Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10850
Peer-review started: May 4, 2021
First decision: July 15, 2021
Revised: August 4, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: December 16, 2021
Processing time: 219 Days and 18 Hours
Radiologic adjacent segment degeneration (ASDeg) can occur after spinal surgery. Adjacent segment disease (ASDis) is defined as the development of new clinical symptoms corresponding to radiographic changes adjacent to the level of previous spinal surgery. Greater pre-existing ASDeg is generally considered to result in more severe ASDis; nonetheless, whether the ASDeg status before index surgery influences the postoperative risk of revision surgery due to ASDis warrants investigation.
To identify possible risk factors for ASDis and verify the concept that greater preexisting ASDeg leads to more severe ASDis.
Data from 212 patients who underwent posterior decompression with Dynesys stabilization from January 2006 to June 2016 were retrospectively analyzed. Patients who underwent surgery for ASDis were categorized as group A (n = 13), whereas those who did not were classified as group B (n = 199). Survival analysis and Cox proportional hazards models were used to compare the modified Pfirrmann grade, University of California-Los Angeles grade, body mass index, number of Dynesys-instrumented levels, and age.
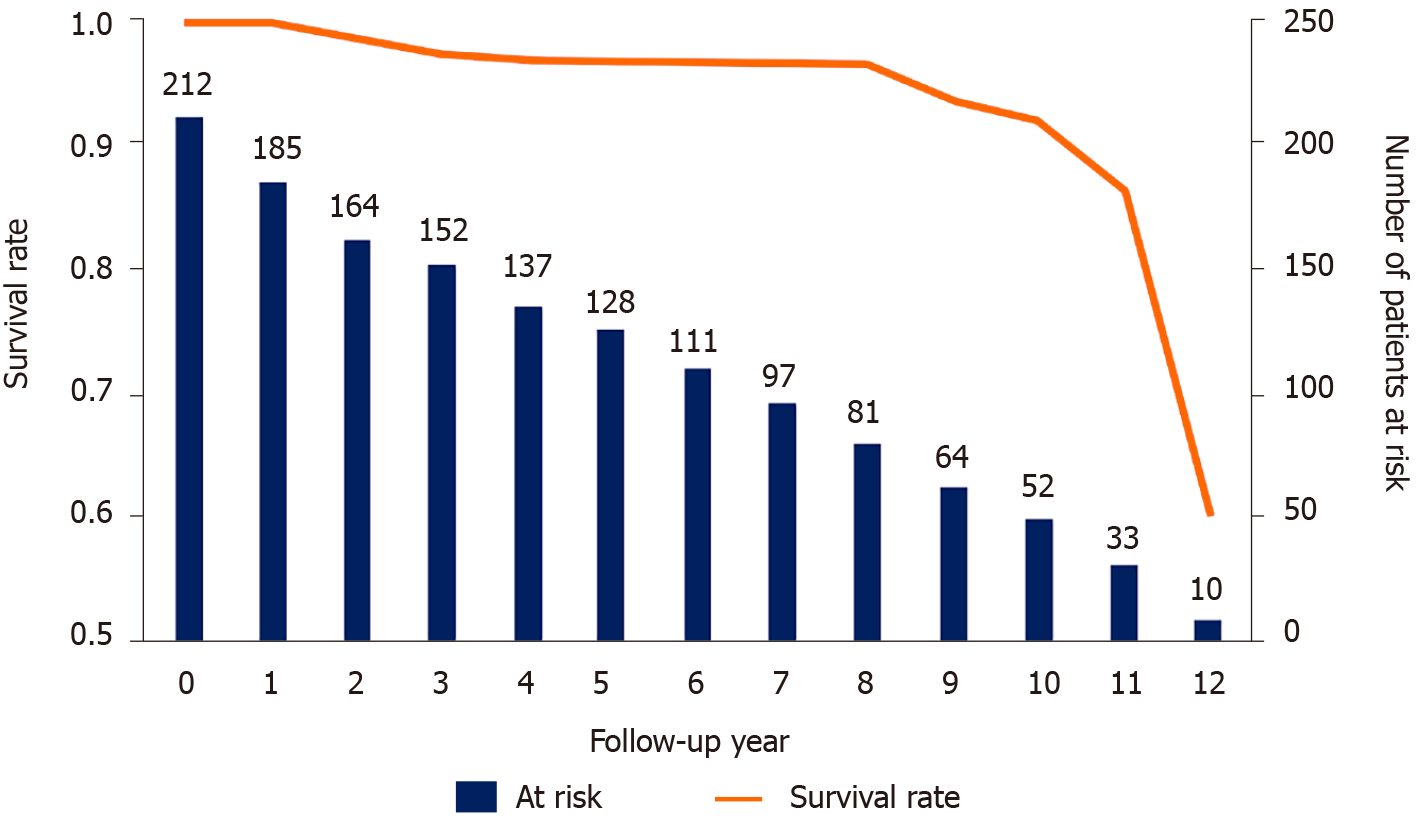
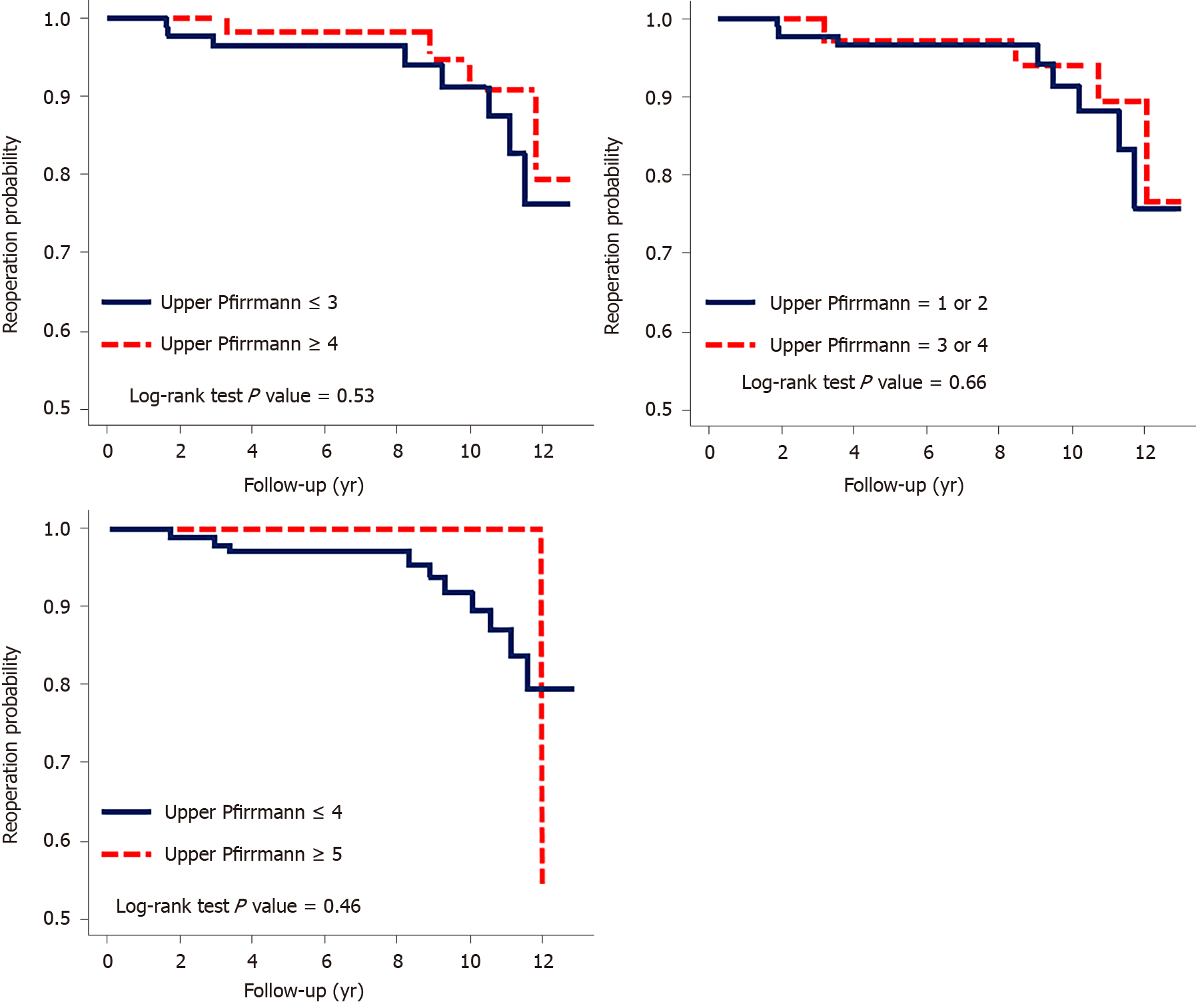
The mean time of reoperation was 7.22 (1.65–11.84) years in group A, and the mean follow-up period was 6.09 (0.10–12.76) years in group B. No significant difference in reoperation risk was observed: Modified Pfirrmann grade 3 vs 4 (P = 0.53) or 4 vs 5 (P = 0.46) for the upper adjacent disc, University of California-Los Angeles grade 2 vs 3 for the upper adjacent segment (P = 0.66), age of < 60 vs > 60 years (P = 0.9), body mass index < 25 vs > 25 kg/m2 (P = 0.3), and sex (P = 0.8).
Greater preexisting upper ASDeg was not associated with a higher rate of reoperation for ASDis after Dynesys surgery. Being overweight tended to increase reoperation risk after Dynesys surgery for ASDis.
Core Tip: Preoperative degeneration status of the adjacent segment did not affect the rate of mid- and long-term follow-up for adjacent segment disease. Dynesys is a reliable implant with respect to preserving the motion of the adjacent segment and reducing the progression of adjacent segment disease.
- Citation: Chen KJ, Lai CY, Chiu LT, Huang WS, Hsiao PH, Chang CC, Lin CJ, Lo YS, Chen YJ, Chen HT. Adjacent segment disease following Dynesys stabilization for lumbar disorders: A case series of mid- and long-term follow-ups. World J Clin Cases 2021; 9(35): 10850-10860
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10850.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10850
Fusion surgery remains the gold standard for degenerative lumbar disorders with instability. The rate of surgical interventions for adjacent segment disease (ASDis) following fusion has been reported to be 3.9% annually and 25%–35% after 10 years[1].
The Dynesys® dynamic stabilization system (Zimmer Inc., Warsaw, IN, United States) was developed to maintain partial motion of instrumented levels and reduce the occurrence of ASDis. This system, which consists of titanium alloy screws connected by an elastic synthetic compound, controls motion in any plane. Several studies have obtained good short- and long-term results after Dynesys surgery when clinical parameters such as the Oswestry Disability Index (ODI), visual analog scale (VAS) score, disc height, and even Cobb’s angle were evaluated. Nonetheless, data on long-term adjacent degeneration from large cohort studies are lacking[2-9]. While the risk of ASDis remains controversial, it most frequently affects the upper adjacent segment after fusion surgery[3].
This study aims to analyze clinical and radiologic outcomes in order to identify possible risk factors for ASDis and verify whether greater preexisting adjacent segment degeneration (ASDeg) could lead to more severe ASDis.
This retrospective study was approved by the institutional review board, the Research Ethics Committee of China Medical University and Hospital in Taichung, Taiwan (protocol no. CMUH108-REC2-133); the need for acquisition of informed consent from patients was waived owing to the retrospective nature of the study. A total of 227 patients with lumbar degenerative disorder (of at least 3 mo duration) refractory to medications or rehabilitation underwent posterior decompression and Dynesys instrumentation surgery from January 2006 to June 2016. Indications for Dynesys surgery are listed in Table 1[10].
| Indications | Patient number |
| Lumbar spondylosis with stenosis | 77 |
| Degenerative spondylolisthesis Meyerding[10] grade I | 98 |
| Degenerative disc disease | 3 |
| Recurrent disc herniation | 9 |
| Adjacent degenerative disease | 1 |
| Degenerative lumbar scoliosis | 2 |
| HIVD (large disc extrusion) | 22 |
All surgeries were performed by the same surgeon; reoperation for ASDis was conducted in the same hospital. Exclusion criteria were as follows: Previous spinal implantation, combination with other implants in the same surgery, and reoperation not performed for ASDis. Overall, 212 patients were included in this study.
The endpoints of this study were reoperation for ASDis or imaging examination at the last follow-up prior to November 2019. Patients’ upper ASDeg grade before Dynesys surgery and the rate of reoperation for ASDis were analyzed.
Age, sex, body mass index (BMI), VAS score for back and leg pain, and ODI were recorded[11].
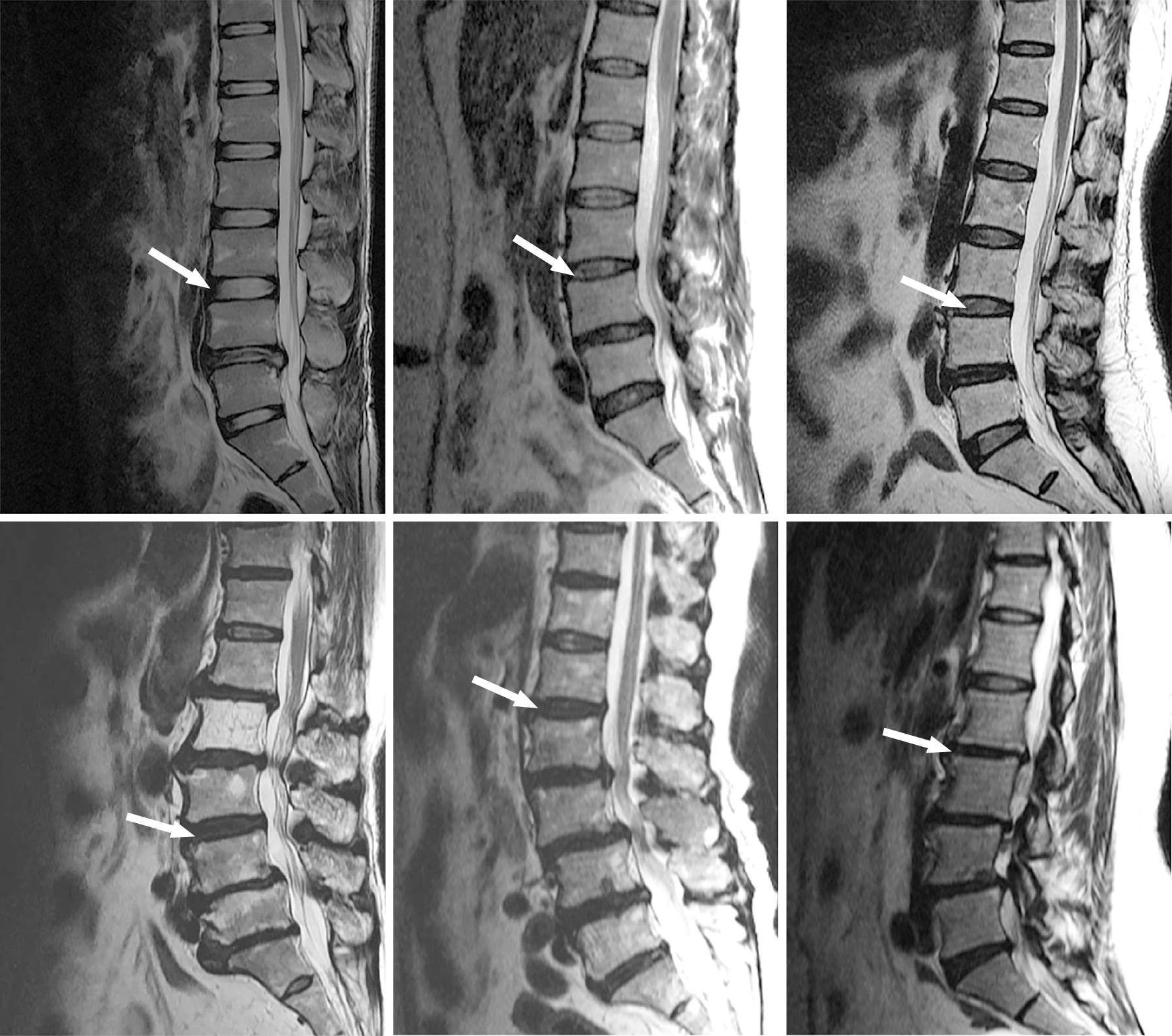
Plain radiography and magnetic resonance imaging were performed prior to Dynesys surgery. The upper ASDeg grade was recorded and compared with that determined by the imaging examination conducted either at the last follow-up or before reoperation for ASDis. The modified Pfirrmann[12] and University of California-Los Angeles (UCLA) grades[1] were used in this study. Analysis of adjacent disc degeneration according to the modified Pfirrmann grade was performed before Dynesys surgery on the upper adjacent segment. Figure 1 shows the magnetic resonance images before Dynesys instrumentation.
The risk of surgical interventions for ASDis was calculated for each year, and Kaplan-Meier survival curves and Cox proportional hazards models with 95% confidence intervals were constructed to determine the independent variables that contributed to the rate of ASDis. Independent variables included age, BMI, and number of Dynesys-instrumented levels.
A total of 212 patients (76 men, 136 women) were included in this study. The mean age was 60.78 (range, 21–82) years. The number of Dynesys-instrumented levels was 2 in 83 patients, 3 in 104 patients, and 4 in 25 patients. The UCLA grade for the upper adjacent segment in these patients was I in 92 patients (43.4%), II in 25 patients (11.8%), grade III in 76 patients (35.8%), and IV in 19 patients (9.0%).
The distribution of modified Pfirrmann grade for the upper adjacent disc was as follows: Grade 1, 20 patients (10%); grade 2, 41 patients (20%); grade 3, 62 patients (30%); grade 4, 54 patients (26%); grade 5, 20 patients (10%); grade 6, 9 patients (4%). No patients with modified Pfirrmann grade 7 or 8 before Dynesys instrumentation were identified in this study. Thirteen patients underwent reoperation for ASDis.
With respect to the association between ASDis and the number of Dynesys-instrumented levels, 9 patients had upper ASDis, 2 had lower ASDis, and 2 had both upper and lower ASDis. Among the 212 patients, the mean UCLA grade was 2.1, mean modified Pfirrmann grade was 3.19, mean age was 60.78 years, mean BMI was 26.29 kg/m2, and mean preoperative ODI was 30.04.
Among patients who did not undergo reoperation, the mean UCLA grade was 2.11, mean modified Pfirrmann grade was 3.19, mean age was 60.89 years, mean BMI was 26.16 kg/m2, preoperative ODI was 30.04, and mean follow-up period was 6.09 years. Among those who underwent reoperation, the mean UCLA grade was 2.08, mean modified Pfirrmann grade was 3.17, mean age was 59.15 years, mean BMI was 28.28 kg/m2, mean preoperative ODI was 30.08, and mean time to reoperation was 7.22 years (Table 2). Indications for reoperation were adjacent stenosis (n = 3), adjacent degenerative spondylolisthesis (n = 5), adjacent disc degeneration (n = 1), and adjacent disc herniation (n = 4). Figure 2 shows patients’ survival distribution. Fifty-four patients were followed for more than 10 years.
| Characteristics | No reoperation (n = 199) | Reoperation (n = 13) | Total patients (n = 212) | |||||||||
| n | Mean | min | Mean | n | Mean | min | Max | n | Mean | min | Max | |
| Before Dynesys surgery | ||||||||||||
| Upper adjacent modified Pfirrmann grade | 194 | 3.19 | 1 | 6 | 12 | 3.17 | 2 | 5 | 206 | 3.19 | 1 | 6 |
| Upper UCLA classification | 199 | 2.11 | 1 | 4 | 13 | 2.08 | 1 | 4 | 212 | 2.10 | 1 | 4 |
| Before reoperation | ||||||||||||
| Upper adjacent modified Pfirrmann grade | / | / | / | / | 12 | 4.58 | 2 | 7 | / | / | / | / |
| Upper UCLA classification | / | / | / | / | 13 | 2.77 | 1 | 4 | / | / | / | / |
| Age | 199 | 60.89 | 21 | 82 | 13 | 59.15 | 41 | 81 | 212 | 60.78 | 21 | 82 |
| Male | 72 | -- | -- | -- | 4 | -- | -- | -- | 76 | |||
| Female | 127 | / | / | / | 9 | / | / | / | 136 | / | / | / |
| VAS score, back | / | 7.67 | 0 | 10 | / | 7.75 | 5 | 10 | / | 7.67 | 0 | 10 |
| VAS score, leg | / | 8.01 | 2 | 10 | / | 7.67 | 6 | 10 | / | 7.98 | 2 | 10 |
| BMI | / | 26.16 | 16 | 38.7 | / | 28.28 | 22.9 | 36.6 | / | 26.29 | 16 | 38.7 |
| Preoperative ODI | 189 | 30.04 | 9 | 48 | / | 30.08 | 22 | 37 | / | 30.04 | 9 | 48 |
| Last imaging follow-up yr (before reoperation) | 199 | 6.09 | 0.10 | 12.76 | / | 7.22 | 1.65 | 11.84 | / | 6.16 | 0.10 | 12.76 |
Patients with a modified Pfirrmann grade ≤ 3 vs ≥ 4 or ≤ 4 vs ≥ 5 for the upper adjacent segment exhibited no significant difference according to the crude hazard ratio determined by the Cox model. The Kaplan-Meier survival analysis of the revision risk revealed no significant difference in the cumulative risk in either comparison, with P values of 0.53 and 0.46, respectively. In the comparison of UCLA grades 1, 2 vs 3, 4, neither the hazard ratio nor the Kaplan-Meier survival analysis revealed significant differences (Table 2 and Figure 3).
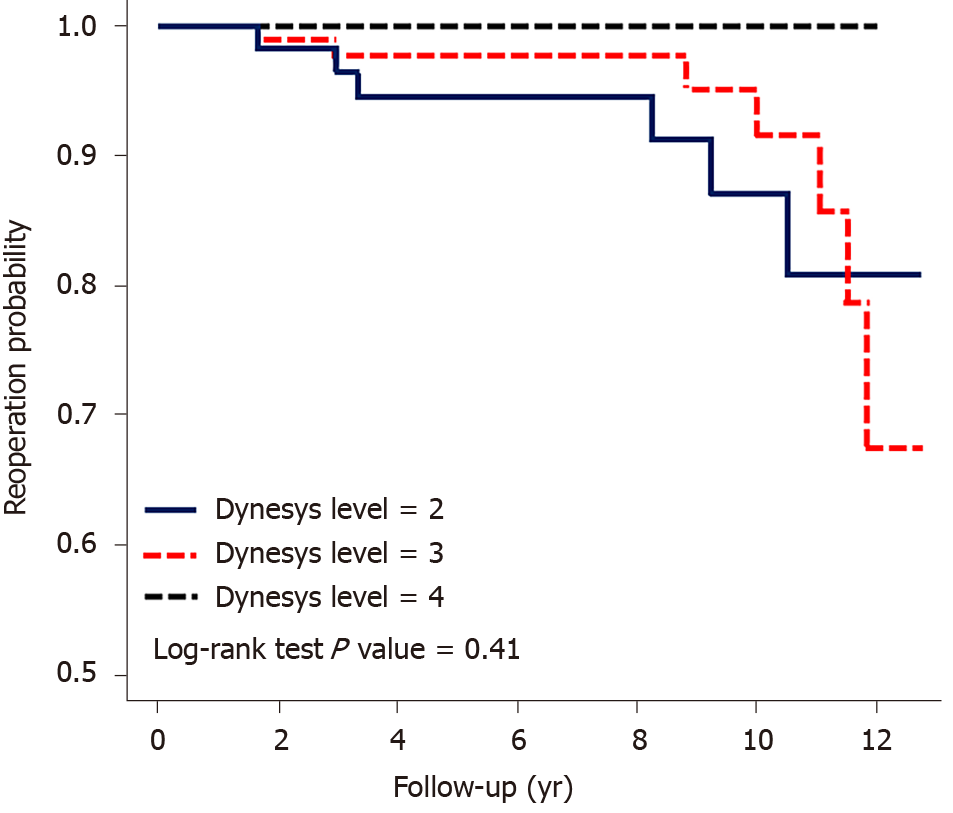
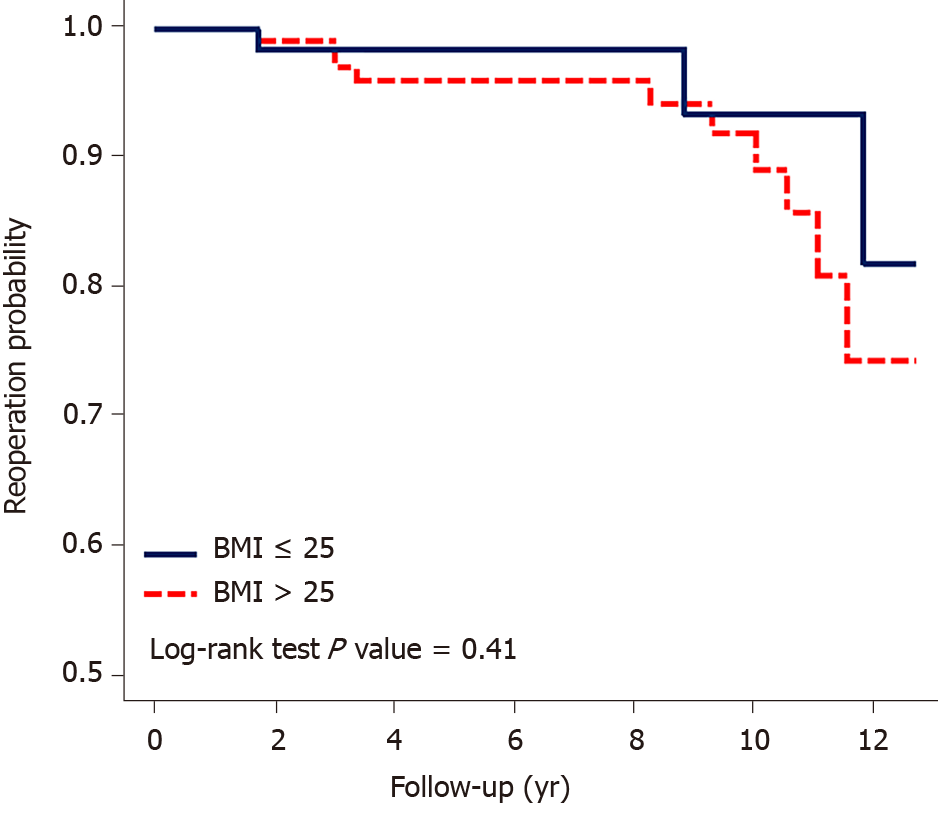
No significant difference was observed between 2 and 3 Dynesys-instrumented levels, with a hazard ratio of 0.51 (0.11–2.43). The P value was 0.41 for the probability of revision. In the comparison of patients aged ≤ 60 and > 60 years, the hazard ratio was 0.94 (0.31–2.82), and the survival probability indicated no significance. In the comparison between BMI ≤ 25 kg/m2 and BMI > 25 kg/m2, the hazard ratio was 2.01 (0.55–7.36), which was also not significant. Moreover, in the Kaplan-Meier survival analysis of revision, BMI > 25 kg/m2 tended to a higher rate of reoperation for ASDis; however, the trend was not significant. Sex and instrumentation of 2 vs 3 levels had no significant effect on the reoperation rate (Table 3, Figures 4 and 5).
| Variables | Reoperation after Dynesys surgery | Crude HR (95%CI) | Adjusted HR (95%CI) | |||
| n/patients | Event/patients | PY | IR | |||
| Upper adjacent modified Pfirrmann grade | 12/206 | |||||
| ≤ 3 | 8/123 | 734.36 | 10.89 | 1 (reference) | 1 (reference) | |
| ≥ 4 | 4/83 | 539.21 | 7.42 | 0.64 (0.19-2.13) | 0.93 (0.22-3.92) | |
| Upper adjacent modified Pfirrmann grade | 12/206 | |||||
| ≤ 4 | 11/177 | 1081.26 | 10.17 | 1 (reference) | 1 (reference) | |
| ≥ 5 | 1/129 | 192.31 | 5.20 | 0.49 (0.06-3.87) | 0.46 (0.05-4.66) | |
| Upper UCLA classification | 13/212 | |||||
| 1, 2 | 8/117 | 737.28 | 10.85 | 1 (reference) | 1 (reference) | |
| 3, 4 | 5/95 | 569.17 | 8.78 | 0.77 (0.25-2.37) | 1.18 (0.31-4.47) | |
| Dynesys level | 13/212 | |||||
| 2 | 6/83 | 468.05 | 12.82 | 1 (reference) | 1 (reference) | |
| 3 | 7/104 | 695.30 | 10.07 | 0.51 (0.11-2.43) | 0.72 (0.19-2.67) | |
| 4 | 0/25 | 143.10 | 0.00 | -- | -- | |
| Age, yr | 13/212 | |||||
| < 60 | 7/92 | 642.72 | 10.89 | 1 (reference) | 1 (reference) | |
| ≥ 60 | 6/120 | 663.73 | 9.04 | 0.94 (0.31-2.82) | 0.69 (0.21-2.24) | |
| Sex | 13/212 | |||||
| Male | 4/76 | 456.05 | 8.77 | 1 (reference) | 1 (reference) | |
| Female | 9/136 | 850.41 | 10.58 | 1.15 (0.35-3.76) | 0.92 (0.27-3.10) | |
| BMI | 13/212 | |||||
| < 25 | 3/80 | 460.16 | 6.52 | 1 (reference) | 1 (reference) | |
| ≥ 25 | 10/132 | 846.29 | 11.82 | 2.01 (0.55-7.36) | 3.90 (0.74-16.95) | |
Preoperative degeneration status of the adjacent disc did not affect the risk of occurrence of ASDis. Furthermore, age, BMI, and sex did not significantly increase the risk of developing ASDis.
Many image grading systems are available to assess degenerative changes. However, the Pfirrmann grading system cannot provide good discrimination in the spine of the elderly, especially in cases of decreased disc height but good T2 signal intensity. Therefore, we applied a modified Pfirrmann grading system as the grading method[1,12,13].
In this study, the mean time to reoperation for ASDis was 7.22 years. For comparison, in a study by Lee et al[3], the time interval from fusion to later non-fusion surgery was 5.6 ± 3 (range, 0.5–10) years. Yeh et al[14] compared the radiologic outcomes of decompression with Dynesys instrumentation with those of microdiscectomy for L4-5 spinal stenosis. The patients in the Dynesys surgery group had a higher grade of facet degeneration than those in the microdiscectomy group. A higher facet fusion rate and decreased range of motion (ROM) at the instrumented level were noted 2 years after surgery. Higher grades of facet degeneration played an important role in increasing the facet fusion rate and decreasing the ROM at the instrumented level. In the same study, which involved more than 3 years of follow-up, the grade of facet degeneration in the Dynesys group was positively correlated with and increased by time[14]. In order to achieve the same ROM, it is necessary for the remaining spinal levels to accept greater load following elimination of motion from the instrumented level, leading to hypermobility and increased stress in the adjacent segments[15]. The use of non-fusion devices is associated with a significantly lower rate of reoperation for ASDis, which has been proven by a meta-analysis[16]. Dynesys implantation lowers the incidence of ASDeg to 9.1% (vs 24.0% in the isolated fusion group)[17].
ASDeg more frequently occurs in the cephalad than caudal direction[1,3]. Lee et al[3] revealed that facet degeneration is a significant risk factor for ASDis. Adjacent disc degeneration is not a risk factor for ASDis in fusion surgery[18].
Degenerative changes affect the bony and soft tissue structures of the spine and may ultimately result in modifications in the spinal motion segment and instantaneous axis of rotation. Considering the functional spinal unit, osteophytes develop as the annulus is distorted and pulled from its bony attachments, resulting in an unstable functional spinal unit and, potentially, in low back pain. The fused spinal segment stress-strain curve indicates that the slope of the elastic zone is steeper than that of the normal functional spinal unit. The ROM increases in early stages but diminishes in later stages of degeneration. Greater ROM is found at the adjacent levels[15,19].
A meta-analysis reported that the rate of reoperation for ASDis was significantly lower in patients treated with a non-fusion device than in those treated with fusion[16]. St-Pierre et al[20] showed that prior ASDis was a significant factor for progressive ASDis after Dynesys surgery.
The risk of revision due to ASDis is twice as high in men as in women[21]. However, in this study, sex did not appear to be significantly related to the reoperation rate. No progression of spondylolisthesis occurred over a 2-year follow-up after dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis; only 1 patient with Dynesys instrumentation at L4-5 had adjacent level instability[6]. Another study reported 1 patient with adjacent instability after instrumentation at L4-5[5].
Nevertheless, in contrast, one report indicated that floating fusion (L4-5) accelerates adjacent degeneration. Radiologic ASDeg at L5-S1 is mostly asymptomatic[22]. In our postoperative 12-year study, the number of levels treated with posterior instrumentation was not a significant indication for revision for ASDis.
BMI > 25 kg/m2 is a risk factor for ASDeg and ASDis[23]. However, BMI cannot be confirmed as a risk factor for reoperation for ASDis[21]. Our results further indicate that BMI > 25 kg/m2 was not significantly associated with ASDis but showed a higher tendency for reoperation.
This study has some limitations. First, it is a retrospective review of medical records at one hospital, representing a single surgeon’s clinical experience with non-fusion dynamic stabilization for degenerative lumbar spine disease, and involves patient follow-up for reoperation at a single hospital. Second, the rate of reoperation for ASDis was relatively low. Strict compliance with conservative measures, such as spinal braces and adjustment of daily activity, is believed to significantly decrease ASDis progression. Third, only 13 patients underwent reoperation; an analysis of a larger number of patients would allow drawing of more precise conclusions. Fourth, before the secondary endpoint of our study (November 2019), more than 20 elderly patients had died due to age, cancer, cardiovascular disease, cardiopulmonary trauma, and other non-Dynesys surgery-related conditions.
The concept of patients with more degenerative changes in adjacent segments being more prone to revision for ASDis appears reasonable. Nonetheless, our results indicate that the modified Pfirrmann grade was not a significant factor that influenced the rate of revision for ASDis. In addition, the number of stabilized levels tended not to affect the rate of reoperation for ASDis and was not a risk factor. Finally, BMI was not a risk factor for ASDis reoperation but displayed a higher tendency towards reoperation; thus, BMI should be considered before surgery. Greater preexisting upper ASDeg was not related to a higher rate of reoperation for ASDis after Dynesys surgery. Being overweight tended to increase the reoperation risk after Dynesys surgery for ASDis.
Dynesys surgery is believed to decrease adjacent segment disease compared to fusion surgery.
The main topics, key problems to be solved, and significance of solving these problems for future research in this field should be described in detail.
To determine the relationship between preoperative adjacent degeneration condition and adjacent segment disease requiring surgery.
This is a retrospective study involving 212 patients. Data on University of California-Los Angeles and modified Pfirrmann grading were analyzed with Kaplan-Meier survival curves and Cox proportional hazards models.
No static significant difference exists between higher vs lower University of California-Los Angeles grades of adjacent segment degeneration (ASDeg). No static significant difference exists between higher vs lower modified Pfirrmann grades of ASDeg.
Greater preexisting upper ASDeg was not related to a higher rate of reoperation for adjacent segment disease following Dynesys surgery.
A cohort study on the relationship between pre-existing ASDeg and surgery for adjacent segment disease is lacking.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Orthopedics
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng B S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Guo X
| 1. | Lautenschlager EP, Harcourt JK, Ploszaj LC. Setting reactions of gypsum materials investigated by x-ray diffraction. J Dent Res. 1969;48:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 534] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 2. | Di Silvestre M, Lolli F, Bakaloudis G, Parisini P. Dynamic stabilization for degenerative lumbar scoliosis in elderly patients. Spine (Phila Pa 1976). 2010;35:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lee SE, Jahng TA, Kim HJ. Clinical Experiences of Non-fusion Dynamic Stabilization Surgery for Adjacent Segmental Pathology after Lumbar Fusion. Int J Spine Surg. 2016;10:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Lee SE, Park SB, Jahng TA, Chung CK, Kim HJ. Clinical experience of the dynamic stabilization system for the degenerative spine disease. J Korean Neurosurg Soc. 2008;43:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine (Phila Pa 1976). 2008;33:E636-E642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine (Phila Pa 1976). 2006;31:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Veresciagina K, Mehrkens A, Schären S, Jeanneret B. Minimum ten-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. J Spine Surg. 2018;4:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Zhang ZC, Li F, Sun TS, Shan JL, Guan K, Zhao GM, Zhang LZ. Long-Term Outcome of Dynesys Dynamic Stabilization for Lumbar Spinal Stenosis. Chin Med J (Engl). 2018;131:2537-2543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11 Suppl 2:S170-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Meyerding HW. Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. J Int Coll Surg. 1956;26:566-591. [PubMed] |
| 11. | Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940-52; discussion 2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3162] [Cited by in RCA: 3760] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 12. | Griffith JF, Wang YX, Antonio GE, Choi KC, Yu A, Ahuja AT, Leung PC. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2007;32:E708-E712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 369] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Tan TL, Borkowski SL, Sangiorgio SN, Campbell PA, Ebramzadeh E. Imaging Criteria for the Quantification of Disc Degeneration: A Systematic Review. JBJS Rev. 2015;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Yeh MY, Kuo CH, Wu JC, Huang WC, Tu TH, Fay LY, Wu CL, Cheng H. Changes of Facet Joints After Dynamic Stabilization: Continuous Degeneration or Slow Fusion? World Neurosurg. 2018;113:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Iorio JA, Jakoi AM, Singla A. Biomechanics of Degenerative Spinal Disorders. Asian Spine J. 2016;10:377-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Ren C, Song Y, Liu L, Xue Y. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures: a meta-analysis. Eur J Orthop Surg Traumatol. 2014;24 Suppl 1:S245-S253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Zhou ZJ, Xia P, Zhao X, Fang XQ, Zhao FD, Fan SW. Can posterior dynamic stabilization reduce the risk of adjacent segment deterioration? Turk Neurosurg. 2013;23:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH, Lee MY. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Liu CL, Zhong ZC, Shih SL, Hung C, Lee YE, Chen CS. Influence of Dynesys system screw profile on adjacent segment and screw. J Spinal Disord Tech. 2010;23:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | St-Pierre GH, Jack A, Siddiqui MM, Henderson RL, Nataraj A. Nonfusion Does Not Prevent Adjacent Segment Disease: Dynesys Long-term Outcomes With Minimum Five-year Follow-up. Spine (Phila Pa 1976). 2016;41:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Rienmüller AC, Krieg SM, Schmidt FA, Meyer EL, Meyer B. Reoperation rates and risk factors for revision 4 years after dynamic stabilization of the lumbar spine. Spine J. 2019;19:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Lee JC, Choi SW. Adjacent Segment Pathology after Lumbar Spinal Fusion. Asian Spine J. 2015;9:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Wang H, Ma L, Yang D, Wang T, Liu S, Yang S, Ding W. Incidence and risk factors of adjacent segment disease following posterior decompression and instrumented fusion for degenerative lumbar disorders. Medicine (Baltimore). 2017;96:e6032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |