Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10838
Peer-review started: April 22, 2021
First decision: May 24, 2021
Revised: June 1, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 16, 2021
Processing time: 231 Days and 23.8 Hours
The resistance rate to antibacterial drugs is the key inhibitor of Helicobacter pylori (H. pylori) eradication treatment.
To evaluate the prevalence and drug resistance of H. pylori based on big data.
Gastric mucosal specimens were collected from naive patients undergoing upper gastrointestinal endoscopy for H. pylori culture and antimicrobial susceptibility testing (AST), including clarithromycin, levofloxacin, metronidazole and amoxicillin. Every 10 years of age was grouped as an age group. The H. pylori infection and resistance were explored based on the age group and gender.
The number of H. pylori-positive specimen was 94509 in 283823 gastric mucosal specimens, with an infection rate of 33.30%. The infection rate increased with age, and males had a higher infection rate than females. The average resistance rate of H. pylori to amoxicillin and metronidazole was 0.21% and 93.72%, which remained stable. The average resistance rate to clarithromycin was 23.99% with an increasing trend from 14.43% to 38.24%. The average resistance rate to levofloxacin was 30.29%, which increased from 17.07% to 39.42% and mostly stabilized after 2017. The resistance rate of H. pylori increased with age, except amoxicillin. H. pylori in females are at higher risk of resistance to metronidazole, but not to amoxicillin, regardless of the age group. Meanwhile, H. pylori in females are at higher risk of resistance to levofloxacin and clarithromycin in the 21-50 age group. The single, dual, triple and quadruple-drug resistance rate was 54.59%, 29.03%, 11.71% and 0.11%, respectively.
The resistance of H. pylori in Taizhou city is serious. Guided by the consensus report, individualized treatment based on AST is recommended.
Core Tip: We shared a 10-year data in prevalence and resistance of Helicobacter pylori (H. pylori) in Taizhou city, Zhejiang province, China. We found that the infection rate increased with age, and males had a higher infection rate than females. The resistance rate of H. pylori to metronidazole, clarithromycin and levofloxacin was increased with age. H. pylori in females are at higher risk of resistance to metronidazole, but not to amoxicillin, regardless of the age group. Meanwhile, H. pylori in females are at higher risk of resistance to levofloxacin and clarithromycin in the 21-50 age group.
- Citation: Zhang Y, Meng F, Jin J, Wang J, Gu BB, Peng JB, Ye LP. Ninety-four thousand-case retrospective study on antibacterial drug resistance of Helicobacter pylori. World J Clin Cases 2021; 9(35): 10838-10849
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10838.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10838
According to the estimates by GLOBOCAN, 1033701 individuals developed gastric cancer, and an estimated 782685 related deaths occurred in 2018[1]. In China, gastric cancer is ranked second for incidence rate and third for mortality rates among all cancers, while in Western Europe, the incidence rate ranks 9th and the mortality rate ranks 6th. In North America, the incidence rate and mortality rates of gastric cancer rank 13th and 9th respectively[2]. Helicobacter pylori (H. pylori) is the most important pathogenic factor of gastric cancer which accounted for 44.2% of new gastric cancer cases globally[2]. H. pylori was estimated to be responsible for more than 78.00% of new gastric cancer cases in 2018, and the total number of H. pylori-related cases is not expected to decrease for decades due to global population growth and aging[3]. Moreover, H. pylori causes substantial morbidity from various peptic ulcer diseases, gastritis and even gastric lymphoma. H. pylori is classified as carcinogenic (Group 1) to humans by the Monographs program of the International Agency for Research on Cancer[4].
Several recent publications have demonstrated the global prevalence of H. pylori infection to be approximately 50%[5,6]. Large differences were observed between areas within large countries, such as China, which is one of the countries with the highest prevalence of H. pylori infection[7]. Wuwei city has a much higher H. pylori prevalence (72.28%) than all other areas in China, followed by Fengkai city (55.90%). Dali city has the lowest H. pylori prevalence of 11.36%[8]. Effective treatment for H. pylori infection with a combination of antibacterial drugs, proton pump inhibitors and bismuth all contribute to a decline in peptic ulcer disease incidence and the gastric cancer mortality rate. The resistance rate to antibacterial drugs is the key inhibitor of H. pylori eradication treatment. Some data that describe the resistance rates of H. pylori indicate a correlation with age[9]. Populations of different ages with various antibiotic usage habits may have different resistances to antibacterial drugs used for H. pylori treatment, and this result will be more apparent when examined by big data analysis.
Thus, big data-based H. pylori prevalence and drug resistance statistical programs are critical. In this study, we shared a 10-year data analysis on the prevalence and resistance of H. pylori in Taizhou city, Zhejiang Province, China.
This multicenter retrospective study was conducted from January 2011 to December 2020 in Taizhou city, Zhejiang Province, China. Gastric mucosal specimens were collected from patients undergoing upper gastrointestinal endoscopy for H. pylori culture and in vitro antimicrobial susceptibility testing (AST). Patients in this study followed the enrollment criteria: (1) With gastrointestinal symptoms such as abdominal pain, bloating, acid reflux and nausea, etc.; (2) Gastroscopic diagnosed as gastric, duodenal peptic ulcer and chronic gastritis with erosion, etc.; (3) No severe damage to heart, liver, and kidney function, and no allergies to antibacterial drugs used in AST; (4) Non-pregnant or breastfeeding women, no gastrointestinal bleeding, perforation, pyloric obstruction, or cancer complications; (5) Have not taken any antibacterial drugs, bismuth, H2 receptor antagonist or proton-pump inhibitors within half a month; and (6) Agree to conduct gastric mucosal tissue sampling, H. pylori culture and in vitro AST, and signed their informed consent. The study had been approved by the ethics committee of Taizhou Hospital of Zhejiang Province.
A gastric mucosal specimen was collected from lesser curvature of the gastric antrum, greater curvature of the gastric body, or the tissue adjacent to the lesion. After biopsy, the specimen was immersed in H. pylori preservation tube containing brain heart infusion. All specimens were transported on dry ice throughout the entire process to Zhiyuan Medical Inspection Institute Co., Ltd. for H. pylori culture, isolation, identification and AST.
Thawed the gastric mucosal specimen at room temperature and fully ground it into homogenate with 600 μL of brain heart infusion in a glass grinder. Spread the tissue homogenate on a Columbia blood plate (OXOID, England) containing 5% sheep blood by streaking. Cultivated the H. pylori in a 37 °C mixed gas incubator (5% O2, 10% CO2 and 85% N2) for 2-3 d. Extended the culture time to 7 d if there was no growth. Picked out and subcultured the monoclonal colonies with typical colony morphology. Then smeared microscopy with the suspected H. pylori strain and carried out the biochemical experiments with urease (Haibo Biotechnology Co., Ltd., China), oxidase (Beijing Luqiao Technology Co., Ltd., China) and catalase (Haibo Biotechnology Co., Ltd., China). Suspected strains with biological morphology and biochemical reactions were identified as H. pylori, and the patient was diagnosed as positive for H. pylori infection. Extended the culture time to 7 d, and if there was still no H. pylori strain growth on the medium, the patient was diagnosed as negative for H. pylori infection.
The plate incorporation assay was used to test the antimicrobial susceptibility of H. pylori strains[10]. The critical points of antibacterial resistance referred to the value of H. pylori clinical susceptibility test in China[11,12]: 1 μg/mL for clarithromycin (National Institute for Food and Drug Control, China), 2 μg/mL for levofloxacin (National Institute for Food and Drug Control, China), 8 μg/mL for metronidazole (National Institute for Food and Drug Control, China), and 2 μg/mL for amoxicillin (Dr. Ehrenstorfer GmbH, Germany).
We diluted and added these 4 kinds of antibacterial drugs to sterile Columbia culture medium containing 5% sheep blood respectively and ensured the concentration of antibacterial drugs to the critical point. After mixing well, poured into the plate to prepare 4 kinds of blood plates containing the antibacterial drugs.
Collected H. pylori strains with good growth status, and diluted the strains with 0.9% normal saline to a concentration of 0.5 McFarland, about 1.5 × 108/mL[10]. Drew 2 μL of the diluted bacterial solution and inoculated on the conventional Columbia blood plate and the Columbia blood plate containing antibacterial drug, respectively. Cultivated the H. pylori in a 37 °C mixed gas incubator (5% O2, 10% CO2 and 85% N2) for 2 d.
Firstly, we observed the growth of H. pylori strains on the conventional blood plate. The H. pylori strains with good growth status proved that its antimicrobial susceptibility results are credible. The H. pylori strains with poor growth status or not growing needed to undergo a new AST. Secondly, we observed the growth of H. pylori strains on the blood plate containing antibacterial drug. An inhibited growth of the H. pylori strain on the blood plate containing antibacterial drug was judged to be sensitive to the antibacterial drug, and the uninhibited growth of the H. pylori strain was judged to be resistant to the antibacterial drug. The NCTC11637 standard strain was selected as the quality control bacteria.
SPSS 19.0 software were used. Using chi-square test to perform statistical analysis on count data. Odds ratio (OR) was used to evaluate risk factors. The Mantel-Haenszel chi-square test was used to evaluate a linear relationship. Breslow-Day method was used for the test of Homogeneity of OR. P < 0.05 indicated that the difference was statistically significant.
Basic information: From 2011 to 2020, a total of 283823 gastric mucosal specimens were cultured from patients with peptic ulcer disease. The ratio of males to females was 1.01:1.00. The ages of the participants ranged from 3 to 97, and the average age was 48.52 ± 14.04. The number of H. pylori-positive specimens was 94509; thus, the overall positive rate of H. pylori infection reached 33.30%. The ratio of male to female patients who were positive for H. pylori was 1.08:1.00. The ages of these patients ranged from 4 to 96, and the average age was 50.79 ± 12.76. Every 10 years of age was grouped as an age group, resulting in division into 8 age groups: ≤ 20 years, 21-30, 31-40, 41-50, 51-60, 61-70, 71-80 and ≥ 81 years; this division was close to a normal distribution (P = 0.383) (Table 1).
| Age group | Infection rate (%) | Tested specimen | H. pylori–positive specimen | Multivariable analysis | ||||||
| Total | Male | Female | Total | Male | Female | OR | 95%CIa | P value | ||
| Total | 33.30 | 283823 | 142851 | 140972 | 94509 | 49139 | 45370 | - | - | - |
| ≤ 20 | 22.49 | 4825 | 2576 | 2249 | 1085 | 624 | 461 | 1.240 | 1.082 1.421 | 0.002 |
| 21-30 | 26.78 | 21681 | 11655 | 10026 | 5807 | 3196 | 2611 | 1.073 | 1.010 1.140 | 0.022 |
| 31-40 | 27.24 | 46803 | 23720 | 23083 | 12750 | 6688 | 6062 | 1.103 | 1.059 1.148 | 0.000 |
| 41-50 | 29.76 | 80907 | 39271 | 41636 | 24078 | 12156 | 11922 | 1.117 | 1.084 1.152 | 0.000 |
| 51-60 | 38.86 | 72229 | 35077 | 37152 | 28066 | 14284 | 13782 | 1.165 | 1.131 1.200 | 0.000 |
| 61-70 | 43.33 | 40951 | 21065 | 19886 | 17744 | 9250 | 8494 | 1.050 | 1.010 1.092 | 0.014 |
| 71-80 | 32.39 | 13939 | 7866 | 6073 | 4515 | 2635 | 1880 | 1.123 | 1.046 1.207 | 0.001 |
| ≥ 81 | 18.65 | 2488 | 1621 | 867 | 464 | 306 | 158 | 1.044 | 0.844 1.292 | 0.690 |
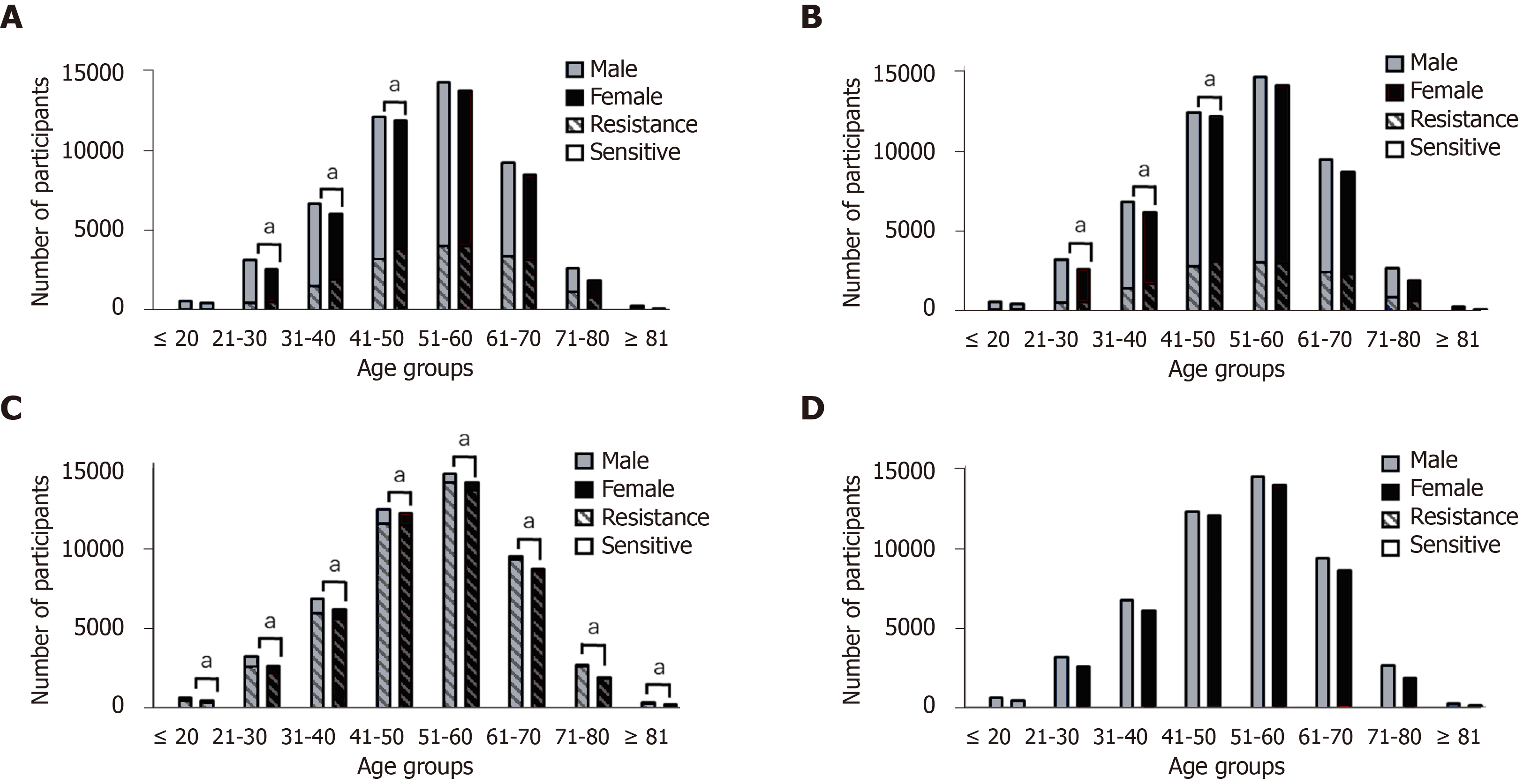
Gender and risk of H. pylori infection: A total of 142851 males and 140972 females were tested for H. pylori infection; of these, 49139 males and 45370 females tested positive for H. pylori infection. Compared with females, males had a higher rate of H. pylori infection (OR = 1.105, 95% confidence interval (CI): 1.088-1.122).
Relationship between age and H. pylori infection: The results of the Mantel-Haenszel chi-square test showed that there was a linear relationship between age and the rate of H. pylori infection (χ2 = 2577.812, P < 0.001). The Pearson-related results showed that r = 0.095, P < 0.001, indicating that the H. pylori infection rate increased with age.
Multivariable analysis of H. pylori infection: The homogeneity of OR test showed P = 0.003, suggesting that the OR values between age groups were heterogeneous, and it was not appropriate to combine the OR values. Therefore, after stratifying according to age group, male gender was determined to be a risk factor for H. pylori infection, except for the population aged ≥ 81-years-old (OR = 1.044, 95%CI: 0.844-1.292, P = 0.690). For the ≤ 20-years-old age group, the risk of H. pylori infection was 1.240 times higher in males than in females (OR = 1.240, 95%CI: 1.082-1.421, P = 0.002). For different age groups between 21-years-old and 80-years-old, the risk of H. pylori infection was 1.073, 1.103, 1.117, 1.165, 1.050, and 1.123 times higher in males than in females, respectively (Table 1).
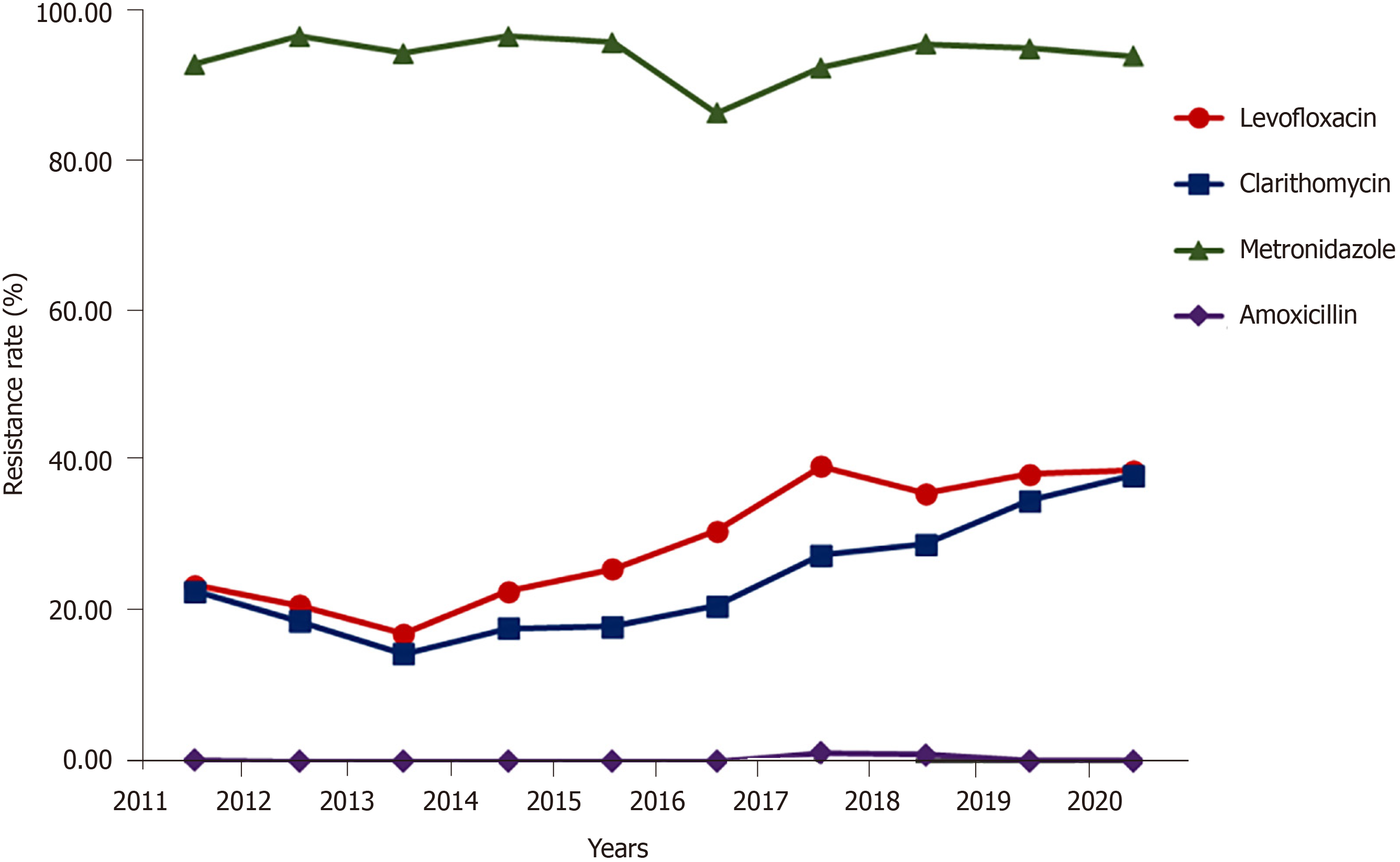
The overall resistance rate of H. pylori: The average resistance rate of H. pylori to amoxicillin was 0.21% and slightly increased in 2017 and 2018 to 1.15% and 0.87%, respectively. The average resistance rate of H. pylori to metronidazole was 93.72%, remained at a high level and fluctuated in the range of 92.71% to 96.92%, except for when the rate fell to 86.64% in 2016. Different from the trends of these two antibacterial drugs, the average resistance rate of H. pylori to clarithromycin was 23.99% and had an increasing trend (from 14.43% in 2013 to 38.24% in 2020) (Figure 1). The average resistance rate of H. pylori to levofloxacin was 30.29%, which increased over time (from 17.07% in 2013 to 39.42% in 2017) and mostly stabilized after 2017. The results of the Mantel-Haenszel chi-square test and Pearson-related results showed a linear increase for resistance to clarithromycin (χ2 = 2389.117, r = 0.159, P < 0.001) and for resistance to levofloxacin (χ2 = 1901.809, r = 0.142, P < 0.001).
Gender and risk of H. pylori resistance rates: The number of males infected with H. pylori who were resistant to levofloxacin was 14051, and the resistance rate was 28.59%, while the number of females infected with H. pylori who were resistant was 14578, with a resistance rate of 32.13%. Compared with that of males, the H. pylori resistance rate was higher in females (OR = 1.182, 95%CI: 1.159-1.216). The resistance rates to clarithromycin in males and females were 22.93% and 25.14%, respectively, and the H. pylori resistance rate to clarithromycin in females was higher than that in males (OR = 1.129, 95%CI: 1.095-1.163). Similarly, the H. pylori resistance rate to metronidazole in females (94.44%) was higher than that in males (93.05%) (OR = 1.267, 95%CI: 1.202-1.337). There was no significant difference in the resistance rate of H. pylori to amoxicillin between different genders (OR = 0.880, 95%CI: 0.663-1.169).
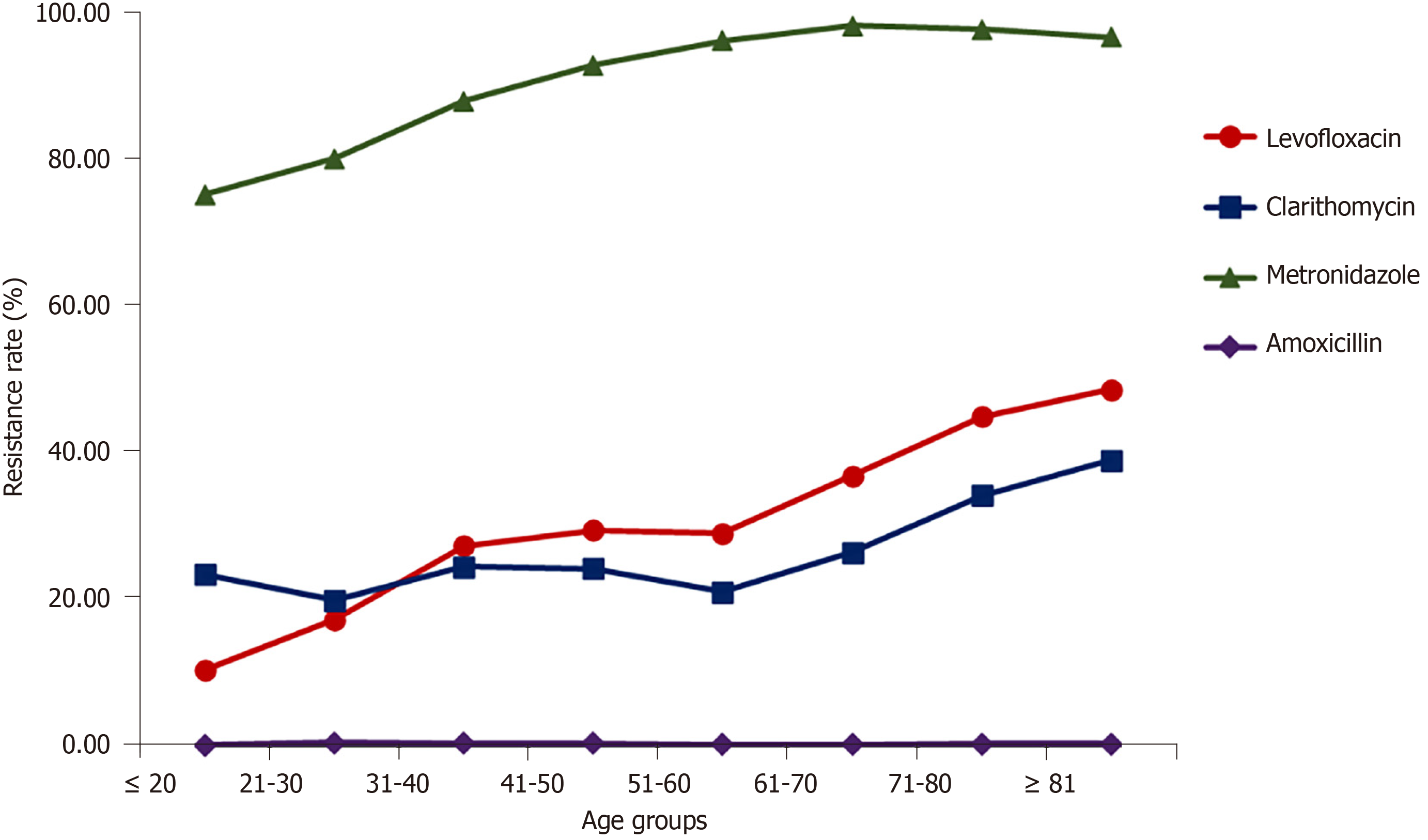
Relationship between age and H. pylori resistance rates: Overall, the resistance rate to amoxicillin was low (< 0.35%). The resistance rate to metronidazole mostly stabilized after increasing from 75.39% to 96.49% in the population < 60-years-old, and the resistance rate of the population ≥ 61-years-old remained at approximately 98%. The resistance rate to clarithromycin increased rapidly in the population ≥ 61 years of age, changing from 21.02% to 39.01%. The resistance rate to levofloxacin continuously increased from 10.32% to 48.71% with increasing age. The results from the Mantel-Haenszel chi-square test and the Pearson relationship showed that there was a linear and positive relationship between age and resistance rate of H. pylori to the three kinds of antibacterial drugs, except for a negative relationship to amoxicillin resistance (χ2 = 1356.563, r = 0.120, P < 0.001 for levofloxacin; χ2 = 153.312, r = 0.040, P < 0.001 for clarithromycin; χ2 = 3685.476, r = 0.197, P < 0.001 for metronidazole; and χ2 = 24.710, r = -0.016, P < 0.001 for amoxicillin) (Figure 2).
Multivariable analysis of resistance rates: Furthermore, we conducted an analysis on the correlation of age and gender with the H. pylori resistance rates to levofloxacin, clarithromycin and metronidazole (Figure 3).
Resistance rate to levofloxacin: The homogeneity of OR test showed P < 0.001, suggesting that the OR values between age groups are heterogeneous, and it is not appropriate to combine OR values. Therefore, after stratifying according to age group, there was no significant difference in the resistance rate of H. pylori to levofloxacin between female and male, aged ≤ 20-years-old and aged ≥ 51-years-old (P > 0.05). However, for the 21-50 age group, H. pylori in female was at a higher risk of resistance to levofloxacin than in male. For the 21-30 age group, the risk of H. pylori developing resistance to levofloxacin was 1.525 times higher in females than in males (OR = 1.525, 95%CI: 1.330-1.748, P < 0.001). For the 31-40 age group, the risk of H. pylori developing resistance to levofloxacin was 1.604 times higher in females than in males (OR = 1.604, 95%CI: 1.483-1.735, P < 0.001), and for the 41-50 age group, the risk was 1.325 times higher in females than in males (OR = 1.325, 95%CI: 1.253-1.400, P < 0.001).
Resistance rate to clarithromycin: The homogeneity of OR test showed P < 0.001. After stratifying according to age group, there was no significant difference in the resistance rate of H. pylori to clarithromycin between females and males, aged ≤ 20-years-old and aged ≥ 51-years-old (P > 0.05). For the 21-50 age groups, H. pylori in females was at a higher risk of resistance to clarithromycin than in males. For the 21-30 age group, the risk of H. pylori developing resistance to clarithromycin was 1.356 times higher in females than in males (OR = 1.356, 95%CI: 1.192-1.544, P < 0.001). For the 31-40 age group, the risk of H. pylori developing resistance to clarithromycin 1.495 times higher in females than in males (OR = 1.495, 95%CI: 1.379-1.622, P < 0.001). For the 41-50 age group, the risk of H. pylori developing resistance to clarithromycin was 1.175 times higher in females than in males (OR = 1.175, 95%CI: 1.107-1.246, P < 0.001).
Resistance rate to metronidazole: The homogeneity of OR test showed P = 0.056, suggesting that the OR values between the age groups are homogeneous. Therefore, after controlling the age groups, H. pylori in females was at a higher risk of the resistance to metronidazole (OR = 1.257, 95%CI: 0.190-1.327, P < 0.001). Thus, regardless of the age group, the H. pylori resistance rate to metronidazole was approximately 1.257 times in females than that in males.
Multidrug resistance of H. pylori to the four antibacterial drugs is shown in Table 2. Among the 94509 positive specimens, the overall resistance rate to the four antibacterial drugs reached 95.44%, of which the single-drug resistance rate was 54.59%, the dual-drug resistance rate was 29.03%, the triple-drug resistance rate was 11.71%, and the quadruple-drug resistance rate was 0.11%. According to the comparison of gender, the single-drug resistance rate in males was significantly higher than that of females (
| Antibacterial drugs | Total | Male | Female |
| Sensitivea | 4314 | 2485 | 1829 |
| LEV | 626 | 362 | 264 |
| CLA | 557 | 332 | 225 |
| MTR | 50413 | 26642 | 23771 |
| AMX | 0 | 0 | 0 |
| LEV + CLA | 444 | 234 | 210 |
| LEV + MTR | 16419 | 8343 | 8076 |
| LEV + AMX | 1 | 1 | 0 |
| CLA + MTR | 10551 | 5599 | 4952 |
| CLA + AMX | 0 | 0 | 0 |
| MTR + AMX | 17 | 14 | 3 |
| LEV + CLA + MTR | 10990 | 5035 | 5955 |
| LEV + CLA + AMX | 0 | 0 | 0 |
| LEV + MTR + AMX | 48 | 25 | 23 |
| CLA + MTR + AMX | 28 | 16 | 12 |
| LEV + CLA + MTR + AMX | 101 | 51 | 50 |
The infection rate of H. pylori in China is approximately 55%[13], while the detection rate of H. pylori culture is only approximately 30%. A variety of reasons may affect the positivity rate of H. pylori culture, including the colonization of H. pylori at the biopsy site[14], the influence of PPIs and antibacterial drugs on microbial cultures[15], and even contamination in the process of microbial culture. However, the drug susceptibility test based on H. pylori culture can provide doctors with guidance for prescribing clinical medication and is the key to individualized treatment. With an annually increasing rate of antibacterial resistance, individualized treatment based on drug susceptibility results is recommended in China. Therefore, the cultivation of H. pylori is still important and is the gold standard for detecting H. pylori infection.
In this study, we shared a 10-year data analysis on the infection and resistance of H. pylori in Taizhou city, Zhejiang Province, China. A total of 283823 gastric mucosal specimens were examined, and the H. pylori infection rate was 33.30%. Age was determined to be related with the H. pylori infection. The population aged ≤ 20-years-old had the lowest infection rate of 22.49%, and the population aged 61-70 years had the highest infection rate of 43.33%. The infection rate increased with age; however, the prevalence of H. pylori infection in children and adolescents is worthy of attention. The reported rates of H. pylori infection among children and adolescents in different areas of China range from 18.62% to 72.28%[16,17]. The "Kyoto global consensus report on H. pylori gastritis" proposes that H. pylori infection is an infectious disease[18], and it can be transmitted through mouth-to-mouth, stomach-to-mouth, feces-to-mouth and other routes. Thus, in addition to the limited awareness of hygiene and health, the traditional eating style (sharing food from the same plates) observed in China may play a role in the transmission of H. pylori. The diagnosis and treatment of H. pylori infection in children and adolescents requires special attention. In the absence of effective treatment, an H. pylori infection may be lifelong.
On the other hand, males were found to have a higher risk of H. pylori infection than that for females, and Dutta et al[19] reported the same results. Some habits, such as smoking, may be indirectly related to this result[20]. In Taizhou city, 48.00% of males smoke, while only 1.19% of females smoke[21]. The habit of smoking may lead to an increase in the secretion of gastric acid and pepsin, thereby destroying the health of the gastric mucosa and promoting the colonization of H. pylori.
H. pylori has high resistance rates to antibacterial drugs in Taizhou city, and these rates have gradually increased over time. The resistance of H. pylori to amoxicillin remains low (0.21%), while that to metronidazole is high (93.72%) throughout China[22,23]. In the 10 years examined in this study, the resistance rates to clarithromycin and levofloxacin significantly increased by 23.81% and 22.35%, respectively. The resistance rate of H. pylori increases with age[9]. In this study, the resistance rate to clarithromycin and metronidazole for the population aged ≥ 81-years-old was 15.60% and 21.59% higher, respectively, than those for the population aged ≤ 20-years-old, and the difference in levofloxacin resistance reached 38.38%. The application of antibacterial drugs in the population is one of the external factors contributing to the development of H. pylori resistance, and the resistance of H. pylori detected for the population aged ≤ 20-years-old, who seldom use antibacterial drugs, may be related to the resistance of the H. pylori itself.
After controlling for age group, females showed a higher incidence of H. pylori resistance to metronidazole[24], which was approximately 1.257 times higher than that of males. This result is in agreement with data presented by Mirzaei et al[25]. Metronidazole is used in a wide variety of applications and is typically used in the treatment of anaerobic infections in the female reproductive system or for treating Trichomonas vaginalis infections[26]. In this study, H. pylori in females was at a higher risk of resistance to clarithromycin and levofloxacin for the population aged 21-50 years; this finding is different from the data presented by Shu et al[27]. A study by Shao et al[24] reported results in agreement with ours. In addition, the incidence of multidrug resistance in females was higher than that in males. These phenomena may be related to hormones, genetic differences, and the frequency of antibiotic use. For levofloxacin and clarithromycin, the younger population, aged ≤ 20-years-old, had a similar medication background. For the population aged ≥ 51-years-old, the physical fitness and immunity of individuals decreases with age, and medications increase and vary. Due to the accumulation of medication over the years, the difference between female and male decreases, while the difference between age group increases. For the population in the middle-aged 21-50 group, their physical fitness is relatively healthy, and their immunity is strong. Therefore, the use of antibacterial agents will affect the resistance rates in H. pylori. Some research indicates that for some less serious infections, such as colds, females are relatively more willing to receive antibiotic treatment, while males are often accustomed to relying on their own immunity. Therefore, female medication habits may be one of the reasons why the resistance rate in females is higher than that in males[28].
Resistance to antibacterial drugs poses a huge challenge to H. pylori eradication therapy. Experts from all over the world have jointly issued some consensus reports, and a number of consensus reports have been published based on the resistance rates of H. pylori in China. The “Fifth Chinese National Consensus Report on the management of H. pylori infection” (“Fifth Chinese National Consensus Report”) indicated that quadruple therapy containing clarithromycin and metronidazole was not recommended in empirical therapy when the dual-drug resistance rate is over 15.00%[29]. In Taizhou city, the dual-drug resistance rate to clarithromycin and metronidazole is 11.16%, although resistance rates to each drug individually are high. Furthermore, the “Fifth Chinese National Consensus Report”, published in December 2016, proposed that treatment therapy containing levofloxacin was not recommended for initial H. pylori eradication because of its high resistance rate[28]. Following the consensus recommendations, the resistance rate to levofloxacin decreased slightly and remained stable after 2017 in Taizhou, which was an obvious result.
The purpose of this study is to study the prevalence and drug resistance of H. pylori. The detection methods for H. pylori infection include microbial culture, pathological testing, 13C urease breath test, nucleic acid testing, etc., and the combined detection of multiple methods can improve the detection rate of H. pylori. However, some of these methods were not widely and continuously used in the early stages of this study. In this paper, we mainly analyzed the data obtained through microbial culture, which can reflect the changes of H. pylori prevalence to a certain extent.
The prevalence and resistance of H. pylori in Taizhou city are serious. The prevalence of H. pylori increased with age, and the male was at a higher risk of H. pylori prevalence. The resistance rate increased with age, and H. pylori in females, age 21-years-old to 50-years-old, was at a higher risk of resistance to levofloxacin and clarithromycin. Guided by the consensus report, individualized treatment based on an AST is recommended.
The trend of Helicobacter pylori (H. pylori) prevalence and antibacterial drug resistance is getting more serious.
To provide guidance on the use of antimicrobial drugs for H. pylori eradication treatment based on the trend of antimicrobial resistance.
Big data-based research of H. pylori prevalence and antimicrobial resistance trends in Taizhou, Zhejiang Province were performed.
Carried out the statistical analysis of the results of gastric mucosal tissue sample culture and drug susceptibility tests in Taizhou in the past 10 years, and explored the differences between different age groups and gender of the H. pylori prevalence and antibacterial drug resistance rates.
The prevalence of H. pylori increased with age, and males were at a higher risk of H. pylori prevalence. The resistance rate increased with age, and H. pylori in females, age 21-years-old to 50-years-old, were at a higher risk of resistance to levofloxacin and clarithromycin.
The prevalence and resistance of H. pylori in Taizhou city are serious.
Guided by the consensus report, individualized treatment based on an AST is recommended.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Apiratwarakul K, Megraud F, Mohammadi M S-Editor: Liu M L-Editor: Filipodia P-Editor: Yu HG
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL; Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1756] [Article Influence: 292.7] [Reference Citation Analysis (0)] |
| 3. | de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1319] [Article Influence: 263.8] [Reference Citation Analysis (0)] |
| 4. | Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2051] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 6. | Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (1)] |
| 7. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 553] [Article Influence: 61.4] [Reference Citation Analysis (2)] |
| 8. | Jin DC, Jiang P, Guo S, Wu SF, Wang Y, Xu K, Qin L. Epidemiological Features of Helicobacter pylori in Chinese Children and adolescents: A Narrative Review. Yixue Yanjiu Zazhi. 2020;49:146-151. [DOI] [Full Text] |
| 9. | Ji Z, Han F, Meng F, Tu M, Yang N, Zhang J. The Association of Age and Antibiotic Resistance of Helicobacter Pylori: A Study in Jiaxing City, Zhejiang Province, China. Medicine (Baltimore). 2016;95:e2831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Shang H, Wang YS, Shen ZY. National guide to clinical laboratory procedures. 4th ed. China: People's Medical Publishing House, 2014. |
| 11. | Li L, Ke Y, Yu C, Li G, Yang N, Zhang J, Li Y. Antibiotic resistance of Helicobacter pylori in Chinese children: A multicenter retrospective study over 7 years. Helicobacter. 2017;22:e12373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Yang NM, Meng F, Xu SH, Jiang Y, Li HZ, Zhang XF, Guo F, Wu JS, Li WP, Ji ZZ, Ye LP, Pan J, Chen GL, Ye B, Mao JL, Lin L, Zhang JK, Wang S, Ouyang H, Zhu XJ, Lyu LH, Yang JH, Shi ZC, Lin CP, Xu F, Wang QY, Mao JB, Li YM. Therapeutic strategies based on clinical big data of antibiotic resistance monitoring of Helicobacter pylori in Zhejiang province. Zhonghua Xiaohua Neijing Zazhi. 2016;33:738-742. [DOI] [Full Text] |
| 13. | Xiao HQ, Lu TT, Wang CL, Zheng BX, Lian M, Wu HY, Jin Y, Liu ZF. Comparison of Three Detection Methods in Detection of Helicobacter Pylori Infection Rate in Children. Erke Yaoxue Zazhi. 2018;24:19-22. [DOI] [Full Text] |
| 14. | Wang JC, Huang DD, Xu XZ, Lin M, Liu QX, Yang NM, Meng F, Wu JS. The value of combined sampling at multiple sites of gastric mucosa for Helicobacter pylori culture. Zhonghua Xiaohua Neijing Zazhi. 2017;34:200-202. [DOI] [Full Text] |
| 15. | Saniee P, Shahreza S, Siavoshi F. Negative Effect of Proton-pump Inhibitors (PPIs) on Helicobacter pylori Growth, Morphology, and Urease Test and Recovery after PPI Removal--An In vitro Study. Helicobacter. 2016;21:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Zhang LH, Zhou YN, Zhang ZY, Zhang FH, Li GZ, Li Q, Wu ZQ, Ren BL, Zou SJ, Wang JX. [Epidemiological study on status of Helicobacter pylori in children and teenagers in Wuwei city, Gansu province]. Zhonghua Yi Xue Za Zhi. 2009;89:2682-2685. [PubMed] |
| 17. | Shu X, Ping M, Yin G, Jiang M. Investigation of Helicobacter pylori infection among symptomatic children in Hangzhou from 2007 to 2014: a retrospective study with 12,796 cases. PeerJ. 2017;5:e2937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1186] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 19. | Dutta AK, Reddy VD, Iyer VH, Unnikrishnan LS, Chacko A. Exploring current status of Helicobacter pylori infection in different age groups of patients with dyspepsia. Indian J Gastroenterol. 2017;36:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Brenner H, Rothenbacher D, Bode G, Adler G. Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ. 1997;315:1489-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Liu LC, Wang LY, Wang X, Chen XX, Xu B, Yu YX. Epidemiological survey on smoking and passive smoking situation among adults in Taizhou City, Zhejiang Province. Zhongguo Jiankang Jiaoyu. 2013;29:341-344. [DOI] [Full Text] |
| 22. | Jiang ZD, He BS, Zhang ZY, Wang SK, Ran D, Wang ZB. Analysis of the Primary and Post-Treatment Antibiotic Resistance of Helicobacter pylori in the Nanjing Area. Curr Pharm Biotechnol. 2021;22:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Hu Y, Zhu Y, Lu NH. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front Cell Infect Microbiol. 2017;7:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Shao Y, Lu R, Yang Y, Xu Q, Wang B, Ye G. Antibiotic resistance of Helicobacter pylori to 16 antibiotics in clinical patients. J Clin Lab Anal. 2018;32:e22339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 25. | Mirzaei N, Poursina F, Faghri J, Talebi M, Khataminezhad MR, Hasanzadeh A, Safaei HG. Prevalence of Resistance of Helicobacter pylori Strains to Selected Antibiotics in Isfahan, Iran. Jundishapur J Microbiol. 2013;6:e6342. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Ben Mansour K, Burucoa C, Zribi M, Masmoudi A, Karoui S, Kallel L, Chouaib S, Matri S, Fekih M, Zarrouk S, Labbene M, Boubaker J, Cheikh I, Hriz MB, Siala N, Ayadi A, Filali A, Mami NB, Najjar T, Maherzi A, Sfar MT, Fendri C. Primary resistance to clarithromycin, metronidazole and amoxicillin of Helicobacter pylori isolated from Tunisian patients with peptic ulcers and gastritis: a prospective multicentre study. Ann Clin Microbiol Antimicrob. 2010;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Shu X, Yin G, Liu M, Peng K, Zhao H, Jiang M. Antibiotics resistance of Helicobacter pylori in children with upper gastrointestinal symptoms in Hangzhou, China. Helicobacter. 2018;23:e12481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Roe CM, McNamara AM, Motheral BR. Gender- and age-related prescription drug use patterns. Ann Pharmacother. 2002;36:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB; Chinese Medical Association, Chinese Society of Gastroenterology. H. pylori and Peptic Ulcer Study Group, Chinese National Helicobacter Pylori Research Cooperative Group. Fifth ChinesNational Consensus Report on the management of Helicobacter pylori infection. Wei Chang Bing Xue. 2017;37:364-378. [DOI] [Full Text] |