14.1 cm/m2. The female high PMTH group consisted of 46 patients with scores higher than 14.1 cm/m2, and the low PMTH group consisted of 18 female patients with scores lower than 14.1 cm/m2 (n = 18). Overall, there were 173 patients in the high PMTH group and 81 patients in the low PMTH group. Comparison of the perioperative data of the two PMTH groups (Tables 4 and 5) found that the patients in the low PMTH group had lower serum BUN levels, more intraoperative RBC and frozen fresh plasma transfusions, and longer ventilator extubation times than those in the high PMTH group (P < 0.05). There were no significant differences in sex, age, preoperative BMI, MELD score, serum hemoglobin, total cholesterol, total lymphocyte count, WBC or PLT count, intraoperative blood loss, anhepatic phase, operation time, length of postoperative ICU stay or hospital stay, postoperative serum bilirubin, Cr, BUN, or INR on days 7 and 14 between the two groups (P > 0.05).
| Group | Low PMTH | High PMTH | P value |
| Cases | 81 | 173 | |
| Age (yr) | 50.6 ± 9.8 | 50.7 ± 8.8 | 0.765 |
| Sex (M:F) | 63:18 | 127:46 | 0.455 |
| BMI (kg/m2) | 23.0 (20.9-26.0) | 24.1 (21.8-26.4) | 0.069 |
| MELD score | 16 (11-21.5) | 14 (10-20) | 0.237 |
| Etiology | 0.712 | ||
| HBV (n) | 41 | 99 | |
| HCV (n) | 2 | 3 | |
| Alcohol (n) | 11 | 14 | |
| NASH (n) | 5 | 10 | |
| Autoimmune disease (n) | 10 | 18 | |
| Cryptogenic (n) | 0 | 3 | |
| Other (n) | 12 | 26 |
| Group | Low PMTH | High PMTH | P value |
| BUN (mmol/L) | 5.98 (4.06-10.22) | 4.64 (3.52-6.03) | 0.001 |
| HB (g/L) | 97.22 ± 21.84 | 102.77 ± 23.35 | 0.085 |
| WBC (cells/mm3) | 5.14 (3.71-8.26) | 4.73 (3.14-6.22) | 0.066 |
| PLT (cells/mm3) | 120.0 (55.5-171.5) | 112.0 (51.0-161.0) | 0.505 |
| Album (g/L) | 33.6 ± 6.1 | 37.1 ± 5.6 | 0.067 |
| Total cholesterol (mg/dL) | 1050.2 ± 520.4 | 1010.1 ± 550.9 | 0.624 |
| Total lymphocytes (cells/mm3) | 820 (510-1320) | 720 (490-1000) | 0.124 |
| Intraoperativedata | |||
| Packed red cell transfusion (units) | 10.0 (8.0-12.0) | 8.0 (6.0-10.0) | 0.003 |
| Frozen fresh;plasm transfusion (mL) | 2000.0 (1800.0-2350.0) | 1800.0 (1400.0-2000.0) | 0.001 |
| Blood loss (mL) | 2000 (1500-2350.0) | 1900.0 (1500.0-2000.0) | 0.180 |
| Anhepatic phase (min) | 45 (35.5-50) | 45.0 (40.0-50.0) | 0.932 |
| Operation time (h) | 7.75 (7.0-8.6) | 7.5 (7.0-8.5) | 0.649 |
| Postoperative data | |||
| Time of ventilatorextubation (h) | 7.0 (4.0-32.5) | 6.0 (4.0-12.5) | 0.043 |
| ICU stay (d) | 4.0 (2.5-4.7) | 3.0 (2.0-4.0) | 0.113 |
| Hospital stay (d) | 29.0 (23.0-31.5) | 28.0 (23.0-35.7) | 0.968 |
| Day 7 TBil (μmol/L) | 41.0 (20.0-63.7) | 37.0 (24.3-62.0) | 0.774 |
| Day 14 TBil (μmol/L) | 29.0 (16.0-43.9) | 27.1 (16.9-44.5) | 0.998 |
| Day 7 AST (U/L) | 18.0 (13.5-32.5) | 25.0 (17.1-38.0) | 0.005 |
| Day 14 AST(U/L) | 24.0 (16.0-27.0) | 28.0 (19.0-42.5) | 0.008 |
| Day 7 ALT (U/L) | 55.0 (32.5-83.5) | 74.0 (48.5-102.5) | 0.002 |
| Day 14 ALT (U/L) | 41.0 (26.0-48.5) | 57.0 (34.0-88.6) | 0.001 |
| Day 7 INR | 1.28 (1.15-1.49) | 1.24 (1.13-1.43) | 0.291 |
| Day 14 INR | 1.29 (1.13-1.29) | 1.31 (1.12-1.51) | 0.891 |
| Day 7 Cr (μmol/L) | 57.0 (50.3-67.8) | 60.0 (50.5-73.0) | 0.196 |
| Day 14 Cr (μmol/L) | 61.0 (48.5-69.0) | 60.0 (48.0-75.0) | 0.982 |
| Day 7 BUN (mmol/L) | 8.47 (5.95-11.4) | 7.83 (6.05-10.41) | 0.264 |
| Day 14 BUN (mmol/L) | 7.2 (4.9-9.0) | 6.04 (4.75-8.15) | 0.203 |
Comparison of the incidence of complications between the two PMTH groups (Table 6) found that the incidence of grade V and total complications in the low PMTH group was higher than that in the high PMTH group (grade V complications, 17.3% vs 2.9%, P < 0.001 and total complications, 58.0% vs 34.7%, P = 0.001). There were no significant differences between the low- and high PMTH groups in the incidence of grade III/IV complications or infection (grade III, 30.9% vs 24.9% and P = 0.313; grade IV, 9.9% vs 6.9%, P = 0.630; and infection, 44.4% vs 37.6%, P = 0.297). There were no significant differences in graft loss between the two groups (P = 1.000).
| Group | Low PMTH | High PMTH | P value |
| Cases | 81 | 173 | |
| Grade III complications | 25 (30.9) | 43 (24.9) | 0.313 |
| Grade IV complications | 8 (9.9) | 12 (6.9) | 0.630 |
| Grade V complications | 14 (17.3) | 5 (2.9) | < 0.001 |
| Total complications | 47 (58.0) | 60 (34.7) | 0.001 |
| Graft loss | 2 (2.5) | 3 (1.7) | 1.000 |
| Infection | 36 (44.4) | 65 (37.6) | 0.297 |
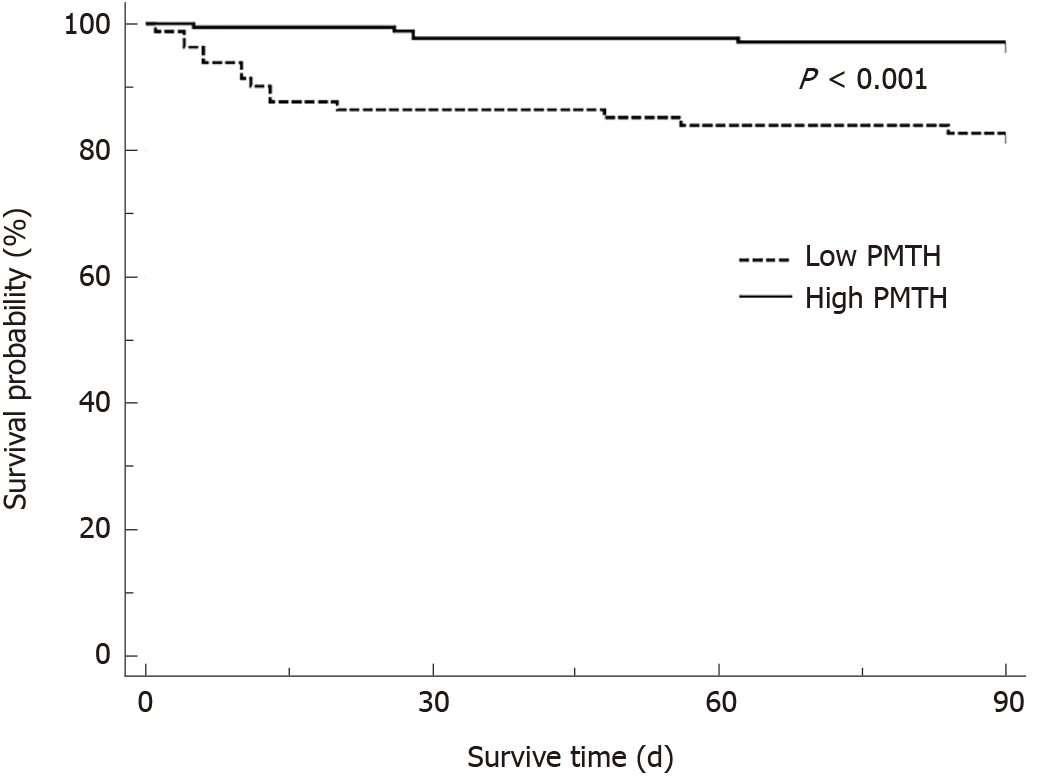
The 3-mo postoperative cumulative survival (Figure 3) was lower in the low PMTH group (82.7%) than in the high PMTH group (97.1%; P < 0.001, 95%CI: 0.830-0.875).
There are many nutritional assessments for liver cirrhosis patients, including BMI, Nutrition Risk Screening 2002 (NRS 2002), the Royal Free Hospital Nutritional Prioritizing Tool (RFH-NPT), upper arm circumference, the laboratory-related index, and muscle mass. BMI is thought to lack accuracy for LT patients because patients with end-stage liver disease usually have varying degrees of ascites that results in weight measurements that do not accurately reflect their nutritional status. In addition, some patients lack accurate weight data before surgery because of a long-term bedridden status. NRS 2002 and the RFH-NPT require assessment of body weight changes and food intake over a long period[14,15]. For patients with poor compliance, accurate data might be difficult to obtain, and some patients require emergency LT because of the severity of their condition, leading to difficulty in completing long-term monitoring. Upper arm circumference is an objective nutritional assessment index that cannot be affected by ascites and peripheral edema. It has been recommended as a screening index for chronic liver disease[16]. However, it is not included in routine preoperative examinations for LT at our center, and there may be some errors that result from variability among those who measure the arm circumference. Therefore, the CONUT score and muscle mass, as objective and convenient assessments, were used to predict the prognosis of LT patients.
The CONUT score is calculated from three indices, albumin, total cholesterol and total lymphocyte count, and represents the energy reserve and immune status of the body. The study results showed that patients with the medium and high CONUT scores had more intraoperative RBC transfusions than those with low CONUT scores, but the operation time and anhepatic period were not significantly different among the three groups, which may be related to anemia before surgery. Anemia is a surrogate marker for hypersplenism, portal hypertension, and malnutrition in cirrhosis patients because an enlarged spleen inactivates RBCs, and esophageal varicose veins caused by portal hypertension lead to upper digestive bleeding. In addition, long-term loss of appetite and protein malabsorption lead to nutritional anemia. Hypersplenism, portal hypertension and malnutrition gradually aggravate anemia as the disease develops. Patients with medium and high CONUT scores had higher postoperative day 7 and day 14 bilirubin and BUN levels. The increase in bilirubin may be highly correlated with postoperative complications, and the high BUN may have been associated with postoperative short-term fasting, which leads to hypercatabolism in patients. Low lymphocyte counts might have been associated with chronic hepatitis B and C virus infections and immune system disorders caused by autoimmune hepatitis, which lead to decreased immunity and increased susceptibility to pneumonia and infection. Enzymes for the synthesis of cholesterol are reduced because of impaired liver function in patients with end-stage liver disease, which leads to a decrease in cholesterol. Cholesterol is a major component of cell membranes and is closely related to cell surface molecules and structural functions and is also a required for the synthesis of steroid hormones[17]. Cholesterol reduction may affect the repair function of cells and the stability of the internal environment, which can lead to some complications.
The pathogenesis of sarcopenia in patients with end-stage liver disease is complex and associated with inadequate intake, changes in energy metabolism, chronic inflammation, hyperammonemia, insulin resistance, and so on. Hyperammonemia and portosystemic shunts can occur in patients with end-stage liver disease, and hyperammonemia can upregulate myostatin, which inhibits protein synthesis and muscle regeneration. Hyperammonemia also promotes oxidative stress and damages mitochondrial function, resulting in impaired protein synthesis[18]. In addition, patients with end-stage liver disease often have insulin resistance, especially in the context of metabolic-related fatty liver disease, leading to reduced muscle protein synthesis and increased catabolism[2]. In patients with end-stage liver disease, the body is in a proinflammatory state, with elevated levels of the proinflammatory cytokines interleukin 6 and tumor necrosis factor, which activate the ubiquitin-proteasome pathway, leading to increased autophagy of skeletal muscle cells and a significant reduction in muscle mass and strength[19]. Measurement of the skeletal muscle of the whole body is the gold standard for diagnosing sarcopenia, but because of the complexity of the measurement, the skeletal muscle at the level of the third lumbar spine or the skeletal muscle index (SMI) is commonly used to reflect the skeletal muscle content instead and is calculated by software[20]. Compared with measurement of the SMI, measurement of the PMTH only requires the transverse thickness of the right psoas at the umbilical plane on CT images. Some studies have shown that the PMTH is highly correlated with the SMI and is an independent risk factor for mortality in patients with cirrhosis. The transverse thickness of the psoas muscle is also correlated with sex, age, and ethnicity. Therefore, PMTH cutoff values of less than 17.9 cm/m2 for male patients and PMTH less than 14.1 cm/m2 for female patients were obtained using the study data and were for the diagnosis of sarcopenia. Patients with a low PMTH had a higher preoperative BUN than patients with a high PMTH, which may be related to skeletal muscle loss and protein metabolism imbalance. Patients with a low PMTH had an increased incidence of postoperative complications. Because patients with sarcopenia have low preoperative protein storage, it is difficult to provide sufficient amino acids for the human body, which leads to a deficiency of amino acids in the postoperative tissue recovery stage, and injuries remain unhealed[21]. Additionally, L-glutamine released by skeletal muscle can activate lymphocytes to strengthen the immune system of muscle, and its reduction leads to a weak immune system[22]. Meanwhile, the patient’s respiratory muscle volume is reduced, which may cause postoperative respiratory failure and septic shock. It was found that patients with a low PMTH need a much longer ventilator extubation time.
Many studies have shown an association between sarcopenia and recipient mortality. The CONUT score predicts mortality after hepatectomy for hepatocellular carcinoma[4]. However, our data is consistent with a previous study showing that the CONUT score did not predict mortality after LT[23]. We think the inconsistent results can be explained by a lack of improvement in some nutrition-related indices after LT, especially an elevation of albumin. The most severe complications (i.e. death) are avoided, but sarcopenia in not improved in the short term.
The two tools used in this study are convenient to use and intuitive for predicting the prognosis of LT. Both have been used in various clinical studies, and the indices considered by the two tools are routinely performed during clinical examinations and are available in clinical practice. As such, we think they have excellent clinical practicability.
There are some study limitations. There were few female patients (n = 64), which may have affected the accuracy of the PMTH cutoff values. The PMTH was measured on CT images taken within 2 mo of surgery; however, for patients with severe conditions, the PMTH may change rapidly, which could have affected the accuracy of measurement. Recent studies have shown that sarcopenia is associated not only with the amount of skeletal muscle but also with the quality of skeletal muscle[24], which can be measured by dual energy X-ray absorption (DEXA), bioelectrical impedance analysis (BIA), grip strength and so on. DEXA and BIA have been used for measurement of skeletal muscle for LT recipients in several studies[3,25-26]. Unfortunately, we do not have related data; in the early stage of primary biliary cirrhosis and primary sclerosing cholangitis, patients have dyslipidemia that leads to hypercholesterolemia, which then gradually decreases as the disease progresses[27,28]. That affects the CONUT score in some patients. In addition, studies have shown that patients with alcoholic cirrhosis have a higher incidence of malnutrition than patients with nonalcoholic cirrhosis[28], probably because ethanol and its metabolites may affect protein synthesis and skeletal muscle autophagy[29], but whether that leads to sarcopenia needs to be further validated by experience with additional cases.
A CONUT score ≥ 5 was associated with the incidence of grade III/IV/V complications and infection after LT. A low PMTH was associated with the incidence of total complications after LT. The CONUT score had no predictive value for short-term patient survival after LT, and the PMTH was predictive of short-term patient survival after LT.
Patients with end-stage liver disease usually have varying degrees of malnutrition, and severe malnutrition may affect the prognosis of patients after liver transplantation (LT). However, whether malnutrition has an impact on the occurrence of postoperative complications in not known, and there is no unified standard for the nutrition assessment of patients waiting for LT. This study included 313 patients from single center in China, and statistically analyzed the predictive value of the two nutrition assessments, the controlling nutritional status (CONUT) score and psoas muscle thickness per height (PMTH) on prognosis in LT.
The study aimed to investigate the relationship between nutrition and prognosis of LT.
This study was designed to find the right nutrition assessment tools of patients waiting for LT and investigate the predictive value of tools on prognosis in LT.
This was a retrospective study that included 313 patients from a single center undergoing orthotopic liver transplantation. Patients were divided into two or three groups, independent sample t tests, Mann-Whitney U or Kruskal-Wallis tests were used to compare intergroup perioperative data. Fisher’s exact or 2 tests were used to compare numbers and percentages of cases. Cumulative 3-mo survival rates were estimated by the Kaplan-Meier method.
Patients in the medium and high CONUT score groups had a lower preoperative serum hemoglobin levels, more intraoperative red blood cell (RBC) transfusions, longer postoperative intensive care unit and hospital stays, higher preoperative day 7 and day 14 serum bilirubin levels, and a higher incidence of postoperative grade III/IV complications and infections than patients in the low CONUT score group. There were no significant differences in the 3-mo cumulative survival rate among the three groups. Patients with a low PMTH had higher levels of preoperative serum urea nitrogen, more intraoperative packed RBC and frozen plasma transfusions, longer postoperative ventilator extubation times, an increased incidence of total postoperative complications, and a lower 3-mo cumulative survival rate than those with a high PMTH.
A CONUT score ≥ 5 was associated with the incidence of grade III/IV/V complications and infection after LT, and a low PMTH was associated with the incidence of total complications after LT. The CONUT score had no predictive value for short-term patient survival after LT, and the PMTH was predictive of short-term patient survival after LT.
We hope to develop a predictive model for poor clinical outcomes of LT that combines the CONUT score and PMTH so that the two tools can be used together to predict outcomes in a wider audience.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Bendary M, Ferrarese A S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Pereira JN, Chactoura J, Nohra F, Diogenes MEL, Bezerra FF. Free and Bioavailable Fractions of Vitamin D: Association with Maternal Characteristics in Brazilian Pregnant Women. J Nutr Metab. 2020;2020:1408659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 3. | Bohannon RW. Grip Strength: An Indispensable Biomarker For Older Adults. Clin Interv Aging. 2019;14:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 545] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 4. | Takagi K, Umeda Y, Yoshida R, Nobuoka D, Kuise T, Fushimi T, Fujiwara T, Yagi T. Preoperative Controlling Nutritional Status Score Predicts Mortality after Hepatectomy for Hepatocellular Carcinoma. Dig Surg. 2019;36:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, Baba Y, Sakamoto Y, Yamashita Y, Yoshida N, Chikamoto A, Baba H. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 6. | Gu DH, Kim MY, Seo YS, Kim SG, Lee HA, Kim TH, Jung YK, Kandemir A, Kim JH, An H, Yim HJ, Yeon JE, Byun KS, Um SH. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 8. | Huguet A, Latournerie M, Debry PH, Jezequel C, Legros L, Rayar M, Boudjema K, Guyader D, Jacquet EB, Thibault R. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: A retrospective cohort study. Nutrition. 2018;51-52:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1696] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. Clinical practice guidelines panel, Wendon, J; Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 11. | Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38-45. [PubMed] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24854] [Article Influence: 1183.5] [Reference Citation Analysis (0)] |
| 13. | Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 518] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 14. | Traub J, Bergheim I, Horvath A, Stadlbauer V. Validation of Malnutrition Screening Tools in Liver Cirrhosis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Wu Y, Zhu Y, Feng Y, Wang R, Yao N, Zhang M, Liu X, Liu H, Shi L, Zhu L, Yang N, Chen H, Liu J, Zhao Y, Yang Y. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr. 2020;124:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 675] [Article Influence: 112.5] [Reference Citation Analysis (2)] |
| 17. | Narwal V, Deswal R, Batra B, Kalra V, Hooda R, Sharma M, Rana JS. Cholesterol biosensors: A review. Steroids. 2019;143:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 19. | Khoshnood A, Nasiri Toosi M, Faravash MJ, Esteghamati A, Froutan H, Ghofrani H, Kalani M, Miroliaee A, Abdollahi A, Yasir A. A survey of correlation between insulin-like growth factor-I (igf-I) levels and severity of liver cirrhosis. Hepat Mon. 2013;13:e6181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Murray TÉ, Williams D, Lee MJ. Osteoporosis, obesity, and sarcopenia on abdominal CT: a review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom Radiol (NY). 2017;42:2376-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 670] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 22. | Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001;131:2515S-22S; discussion 2523S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Pravisani R, Mocchegiani F, Isola M, Lorenzin D, Adani GL, Cherchi V, Righi E, Terrosu G, Vivarelli M, Risaliti A, Baccarani U. Controlling Nutritional Status score does not predict patients' overall survival or hepatocellular carcinoma recurrence after deceased donor liver transplantation. Clin Transplant. 2020;34:e13786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7810] [Article Influence: 1301.7] [Reference Citation Analysis (1)] |
| 25. | Kaido T, Tamai Y, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Hammad A, Inagaki N, Uemoto S. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition. 2017;33:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, Castaing D, Antonini T, Coilly A, Samuel D, Cherqui D, Vibert E. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017;23:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | A Clinical Review of Primary Biliary Cholangitis. Gastroenterol Nurs. 2020;43:E100-E101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, Carey W, Alkhouri N. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol. 2017;112:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 29. | Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (1985). 2014;117:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |