Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10816
Peer-review started: January 28, 2021
First decision: June 15, 2021
Revised: June 27, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 16, 2021
Processing time: 315 Days and 16.5 Hours
Carotid artery cross-clamping during carotid endarterectomy (CEA) may damage local cerebral perfusion and induce cerebral ischemia–reperfusion injury to activate local inflammatory responses. Neutrophil-to-lymphocyte ratio (NLR) is an indicator that reflects systemic inflammation. However, the correlation between NLR and complications after CEA remains unclear.
To investigate the association between NLR and major complications after surgery in patients undergoing CEA.
This retrospective cohort study included patients who received CEA between January 2016 and July 2018 at Beijing Tiantan Hospital. Neutrophil and lymphocyte counts in whole blood within 24 h after CEA were collected. The primary outcome was the composite of major postoperative complications including neurological, pulmonary, cardiovascular and acute kidney injuries. The secondary outcomes included infections, fever, deep venous thrombosis, length of hospitalization and cost of hospitalization. Statistical analyses were performed using EmpowerStats software and R software.
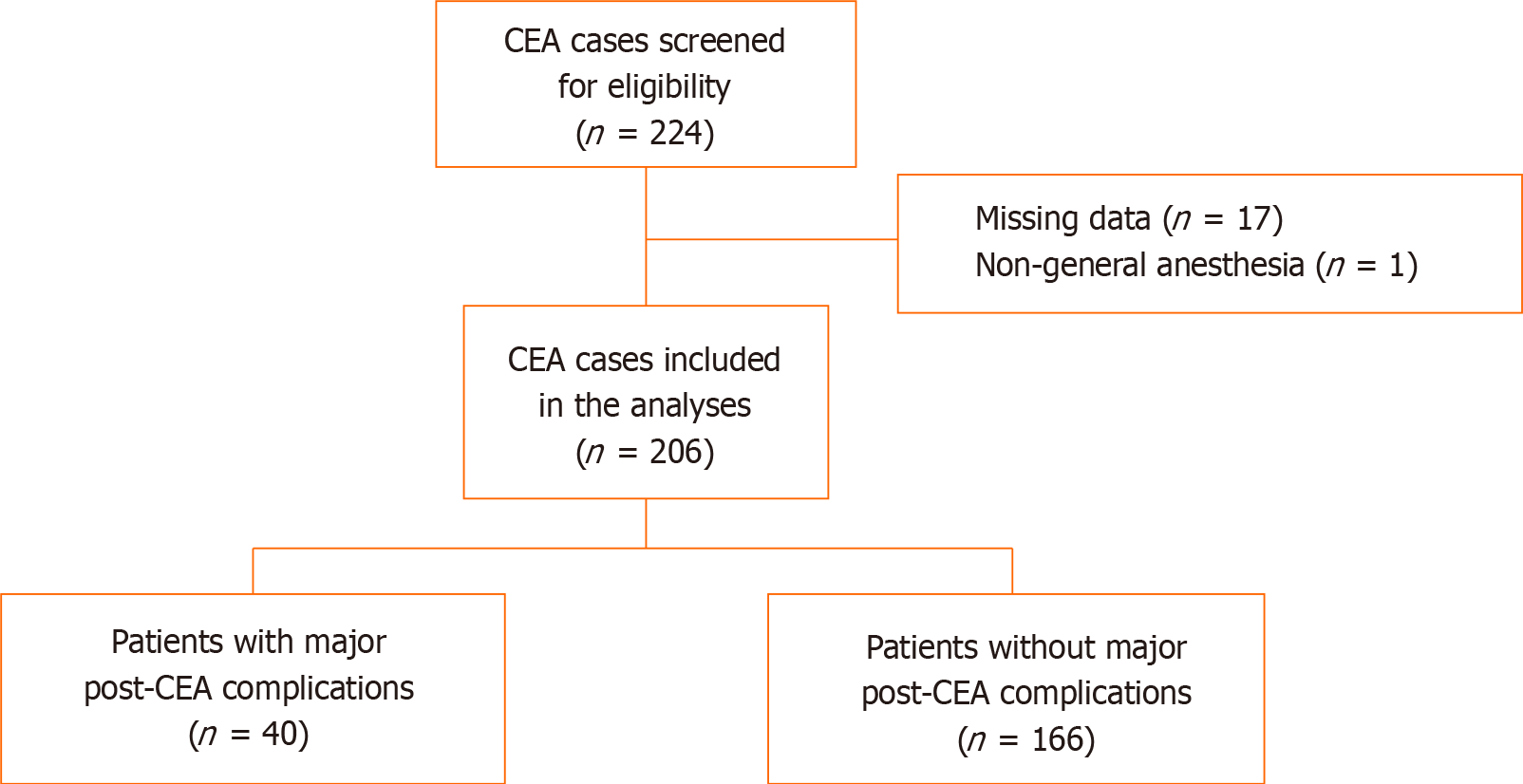
A total of 224 patients who received CEA were screened for review and 206 were included in the statistical analyses; of whom, 40 (19.42%) developed major postoperative complications. NLR within 24 h after CEA was significantly correlated with major postoperative complications (P = 0.026). After confounding factors were adjusted, the odds ratio was 1.15 (95%CI: 1.03–1.29, P = 0.014). The incidence of major postoperative complications in the high NLR group was 8.47 times that in the low NLR group (P = 0.002).
NLR is associated with major postoperative complications in patients undergoing CEA.
Core tip: We retrospectively evaluated the association between neutrophil-to-lymphocyte ratio (NLR) and major postoperative complications in patients undergoing carotid endarterectomy (CEA). Nearly 20% of patients developed major postoperative complications. NLR within 24 h after CEA was significantly correlated with major postoperative complications. The incidence of major postoperative complications in the high NLR group was 8.47 times that in the low NLR group after confounding factors were adjusted. Since early detection and early treatment help improve outcomes for CEA, inflammatory markers such as NLR may also become potential treatment targets.
- Citation: Yu Y, Cui WH, Cheng C, Lu Y, Zhang Q, Han RQ. Association between neutrophil-to-lymphocyte ratio and major postoperative complications after carotid endarterectomy: A retrospective cohort study. World J Clin Cases 2021; 9(35): 10816-10827
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10816.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10816
Carotid endarterectomy (CEA) is a classic surgical method for treating carotid artery stenosis. Occlusion and opening of the carotid artery during CEA may damage local cerebral perfusion and induce cerebral ischemia–reperfusion injury to activate local inflammatory responses[1]. Even after CEA, inflammatory responses in the whole body and carotid plaque tissue may still exist. Serum inflammatory and anti-inflammatory cytokines increase at 6 to 24 h after CEA. Compared with asymptomatic patients, patients with symptomatic carotid artery stenosis have higher concentrations of inflammatory markers in serum and tissues[2]. The elevation of perioperative inflammatory markers suggests an increase in the risk of early carotid artery restenosis after CEA[3]. Inflammatory markers can also become treatment targets[4].
Neutrophil-to-lymphocyte ratio (NLR) is an indicator that reflects systemic inflammation, which has been demonstrated to be an independent and convenient predictor of all-cause death or adverse events in many diseases[5-8]. Endothelial dysfunction is the early stage of atherosclerosis formation[9]. NLR is positively correlated with carotid intima–media thickness, and an increase in NLR may be associated with endothelial dysfunction[10]. NLR > 2.6 is an independent predictor of symptomatic carotid artery disease[11]. In patients receiving CEA for significant carotid artery stenosis, NLR is significantly correlated with the characteristics of vulnerable atherosclerotic carotid plaques on preoperative magnetic resonance angiography[12]. However, the correlation between NLR and complications after CEA remain unclear.
Therefore, we undertook this study to clarify whether NLR was significantly associated with major organ dysfunction after surgery in patients undergoing CEA.
This single-center retrospective cohort study was approved by the Ethics Committee of Beijing Tiantan Hospital (KY2017-024-01). Given the retrospective nature of the study, the Ethics Committee waived the need for written informed consent and no registration was required. Consecutive patients who underwent elective CEA between January 2016 and July 2018 at Beijing Tiantan Hospital were screened for eligibility. Characteristics of the patients at baseline, neuroimaging data, intraoperative anesthesia management, postoperative complications and length of hospitalization were acquired from the medical record system. Patients were excluded for the following reasons: incomplete data obtained from medical records; severe anemia (hemoglobin < 9 g/dL) before surgery; nongeneral anesthesia; and massive hemorrhage during surgery.
The method for anesthesia involved intravenous–inhalation anesthesia or total intravenous anesthesia. Intraoperative fluid management involving crystalloids, colloids, blood loss and urine output was collected. Intraoperative blood pressure fluctuations were addressed as follows. The noninvasive blood pressure of the upper limbs was measured and recorded every 5 min during surgery. The mean systolic blood pressure (meanSBP), SD of systolic blood pressure (SDSBP), mean diastolic blood pressure (meanDBP), and SD of diastolic blood pressure (SDDBP) from entering to exiting the operating room were calculated to obtain the coefficient of variation in systolic blood pressure (CVSBP) and diastolic blood pressure (CVDBP). The coefficient of variation = SD/mean value × 100%[13]. Besides, intraoperative vasoactive drugs use including vasopressors and antihypertensive agents was also collected.
Complete blood count (CBC) was collected at admission and repeated after surgery. Neutrophil and lymphocyte counts in whole blood at admission and within 24 h after CEA were extracted from the medical record system. Preoperative basal NLR values and that within 24 h after CEA were calculated. By using the North American Symptomatic Carotid Endarterectomy Trial criteria, the degree of carotid artery stenosis was independently measured by two trained radiologists blinded to clinical data[14].
The composite risk of major postoperative complications was adopted as the primary outcome, similar to those used in previous studies[15-17]. Major postoperative complications included neurological, pulmonary and cardiovascular complications and acute kidney injury (AKI). Neurological complications were defined by new focal neurological deficits confirmed by radiology. Pulmonary complications were defined by a new-onset requirement for oxygen or respiratory support[15]. Cardiovascular complications included new-onset myocardial infarction validated by cardiac enzymes, atrial or ventricular arrhythmias and heart failure. According to the Kidney Disease Improving Global Outcomes, AKI was defined as an increase in serum creatinine > 0.3 mg/dL within 48 h after surgery or serum creatinine value 1.5-fold the preoperative baseline value[18]. If the patient had one or more of the above complications, development of major postoperative complications was considered. No assumptions were made to process missing data, and statistical analyses were conducted for patients with complete data.
Secondary outcomes included fever, surgical site infections, urinary infections, deep venous thrombosis (DVT), length of stay in the intensive care unit (ICU), length of hospitalization and cost of hospitalization. A postoperative fever was considered if the axillary temperature was > 38°C. Surgical site infections were determined if wound cultures were positive. Urinary infections were defined as typical symptoms and signs confirmed by routine urine tests. DVT was diagnosed using the color Doppler ultrasound.
Statistical analyses were performed using EmpowerStats software and R software (R version 3.4.3). Continuous variables were examined using the independent-samples t-test or Kruskal–Wallis test and expressed as mean ± s or median (interquartile range). Analysis of categorical variables was performed using the χ2test and presented as a percentage. After adjusting the confounding factors, smooth curve fitting was used for analyzing the relationship between NLR and post-CEA major complications. By logistic regressions, odds ratios (ORs) and 95%CIs were calculated to assess the association of NLR within 24 h after surgery with postoperative major complications. Model I was adjusted for sex and age. Model II was adjusted for sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) grade, preoperative combined heart disease, anesthesia method, degree of stenosis on the surgical side, degree of stenosis on the contralateral side, operating time, intraoperative intake and output, duration of carotid artery occlusion, CVSBP, and CVDBP. P < 0.05 indicated that a difference had statistical significance.
This retrospective cohort study screened 224 patients. A total of 17 patients did not have CBC on postoperative day 1 and one patient underwent CEA with cervical plexus block. Therefore, 18 patients were excluded and the data for 206 patients were included in the statistical analyses (Figure 1).
Patients were divided into a group with major post-CEA complications (PC group) and a group without major post-CEA complications (WOPC group) according to whether major PC group were present. Baseline characteristics in the two groups are provided in Table 1. The percentage of patients with combined preoperative heart diseases in the PC group (40.0%) was significantly higher than that in the WOPC group (24.10%, P = 0.042). The differences in age, sex, BMI, degree of carotid artery stenosis on the surgical side, degree of carotid artery stenosis on the contralateral side, anesthesia method, duration of carotid artery occlusion, intraoperative intake and output volume, intraoperative blood pressure fluctuation, and use of vasoactive drugs between the two groups were all nonsignificant.
| Total | WOPC group | PC group | P | |
| No. of cases | 206 | 166 | 40 | |
| Age (yr) | 62.0 ± 7.2 | 61.6 ± 7.3 | 63.5 ± 6.8 | 0.151 |
| Sex | 0.616 | |||
| Male | 175 (84.95%) | 140 (84.34%) | 35 (87.50%) | |
| Female | 31 (15.05%) | 26 (15.66%) | 5 (12.50%) | |
| BMI (kg/m2) | 25.33 ± 2.89 | 25.25 ± 2.85 | 25.63 ± 3.04 | 0.461 |
| Smoking history | 118 (57.56%) | 97 (58.79%) | 21 (52.50%) | 0.470 |
| ASA grade | 0.521 | |||
| Grade II | 157 (81.07%) | 136 (81.93%) | 31 (77.50%) | |
| Grade III | 39 (18.93%) | 30 (18.07%) | 9 (22.50%) | |
| Preoperative combined diseases | ||||
| Hypertension | 146 (70.87%) | 114 (68.67%) | 32 (80.0%) | 0.157 |
| Diabetes mellitus | 74 (35.92%) | 61 (36.75%) | 13 (32.50%) | 0.615 |
| Heart disease | 56 (27.18%) | 40 (24.10%) | 16 (40.0%) | 0.042a |
| Respiratory disease | 11 (5.34%) | 10 (6.02%) | 1 (2.50%) | 0.374 |
| Neurological disease | 96 (46.60%) | 78 (46.99%) | 18 (45.0%) | 0.821 |
| Kidney disease | 4 (1.94%) | 4 (2.41%) | 0 | 0.321 |
| Degree of carotid artery stenosis on the surgical side | 0.449 | |||
| Mild/moderate stenosis | 3 (1.46%) | 2 (1.20%) | 1 (2.5%) | |
| Severe stenosis | 201 (97.57%) | 163 (98.19%) | 38 (95.0%) | |
| Occlusion | 2 (0.97%) | 1 (0.60%) | 1 (2.50%) | |
| Degree of carotid artery stenosis on the contralateral side | 0.146 | |||
| Mild/moderate stenosis | 169 (85.35%) | 132 (83.02%) | 37 (94.87%) | |
| Severe stenosis | 21 (10.61%) | 19 (11.95%) | 2 (5.13%) | |
| Occlusion | 8 (4.04%) | 8 (5.03%) | 0 | |
| Operating time (min) | 141.33 ± 40.96 | 138.39 ± 38.78 | 153.57 ± 47.62 | 0.129 |
| Duration of carotid artery occlusion (min) | 22.00 (18.0–44.0) | 22.00 (17.0–43.0) | 27.0 (18.50–49.50) | 0.328 |
| Anesthesia method | ||||
| TIVA | 163 (79.13%) | 129 (77.71%) | 34 (85.0%) | 0.309 |
| Combined intravenous–inhalation anesthesia | 43 (20.87%) | 37 (22.29%) | 6 (15.0%) | |
| Intraoperative intake and output volume (mL) | 897.57 ± 333.40 | 911.85 ± 331.01 | 839.75 ± 341.01 | 0.221 |
| meanSBP | 128.82 ± 12.92 | 128.37 ± 12.77 | 130.69 ± 13.54 | 0.309 |
| SDSBP | 20.26 ± 4.91 | 20.09 ± 5.01 | 20.93 ± 4.48 | 0.333 |
| CVSBP | 0.16 ± 0.04 | 0.16 ± 0.04 | 0.16 ± 0.03 | 0.550 |
| meanDBP | 69.61 ± 7.83 | 69.80 ± 7.77 | 68.82 ± 8.14 | 0.480 |
| SDDBP | 10.23 ± 2.70 | 10.18 ± 2.81 | 10.42 ± 2.26 | 0.612 |
| CVDBP | 0.15 ± 0.04 | 0.15 ± 0.04 | 0.15 ± 0.04 | 0.372 |
| Intraoperative use of vasopressors | 84 (40.78%) | 65 (39.16%) | 19 (47.50%) | 0.335 |
| Intraoperative use of antihypertensive agents | 73 (35.44%) | 63 (37.95%) | 10 (25.0%) | 0.124 |
Outcome variables stratified by major postoperative complications are shown in Table 2. After CEA, 16 patients (7.77%) developed neurological complications, six (2.91%) developed cardiac complications, 14 (6.80%) developed respiratory complications, and nine (4.37%) developed renal complications. A total of 40 patients (19.42%) developed major postoperative complications. The incidence of fever, surgical site infections, urinary infections and DVT was 4.85%, 1.46%, 1.46% and 2.43%, respectively. The cost of hospitalization in the PC group was significantly higher than that in the WOPC group (P < 0.001). More patients in the PC group suffered from fever and DVT (P < 0.001). The differences in the length of stay in the ICU, the length of hospitalization, surgical site infections and urinary infections were not significant.
| Total | WOPC group | PC group | P | |
| No. of cases | 206 | 166 | 40 | |
| Major postoperative complications | 40 (19.42%) | 0 | 40 (100%) | |
| Neurological complications | 16 (7.77%) | 0 | 16 (40.0%) | < 0.001a |
| Cardiac complications | 6 (2.91%) | 0 | 6 (15.0%) | < 0.001a |
| Respiratory complications | 14 (6.80%) | 0 | 14 (35.0%) | < 0.001a |
| AKI | 9 (4.37%) | 0 | 9 (22.50%) | < 0.001a |
| Fever | 10 (4.85%) | 4 (2.41%) | 6 (15.0%) | < 0.001a |
| Surgical site infections | 3 (1.46%) | 1 (0.60%) | 2 (5.0%) | 0.097 |
| Urinary infections | 3 (1.46%) | 1 (0.60%) | 2 (5.0%) | 0.097 |
| DVT | 5 (2.43%) | 1 (0.60%) | 4 (10.0%) | < 0.001a |
| Length of stay in the ICU (d) | 1.0 (0–1.0) | 1.0 (0–1.0) | 1.0 (1.0–1.0) | 0.055 |
| Hospitalization stay (d) | 15.56 ± 4.30 | 15.16 ± 3.63 | 17.20 ± 6.17 | 0.105 |
| Cost of hospitalization (CNY) | 24085.15 (21694.72–28395.65) | 23786.67 (21568.68–27139.36) | 27127.94 (22326.75–31629.55) | < 0.001a |
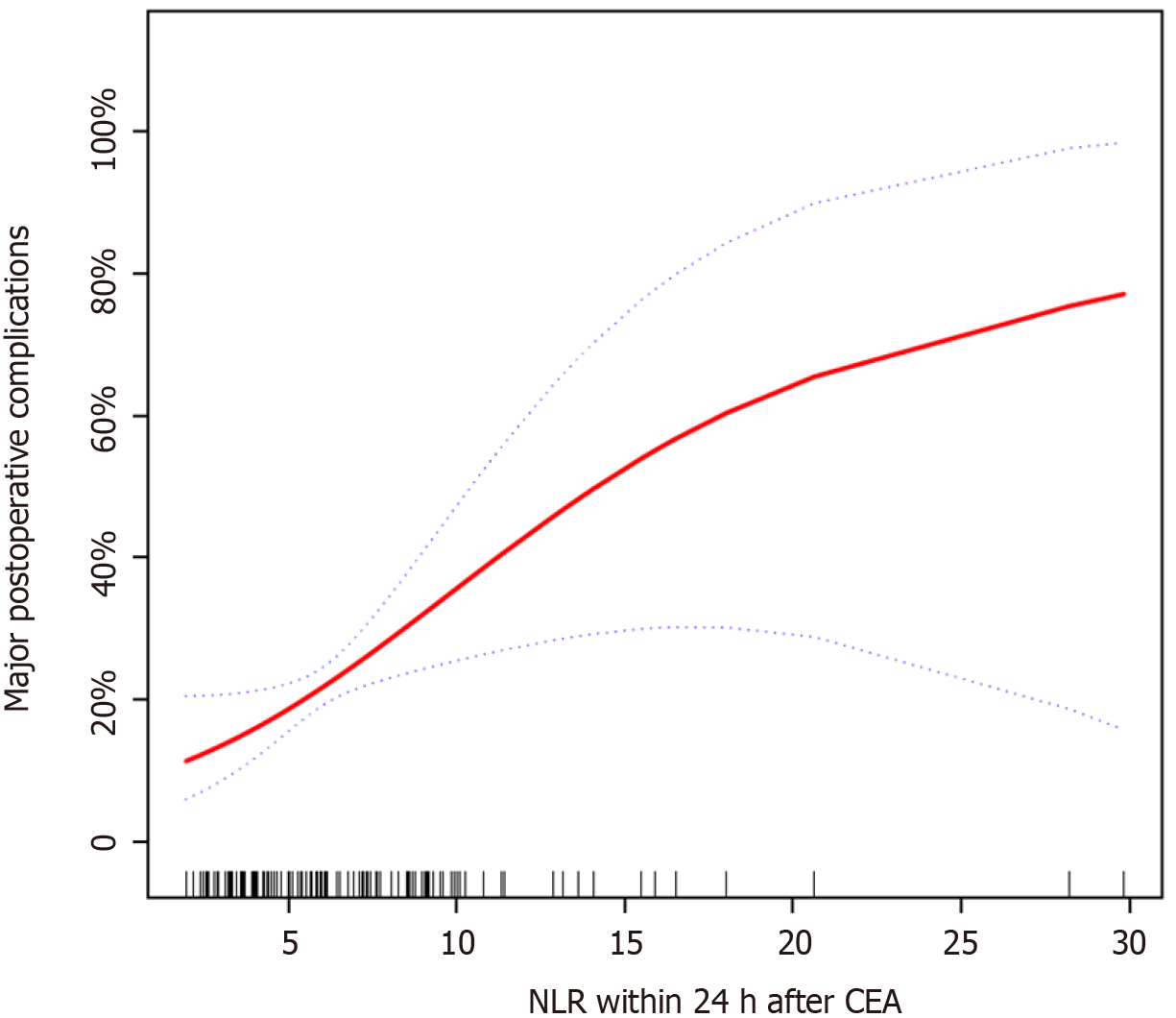
The risk factors associated with post-CEA complications involving vital organs are presented in Table 3. Operating time was significantly correlated with major post-CEA complications (P = 0.038). NLR within 24 h after CEA was also significantly correlated with post-CEA complications (P = 0.026). Figure 2 showed the correlation between NLR within 24 h after CEA and major postoperative complications. NLR within 24 h after CEA and major postoperative complications showed a curvilinear relationship (P = 0.025, degree of freedom = 1.495). With the increase in NLR within 24 h after CEA, the incidence of major postoperative complications gradually increased.
| Statistical value | Post-CEA major complications | P | |
| Age (yr) | 62.0 ± 7.2 | 1.04 (0.99, 1.09) | 0.151 |
| Sex | 0.616 | ||
| Male | 175 (84.95%) | 1.0 | |
| Female | 31 (15.05%) | 0.77 (0.28, 2.15) | |
| BMI (kg/m2) | 25.33 ± 2.89 | 1.05 (0.93, 1.17) | 0.459 |
| ASA grade | 0.522 | ||
| Grade II | 167 (81.07%) | 1.0 | |
| Grade III | 39 (18.93%) | 1.32 (0.57, 3.05) | |
| Anesthesia methods | 0.312 | ||
| TIVA | 163 (79.13%) | 1.0 | |
| Combined intravenous–inhalation anesthesia | 43 (20.87%) | 0.62 (0.24, 1.58) | |
| Degree of carotid artery stenosis on the surgical side | |||
| Mild/moderate stenosis | 3 (1.46%) | 1.0 | |
| Severe stenosis | 201 (97.57%) | 0.47 (0.04, 5.28) | 0.538 |
| Occlusion | 2 (0.97%) | 2.00 (0.05, 78.25) | 0.711 |
| Degree of carotid artery stenosis on the contralateral side | |||
| Mild/moderate stenosis | 169 (85.35%) | 1.0 | |
| Severe stenosis | 21 (10.61%) | 0.38 (0.08, 1.69) | 0.201 |
| Occlusion | 8 (4.04%) | 0 (0, Inf) | 0.986 |
| Operating time (min) | 141.33 ± 40.96 | 1.01 (1.0, 1.02) | 0.038a |
| Intraoperative intake and output volume (mL) | 897.57 ± 333.40 | 1.00 (1.0, 1.00) | 0.221 |
| Duration of carotid artery occlusion | 22.0 (18.0–44.0) | 1.01 (0.99, 1.03) | 0.327 |
| CVSBP | 0.16 ± 0.04 | 16.36 (0.00, 150500.70) | 0.548 |
| CVDBP | 0.15 ± 0.04 | 40.21 (0.01, 132798.95) | 0.372 |
| Preoperative NLR | 2.08 ± 0.94 | 1.21 (0.87, 1.69) | 0.264 |
| NLR within 24 h after CEA | 5.68 (3.93–8.91) | 1.09 (1.01, 1.17) | 0.026a |
Multiple logistic regression showed that NLR within 24 h after CEA and major postoperative complications were correlated (Table 4). After confounding factors were adjusted, the OR = 1.15 (95%CI: 1.03–1.29, P = 0.014). The patients were divided into three groups according to their NLR tertiles within 24 h after CEA; namely, high NLR group (7.66–29.85), middle NLR (4.63–7.65), and low NLR (1.61–4.62). The incidence of post-CEA complications involving vital organs in the high NLR group was 8.47 times that in the low NLR group (P = 0.002). The differences in major postoperative complications (P = 0.015), fever (P = 0.040) and cost of hospitalization (P = 0.032) were significant among NLR tertile groups (Table 5).
| Exposure factors | Unadjusted | P value | Model I | P value | Model II | P |
| NLR | 1.09 (1.01, 1.17) | 0.026a | 1.08 (1.00, 1.16) | 0.041a | 1.15 (1.03, 1.29) | 0.014a |
| NLR tertile groups | ||||||
| Low NLR group | 1.0 | 1.0 | 1.0 | |||
| Middle NLR group | 1.60 (0.63, 4.09) | 0.322 | 1.52 (0.59, 3.92) | 0.386 | 3.99 (1.03, 15.49) | 0.046a |
| High NLR group | 2.92 (1.21, 7.02) | 0.017a | 2.88 (1.17, 7.09) | 0.022a | 8.47 (2.20, 32.63) | 0.002a |
| Total | Low NLR group | Middle NLR group | High NLR group | P | |
| No. of cases | 206 | 68 | 69 | 69 | |
| Major postoperative complications | 40 (19.42%) | 8 (11.76%) | 11 (15.94%) | 21 (30.43%) | 0.015a |
| Neurological complications | 16 (7.77%) | 4 (5.88%) | 4 (5.80%) | 8 (11.59%) | 0.346 |
| Cardiac complications | 6 (2.91%) | 0 (0.00%) | 3 (4.35%) | 3 (4.35%) | 0.218 |
| Respiratory complications | 14 (6.80%) | 2 (2.94%) | 5 (7.25%) | 7 (10.14%) | 0.242 |
| AKI | 9 (4.37%) | 2 (2.94%) | 1 (1.45%) | 6 (8.70%) | 0.089 |
| Fever | 10 (4.85%) | 1 (1.47%) | 2 (2.90%) | 7 (10.14%) | 0.040a |
| Surgical site infections | 3 (1.46%) | 1 (1.47%) | 1 (1.45%) | 1 (1.45%) | 1.000 |
| Urinary infections | 3 (1.46%) | 0 (0.00%) | 1 (1.45%) | 2 (2.90%) | 0.367 |
| DVT | 5 (2.43%) | 2 (2.94%) | 1 (1.45%) | 1 (1.45%) | 0.766 |
| Length of stay in the ICU (d) | 1.0 (0–1.0) | 1.0 (0–1.0) | 1.0 (0–1.0) | 1.0 (1.0–1.0) | 0.079 |
| Hospitalization stay (d) | 15.56 ± 4.30 | 15.44 ± 4.40 | 15.28 ± 4.27 | 15.96 ± 4.26 | 0.627 |
| Cost of hospitalization (CNY) | 26886.26 ± 11277.49 | 24371.70 ± 5233.73 | 26837.07 ± 12681.34 | 29413.56 ± 13520.49 | 0.032a |
This study showed that 19.42% of patients developed major postoperative complications involving the neurological, cardiac and respiratory systems as well as AKI. NLR within 24 h after CEA was significantly correlated with major postoperative complications. The incidence of major postoperative complications in the high NLR group was much higher than that of in the low NLR group after confounding factors were adjusted.
For complications involving the neurological system, NLR can predict and affect clinical outcomes of stroke. Neutrophils are the first cells that invade injured tissues after focal cerebral ischemia. Their proinflammatory feature enhances tissue injury and may cause cerebral ischemia through the induction of thrombosis. Therefore, inflammatory markers may be potential targets for the treatment and prevention of stroke[19]. Within 48–72 h after acute ischemic stroke, patients with NLR ≥ 4.58 were 5.58 times more likely to have a poor outcome than patients with NLR < 4.58[20]. NLR independently predicted 3-month neurological outcomes and symptomatic intrac
A considerable proportion of patients with carotid stenosis also have coronary heart disease. NLR is considered a potential indicator of cardiovascular events. Durmuş et al[24] studied the relationship between NLR and the development of myocardial injury after noncardiac surgery (MINS), which showed that NLR in the MINS group was significantly higher than that in the non-MINS group[24]. For coronary artery disease patients with low high-sensitivity C-reactive protein levels, the elevation of NLR levels could independently predict their long-term outcomes[25]. One post hoc analysis studied patients with coronary heart disease who underwent noncardiac surgery. The results showed that NLR was significantly correlated with major adverse cardiovascular and cerebrovascular events, which were defined as the composite endpoint of death, myocardial ischemia, myocardial infarction, MINS, or embolic or thrombotic stroke within 30 d after surgery[5]. Systemic inflammation plays a critical role in the pathogenesis of cardiovascular diseases. Preoperative NLR > 4 was associated with perioperative myocardial injury (OR = 2.56), indicating that systemic inflammation might be associated with the development of perioperative myocardial injury[26].
Elevated NLR on postoperative day 2 was significantly correlated with higher in-hospital mortality, pneumonia, ICU readmission and prolonged ICU stay after cardiac surgery[27]. A study by Lee et al[28] showed that NLR in pneumonia patients in the ICU was significantly higher than that in pneumonia patients in a ward and healthy controls. Compared with the C-reaction protein level, NLR might be a better indicator for evaluating the severity of pneumonia[28]. Another study also proved that NLR was significantly correlated with the pneumonia severity index[29]. Nam et al[30] confirmed that a higher NLR could predict stroke-associated pneumonia in patients with acute ischemic stroke. Moreover, NLR was higher in patients with severe pneumonia[30]. Feng et al[31] studied patients on mechanical ventilation for > 72 h and showed that NLR levels could be used to assess risk factors for mortality caused by ventilator-associated pneumonia[31].
AKI results from a complex interaction between hemodynamic, toxic and inflammatory factors[32]. Long-term follow-up showed that NLR was an independent predictor of kidney function decline among individuals with diabetes and prediabetes[33,34]. The elevation of NLR immediately after cardiac surgery and on postoperative day 1 was associated with an increased risk of postoperative AKI and 1-year mortality; NLR could assist with the risk stratification of AKI and mortality in high-risk surgical patients[7,35,36]. High NLR levels were associated with increased risks of 30- and 90-day mortality in AKI patients; compared with the lower NLR group (NLR < 5.55), the hazard ratio in the higher NLR group (NLR > 12.14) was 1.37[37]. One recent systematic review and meta-analysis showed that when NLR was used to predict AKI, the sensitivity was 0.736, and the specificity was 0.686, indicating that NLR was a reliable biomarker for the early detection of AKI[38]. One prospective study evaluated the accuracy of a single emergency department measurement of NLR for the early diagnosis of AKI. The results showed that compared with normal controls, patients with AKI had a higher NLR. When the NLR cut-off value was 0.55, the sensitivity was 0.78, the specificity was 0.65, and the OR was 6.423[39].
This study had several limitations. First, the small sample size and the low event rates might have increased the probability of committing a type II error and thus decrease the power of a hypothesis test. Second, this was a retrospective cohort study. The authenticity and completeness of medical records directly affected the reliability of the results.
NLR within 24 h after CEA was associated with major postoperative complications. The incidence of major postoperative complications in the high NLR group was 8.47 times that in the low NLR group. Future prospective studies are needed for further evaluation.
Carotid artery cross-clamping during carotid endarterectomy (CEA) may induce cerebral ischemia–reperfusion injury to activate local inflammatory responses.
There is no consensus on the correlation between neutrophil-to-lymphocyte ratio (NLR) and complications after CEA.
This study aimed to evaluate the association between NLR and major complications after surgery in patients undergoing CEA.
The demographics, neutrophil and lymphocyte count in whole blood and postoperative outcomes of patients undergoing CEA were retrospectively analyzed.
NLR within 24 h after CEA was significantly correlated with major postoperative complications. The incidence of major postoperative complications in the high NLR group was 8.47 times of that in the low NLR group.
NLR is associated with major postoperative complications in patients undergoing CEA.
Since early detection and early treatment help improve outcomes, inflammatory markers may become potential treatment targets for patients undergoing CEA.
We acknowledge Dr. Xing-Lin Chen (Department of Epidemiology and Biostatistics, Empower U, X&Y solutions Inc., Boston, USA) for her excellent technical assistance in statistics. We also thank Dr. Kai-Ying Zhang (Department of Anesthesiology, The University of Texas Health Science Center at Houston, USA) for her help with English editing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kosuga T, Spartalis M S-Editor: Fan JR L-Editor: Kerr C P-Editor: Wang LYT
| 1. | Kragsterman B, Bergqvist D, Siegbahn A, Parsson H. Carotid Endarterectomy Induces the Release of Inflammatory Markers and the Activation of Coagulation as Measured in the Jugular Bulb. J Stroke Cerebrovasc Dis. 2017;26:2320-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Gabrile SA, Antonangelo L, Capelozzi VR, Beteli CB, DE Camargo O Jr, Braga DE Aquino JL, Caffaro RA. Analysis of the acute systemic and tissue inflammatory response following carotid endarterectomy. Int Angiol. 2016;35:148-156. [PubMed] |
| 3. | Tanaskovic S, Radak D, Aleksic N, Calija B, Maravic-Stojkovic V, Nenezic D, Ilijevski N, Popov P, Vucurevic G, Babic S, Matic P, Gajin P, Vasic D, Rancic Z. Scoring system to predict early carotid restenosis after eversion endarterectomy by analysis of inflammatory markers. J Vasc Surg. 2018;68:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Radak D, Djukic N, Tanaskovic S, Obradovic M, Cenic-Milosevic D, Isenovic ER. Should We be Concerned About the Inflammatory Response to Endovascular Procedures? Curr Vasc Pharmacol. 2017;15:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Larmann J, Handke J, Scholz AS, Dehne S, Arens C, Gillmann HJ, Uhle F, Motsch J, Weigand MA, Janssen H. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc Disord. 2020;20:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Wang X, Fan X, Ji S, Ma A, Wang T. Prognostic value of neutrophil to lymphocyte ratio in heart failure patients. Clin Chim Acta. 2018;485:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Weedle RC, Da Costa M, Veerasingam D, Soo AWS. The use of neutrophil lymphocyte ratio to predict complications post cardiac surgery. Ann Transl Med. 2019;7:778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 9. | Ünlü M, Arslan Z. The Relation Between Neutrophil-Lymphocyte Ratio and Endothelial Dysfunction. Angiology. 2015;66:694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Balta S, Ozturk C, Balta I, Demirkol S, Demir M, Celik T, Iyisoy A. The Neutrophil-Lymphocyte Ratio and Inflammation. Angiology. 2016;67:298-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Bhat TM, Afari ME, Garcia LA. Neutrophil lymphocyte ratio in peripheral vascular disease: a review. Expert Rev Cardiovasc Ther. 2016;14:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Mazzaccaro D, Ambrogi F, Milani V, Modafferi A, Marrocco-Trischitta MM, Malacrida G, Righini P, Nano G. Correlation of Clinical and Ultrasound Variables to Vulnerability of Carotid Plaques in Patients Submitted to Carotid Endarterectomy. Ann Vasc Surg. 2020;67:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Chang JY, Jeon SB, Jung C, Gwak DS, Han MK. Postreperfusion Blood Pressure Variability After Endovascular Thrombectomy Affects Outcomes in Acute Ischemic Stroke Patients With Poor Collateral Circulation. Front Neurol. 2019;10:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2514] [Cited by in RCA: 2268] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 15. | Grocott MP, Browne JP, Van der Meulen J, Matejowsky C, Mutch M, Hamilton MA, Levett DZ, Emberton M, Haddad FS, Mythen MG. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol. 2007;60:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Glousman BN, Sebastian R, Macsata R, Kuang X, Yang A, Patel D, Amdur R, Ricotta J, Sidawy AN, Nguyen BN. Carotid endarterectomy for asymptomatic carotid stenosis is safe in octogenarians. J Vasc Surg. 2020;71:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Manning MW, Cooter M, Mathew J, Alexander J, Peterson E, Ferguson TB Jr, Lopes R, Podgoreanu M. Angiotensin Receptor Blockade Improves Cardiac Surgical Outcomes in Patients With Metabolic Syndrome. Ann Thorac Surg. 2017;104:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Gameiro J, Agapito Fonseca J, Jorge S, Lopes JA. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Ruhnau J, Schulze J, Dressel A, Vogelgesang A. Thrombosis, Neuroinflammation, and Poststroke Infection: The Multifaceted Role of Neutrophils in Stroke. J Immunol Res. 2017;2017:5140679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Petrone AB, Eisenman RD, Steele KN, Mosmiller LT, Urhie O, Zdilla MJ. Temporal dynamics of peripheral neutrophil and lymphocytes following acute ischemic stroke. Neurol Sci. 2019;40:1877-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Duan Z, Wang H, Wang Z, Hao Y, Zi W, Yang D, Zhou Z, Liu W, Lin M, Shi Z, Lv P, Wan Y, Xu G, Xiong Y, Zhu W, Liu X; ACTUAL Investigators. Neutrophil-Lymphocyte Ratio Predicts Functional and Safety Outcomes after Endovascular Treatment for Acute Ischemic Stroke. Cerebrovasc Dis. 2018;45:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Goodson CM, Rosenblatt K, Rivera-Lara L, Nyquist P, Hogue CW. Cerebral Blood Flow Autoregulation in Sepsis for the Intensivist: Why Its Monitoring May Be the Future of Individualized Care. J Intensive Care Med. 2018;33:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Masse MH, Richard MA, D'Aragon F, St-Arnaud C, Mayette M, Adhikari NKJ, Fraser W, Carpentier A, Palanchuck S, Gauthier D, Lanthier L, Touchette M, Lamontagne A, Chénard J, Mehta S, Sansoucy Y, Croteau E, Lepage M, Lamontagne F. Early Evidence of Sepsis-Associated Hyperperfusion-A Study of Cerebral Blood Flow Measured With MRI Arterial Spin Labeling in Critically Ill Septic Patients and Control Subjects. Crit Care Med. 2018;46:e663-e669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Durmuş G, Belen E, Can MM. Increased neutrophil to lymphocyte ratio predicts myocardial injury in patients undergoing non-cardiac surgery. Heart Lung. 2018;47:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wada H, Dohi T, Miyauchi K, Nishio R, Takeuchi M, Takahashi N, Endo H, Ogita M, Iwata H, Kasai T, Okazaki S, Isoda K, Suwa S, Daida H. Neutrophil to Lymphocyte Ratio and Long-Term Cardiovascular Outcomes in Coronary Artery Disease Patients with Low High-Sensitivity C-Reactive Protein Level. Int Heart J. 2020;61:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Ackland GL, Abbott TEF, Cain D, Edwards MR, Sultan P, Karmali SN, Fowler AJ, Whittle JR, MacDonald NJ, Reyes A, Paredes LG, Stephens RCM, Del Arroyo AG, Woldman S, Archbold RA, Wragg A, Kam E, Ahmad T, Khan AW, Niebrzegowska E, Pearse RM. Preoperative systemic inflammation and perioperative myocardial injury: prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2019;122:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Giakoumidakis K, Fotos NV, Patelarou A, Theologou S, Argiriou M, Chatziefstratiou AA, Katzilieri C, Brokalaki H. Perioperative neutrophil to lymphocyte ratio as a predictor of poor cardiac surgery patient outcomes. Pragmat Obs Res. 2017;8:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Song S, Yoon SY, Lim CS, Song JW, Kim HS. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic markers for pneumonia severity. Br J Biomed Sci. 2016;73:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Huang Y, Liu A, Liang L, Jiang J, Luo H, Deng W, Lin G, Wu M, Li T, Jiang Y. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol. 2018;64:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, Yoon BW. High Neutrophil-to-Lymphocyte Ratio Predicts Stroke-Associated Pneumonia. Stroke. 2018;49:1886-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 31. | Feng DY, Zhou YQ, Zhou M, Zou XL, Wang YH, Zhang TT. Risk Factors for Mortality Due to Ventilator-Associated Pneumonia in a Chinese Hospital: A Retrospective Study. Med Sci Monit. 2019;25:7660-7665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Gameiro J, Fonseca JA, Dias JM, Milho J, Rosa R, Jorge S, Lopes JA. Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery. BMC Nephrol. 2018;19:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Pektezel MY, Yilmaz E, Arsava EM, Topcuoglu MA. Neutrophil-to-Lymphocyte Ratio and Response to Intravenous Thrombolysis in Patients with Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2019;28:1853-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Li J, Li T, Wang H, Yan W, Mu Y. Neutrophil-lymphocyte ratio as a predictor of kidney function decline among individuals with diabetes and prediabetes: A 3-year follow-up study. J Diabetes. 2019;11:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Kim WH, Park JY, Ok SH, Shin IW, Sohn JT. Association Between the Neutrophil/Lymphocyte Ratio and Acute Kidney Injury After Cardiovascular Surgery: A Retrospective Observational Study. Medicine (Baltimore). 2015;94:e1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Parlar H, Şaşkın H. Are Pre and Postoperative Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio Associated with Early Postoperative AKI Following CABG? Braz J Cardiovasc Surg. 2018;33:233-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Fan LL, Wang YJ, Nan CJ, Chen YH, Su HX. Neutrophil-lymphocyte ratio is associated with all-cause mortality among critically ill patients with acute kidney injury. Clin Chim Acta. 2019;490:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Chen D, Xiao D, Guo J, Chahan B, Wang Z. Neutrophil-lymphocyte count ratio as a diagnostic marker for acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. 2020;24:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Abu Alfeilat M, Slotki I, Shavit L. Single emergency room measurement of neutrophil/lymphocyte ratio for early detection of acute kidney injury (AKI). Intern Emerg Med. 2018;13:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |