Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10805
Peer-review started: June 1, 2021
First decision: June 25, 2021
Revised: June 26, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: December 16, 2021
Processing time: 191 Days and 23.6 Hours
Deep endometriosis (DE) is the most aggressive subtype of endometriosis. The diagnosis may be challenging, and no biomarkers that can discriminate women with DE from those without DE have been developed.
To evaluate the role of blood hemostatic parameters and inflammatory indices in the prediction of DE.
This case-control study was performed at the Women’s Hospital, Zhejiang University School of Medicine between January 2015 and December 2016. Women with DE and women with benign gynecologic disease (control group) eligible for gynecological surgery were enrolled. Routine plasma hemostatic parameters and inflammatory indices were obtained before surgery. Univariate and multivariate analysis were performed. Receiver operating characteristic (ROC) curves were generated, and areas under the curve (AUC) were calculated to assess the predictive values of the selected parameters.
A total of 126 women were enrolled, including 31 with DE and 95 controls. Plasma fibrinogen (Fg, P < 0.01), international normalized ratio (P < 0.05), and C-reactive protein levels (P < 0.01) were significantly higher in women with DE compared with controls. Plasma hemoglobin (HB) levels (P < 0.05) and shortened thrombin time (P < 0.05) were significantly lower in women with DE than in controls. Plasma Fg levels [adjusted OR (aOR) 2.12, 95%confidence interval (CI): 1.31-3.75] and plasma HB levels (aOR 0.48, 95%CI: 0.29-0.78) were significantly associated with DE (both P < 0.05). ROC analysis showed that the diagnostic value of Fg or HB alone for DE was limited. The AUC of the combination of both markers as a dual marker index was 0.773 with improved sensitivity (67.7%) and specificity (78.9%) at cutoffs of 3.09 g/L and 126 g/L, respectively.
The combination of Fg and HB was a reliable predictor of DE. A larger study is needed to confirm the findings.
Core Tip: Deep endometriosis (DE) is the most aggressive subtype of endometriosis. Prompt diagnosis of DE is challenging, and developing new approaches for DE prediction before surgical intervention is of key importance for controlling the disease. Herein, we performed a case-control study to evaluate the role of plasma hemostatic parameters and inflammatory indices in predicting DE. Our results revealed that the combination of fibrinogen and hemoglobin had a good predictive value for DE before surgical intervention.
- Citation: Chen ZY, Zhang LF, Zhang YQ, Zhou Y, Li XY, Huang XF. Blood tests for prediction of deep endometriosis: A case-control study. World J Clin Cases 2021; 9(35): 10805-10815
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10805.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10805
Endometriosis, which is characterized by the presence of endometrial glands and stroma at ectopic sites, affects approximately 10% of women of reproductive age. Up to 80% of women with endometriosis suffer from chronic pain, and up to 50% of women suffer from infertility. Endometriosis-related productivity loss and decreased quality of life lead to a heavy economic burden[1]. Endometriosis can be classified as superficial endometriosis (SUP), ovarian endometrioma (OMA), and deep endometriosis (DE)[2]. DE is the most aggressive of the three subtypes that constitute endometriosis. It is defined as an endometriotic lesion penetrating a depth of > 5 mm and showing aggressive behavior[3]. It can affect the uterosacral ligaments, parametrium, bladder, and bowel. Patients with DE usually present with severe pelvic pain and low fertility. The heterogeneity of the disease makes the diagnosis of DE a clinical challenge[4-6] that may be delayed for more than 8 years[7]. Accidental intraoperative diagnosis of DE is also common. DE often requires surgical therapy, and a high incidence of surgical morbidity of DE has been reported[8]. Therefore, developing new approaches for predicting DE before surgery is of crucial importance.
In previous years, symptoms and clinical history, pelvic examination, blood tests, transvaginal ultrasound, and magnetic resonance imaging (MRI) have been proposed for the preoperative prediction of endometriosis[9-13]. However, the clinical present
The most widely accepted etiologic mechanism of endometriosis is retrograde menstruation resulting in ectopic implantation of endometrium in the pelvic cavity. The ectopic implanted endometrium can lead to recurrent bleeding, subsequent repeated tissue injury, and inflammation[14]. Endometriosis has also been associated with increased activation of the coagulation system and fibrinolysis system, and elevated plasma fibrinogen (Fg) levels, shortened thrombin time (TT), shortened activated partial thromboplastin time (APTT), shortened prothrombin time (PT), and increased expression of urokinase-type plasminogen activator and plasminogen activator inhibitors[15-19]. The data indicate that women with endometriosis might have a potential hypercoagulable state. Additionally, a high concentration of proinflammatory cytokines was reported in women with endometriosis[20-21]. Nevertheless, whether these routine hemostatic parameters and inflammatory indices have any predictive value in terms of preoperative diagnosis of DE has not yet been determined. This study was conducted to assess whether DE could be identified by routine hematological parameters before surgery. The study objectives were to estimate the predictive values of routine hemostatic parameters and inflammatory indices for DE.
A case-control study was performed at the Women’s Hospital, Zhejiang University School of Medicine between January 2015 and December 2016. Approval for this study was obtained from the Institutional Ethics Committee at Women’s Hospital School of Medicine, Zhejiang University (IRB-20200049-R). Data were retrospectively retrieved from an electronic database. Inclusion criteria for the DE group were: (1) 18 to 40 years of age[13]; and (2) DE defined as endometriotic lesions that infiltrated the uterosacral ligaments by > 5 mm and muscularis propria (bladder, intestine, ureters). Lesions were confirmed by pathology. Patients with DE who simultaneously had SUP or OMA were also included[2,14]. Exclusion criteria for the DE group were: (1) A history of abnormal uterine bleeding in the previous 3 mo; (2) A history of acute inflammation, suspected infectious disease, malignancy, metabolic diseases, and autoimmune disease in the previous 3 mo; (3) Pregnancy; (4) Hormonal therapy, including oral contraceptives, gonadotropin-releasing hormone analogs, or any other hormonal treatment, antithrombotic and hemostatic agents, and herbal compounds during the previous 3 mo; (5) Medical emergencies; and (6) With non-fasting lipid profiles.
Women between 18 and 40 years of age with surgical treatment at the same time for benign gynecologic diseases, including benign ovarian tumors, tubal infertility, cervical intraepithelial neoplasia, and intrauterine adhesion, but without any evidence of endometriosis, were recruited as controls. Detailed histories, thorough physical examinations of the abdominal-pelvic cavity, and sonography screening were performed by designated experts. The exclusion criteria were the same as for the DE group. Women to be enrolled in the controls with suspected endometriosis presenting dysmenorrhea or tenderness in the pelvic cavity or were also excluded.
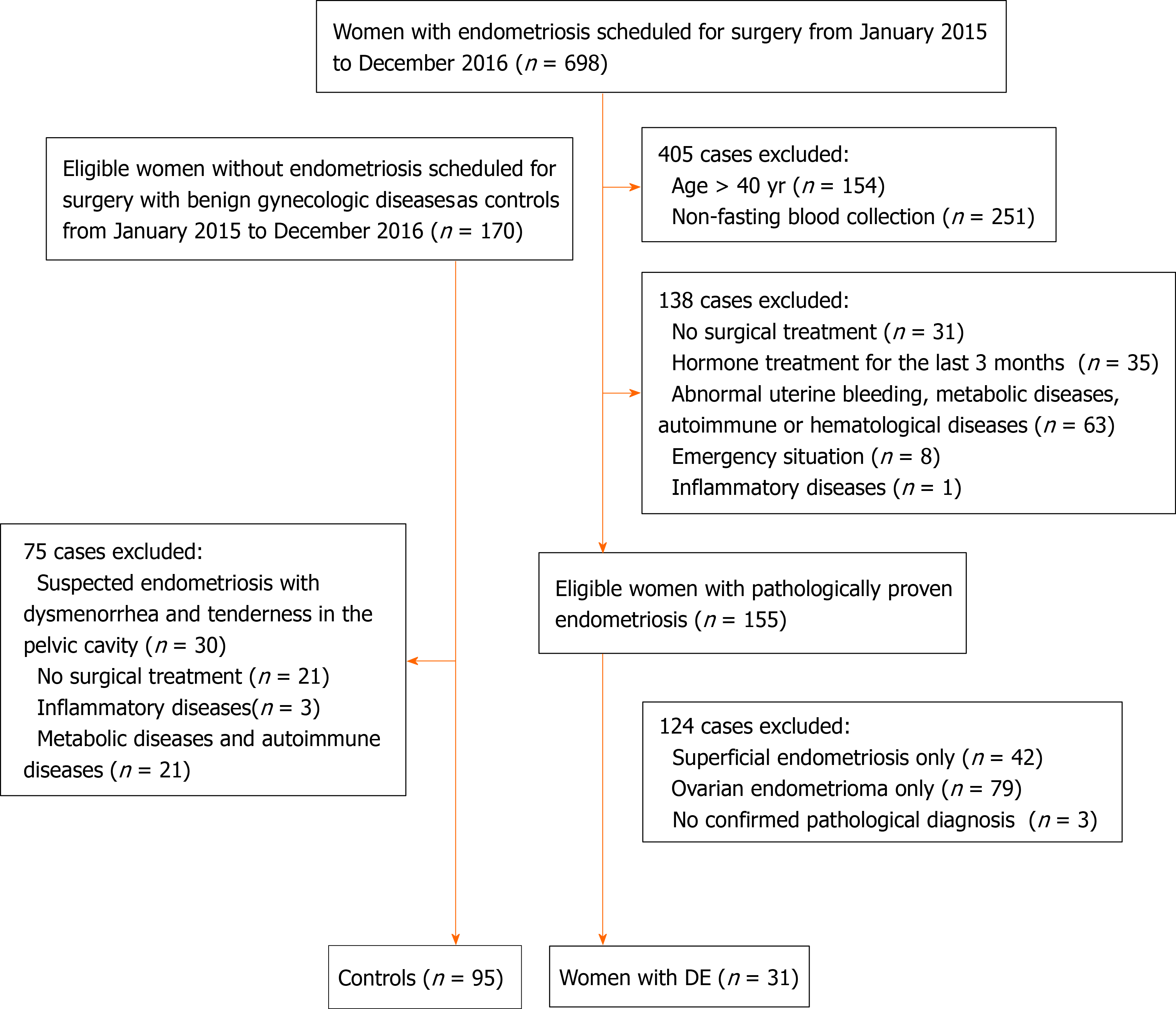
During the study period, 698 patients with endometriosis were scheduled for surgery in the general gynecology department. However, 667 women were excluded because they did not meet the selection criteria, 154 who were > 40 years of age, 251 with non-fasted blood collection, 138 with no surgical treatment, with hormonal treatment, or complicated by other diseases, 124 with pathologically proven endometriosis but not DE phenotype. The remaining 170 eligible women without endometriosis were enrolled; 75 cases were excluded, 30 because of suspected endometriosis, 21 with no surgical treatment, and 24 complicated by inflammatory, or metabolic, or autoimmune diseases.
All participants had routine peripheral blood tests before surgery. Blood samples with ethylenediaminetetraacetic acid (EDTA) as the anticoagulant were used to obtain a complete blood count, platelet count, HB level, neutrophil count, and lymphocyte count with a, autoanalyzer (Beckman, Coulter LH750). Coagulative parameters, including PT, TT, APTT, and Fg were determined with an automatic blood coagulation analyzer (STAGO, Evolution ISTA-R-IV, Germany). The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), international normalized ratio (INR), platelet distribution width, neutrophil number, lymphocyte number, and mean platelet volume were also calculated. Considering crosstalk between lipid metabolism and coagulation function, serum lipid profiles including total cholesterol, total triglycerides, high-density lipoprotein, and low-density lipoprotein were tested and measured by enzymatic colorimetric assay after an overnight fast of 12 h. C-reactive protein (CRP) levels were simultaneously determined. The blood samples without EDTA were centrifuged at 3500 g, and serum supernatants were collected. CRP levels were assayed in fresh serum using an immunoturbidimetric assay (Abbott, Architect C16000). Intra- and interassay coefficients of variation for all measurements were 5% and 10%, respectively.
Continuous data were reported as means ± SD for normally distributed variables, and variables that were not normally distributed were reported as medians and range. Between-group differences of variables with a normal distribution were tested by analysis of variance and Student’s t-test. For variables with a non-normal distribution, differences were compared with Kruskal–Wallis and Wilcoxon tests. Categorical variables were reported as n (%), and the χ2-square test was used to compare the distribution across different groups. Stepwise logistic regression was used to assess the association of hemostatic profiles with the presence of DE. All indices of interest such as age, body mass index (BMI), history of delivery and abortion, inflammatory indices, and serum lipid profiles that could cause a confounding bias were entered into the initial model as potential risk factors with SLE = 0.05 and SLS = 0.10. The final model was built using all significant variables in the multivariate analysis. Receiver operating characteristic (ROC) curves were constructed, and the area under curves was calculated to determine the predictive power of the independent risk factors. The statistical analysis were conducted with SAS, version 9.4 (SAS Institute, Cary, NC, United States). P values < 0.05 were considered statistically significant.
A total of 126 women were enrolled in this study, 31 with DE and 95 without DE (Figure 1). The indications for surgery in the DE patients were a pelvic mass, history of infertility, pelvic pain with failed analgesics. DE involved the uterosacral ligament in 26 (83.9%) patients, the colorectal septum in two (6.5%), the ureter in one (3.2%), and the sigmoid in two (6.5%). Adenomyosis was suspected in 13 women with no uterine fibroids on transvaginal ultrasound. The indications for surgery in controls were benign ovarian tumors and tubal infertility (54 cases), cervical intraepithelial neoplasia (28 cases), and intrauterine adhesion (13 cases). Baseline clinical characteristics are shown in Table 1. There were no differences in age, BMI, parity, abortion, and lipid profiles between the study and the control groups.
| Variable | Control group (n = 95) | DE (n = 31) | P value |
| Age (yr), mean ± SD | 31.57 ± 5.03 | 32.10 ± 5.13 | 0.61 |
| BMI (kg/m2), mean ± SD | 21.37 ± 2.67 | 20.36 ± 2.17 | 0.06 |
| Parity, n (%) | 0.41 | ||
| 0 | 35 (71.43) | 14 (28.57) | |
| ≥ 1 | 60 (77.92) | 17 (22.08) | |
| Abortion, n (%) | 0.90 | ||
| 0 | 50 (75.76) | 16 (24.24) | |
| 1 | 18 (72.00) | 7 (28.00) | |
| ≥ 2 | 27 (77.14) | 8 (22.86) | |
| TG (mmol/L), median (Q1-Q3) | 0.80 (0.64-1.12) | 0.81 (0.57-0.99) | 0.33 |
| TC (mmol/L), median (Q1-Q3) | 4.27 (3.80-4.82) | 4.09 (3.80-4.77) | 0.80 |
| HDL (mmol/L), median (Q1-Q3) | 1.32 (1.14-1.59) | 1.39 (1.19-1.55) | 0.70 |
| LDL (mmol/L), median (Q1-Q3) | 2.16 (1.83-2.60) | 2.16 (1.81-2.69) | 0.98 |
Plasma Fg (P < 0.01), INR (P < 0.05), and CRP levels (P < 0.01) of women with DE were significantly higher than those in controls. Plasma HB levels (P < 0.05) and TT (P < 0.05) of women with DE were significantly lower than those in controls. Differences between the other hematological parameters in the two groups were not significant (Table 2).
| Variable | Controls (n = 95) | DE (n = 31) | P value |
| PT (s) | 12.91 ± 0.61 | 13.11 ± 0.59 | 0.11 |
| INR | 1.01 (0.98-1.04) | 1.05 (1.00-1.07) | 0.03 |
| APTT (s) | 36.11 ± 3.16 | 36.84 ± 3.18 | 0.27 |
| TT (s) | 15.56 ± 0.61 | 15.29 ± 0.56 | 0.03 |
| Fg (g/L) | 2.83 (2.53-3.14) | 3.09 (2.73-3.97) | 0.01 |
| PLT (109/L) | 228.00 (185.00-258.00) | 235.00 (212.00-262.00) | 0.32 |
| HB (g/L) | 130.00 (124.00-136.00) | 126.00 (115.00-130.00) | 0.02 |
| WN (109/L) | 3.90 (3.00-4.80) | 3.30 (2.80-4.10) | 0.27 |
| WL (109/L) | 1.65 (1.30-2.00) | 1.60 (1.30-1.80) | 0.34 |
| MPV (fL) | 8.60 (7.90-9.70) | 8.40 (7.80-8.90) | 0.23 |
| PCT (%) | 0.19 (0.17-0.22) | 0.20 (0.18-0.23) | 0.21 |
| NLR | 2.16 (1.59-3.20) | 2.31 (1.58-2.78) | 0.86 |
| PLR | 130.00 (108.82-180.00) | 154.71 (128.95-179.09) | 0.08 |
| CRP (mg/L) | 12.91 ± 0.61 | 13.11 ± 0.59 | 0.01 |
Multivariate analysis (Table 3) showed that plasma Fg levels [odds ratio (OR) 1.67, 95%CI: 1.13-2.46], PT (OR 1.63, 95%CI: 1.12-2.38), plasma HB levels (OR 0.63, 95%CI: 0.42-0.92), and TT (OR 0.69, 95%CI: 0.48-0.99) were significantly associated with the presence of DE (all P < 0.05). No relationships between the other hematological parameters and the presence of DE were found. APTT, INR, NLR, and PLR were not included in the multivariable logistic models, considering that they overlapped with other parameters.
| Variables | β (SE) | P value | OR (95%CI) |
| PT | 0.49 (0.19) | 0.01 | 1.63 (1.12, 2.38) |
| Fg | 0.51 (0.20) | 0.01 | 1.67 (1.13, 2.46) |
| TT | −0.38 (0.19) | 0.04 | 0.69 (0.48, 0.99) |
| PLT | 0.23 (0.19) | 0.24 | 1.26 (0.86, 1.83) |
| HB | −0.47 (0.20) | 0.02 | 0.63 (0.42, 0.92) |
| WN | −0.22 (0.19) | 0.25 | 0.81 (0.56, 1.16) |
| WL | −0.17 (0.19) | 0.37 | 0.85 (0.59, 1.22) |
| MPV | −0.22 (0.18) | 0.24 | 0.81 (0.56, 1.15) |
| PCT | 0.24 (0.18) | 0.18 | 1.27 (0.89, 1.8) |
| CRP | −0.19 (0.20) | 0.34 | 0.82 (0.55, 1.22) |
After adjusting for potentially confounding factors including age, BMI, history of delivery and abortion, and serum lipid profiles, plasma Fg levels [adjusted OR (aOR) 2.12, 95%CI: 1.31-3.75] and plasma HB levels (aOR 0.48, 95%CI: 0.29-0.78) remained significantly associated with the presence of DE (both P < 0.05, Table 4). The relationship between PT/TT and DE was no longer significant.
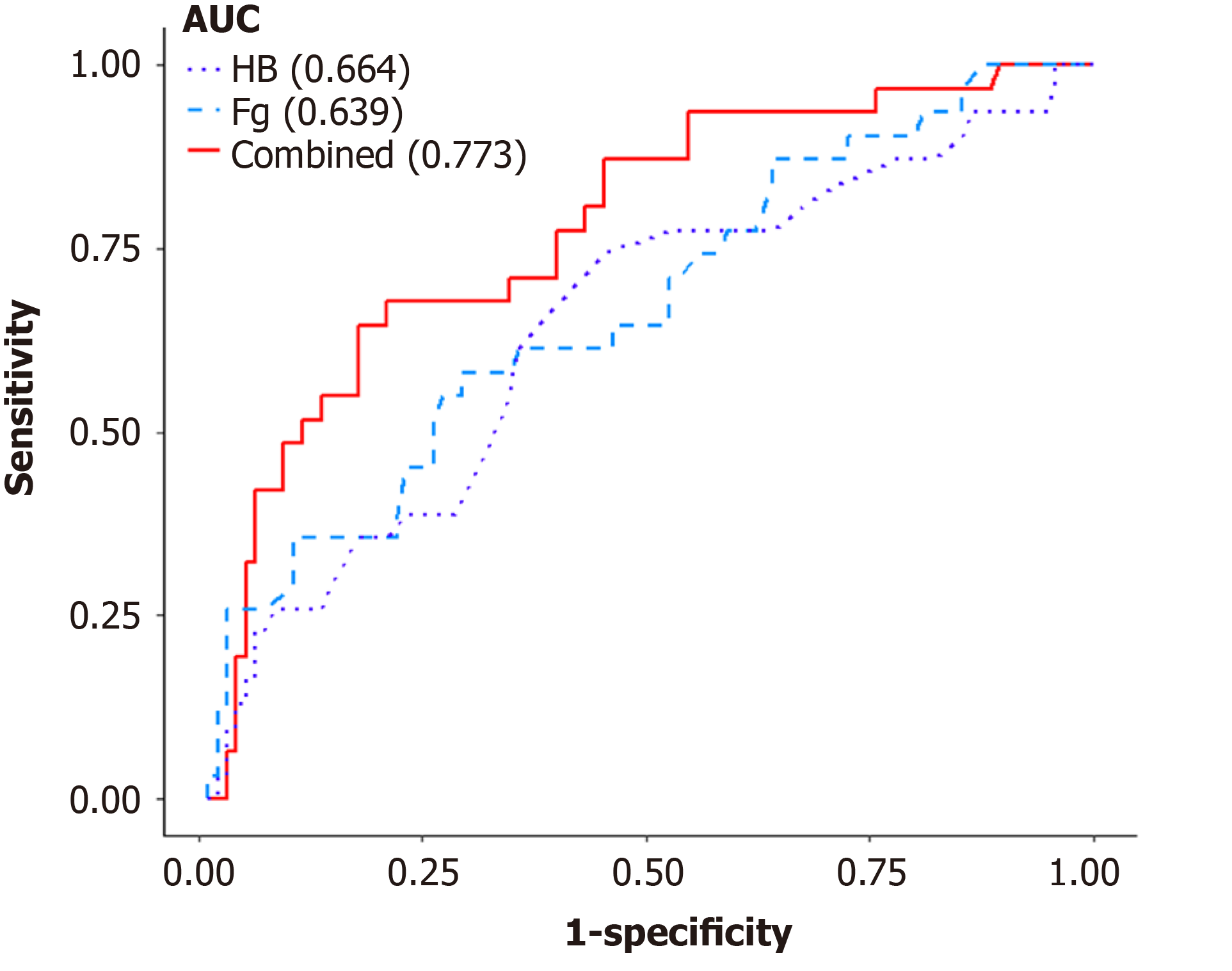
The predictive performance of Fg and HB for DE was investigated using ROC analysis. The area under the curve (AUC) of Fg was 0.639 (95%CI: 0.524-0.755, sensitivity = 58.1%, specificity = 70.5%), and that of HB was 0.664 (95%CI: 0.552-0.776, sensitivity = 64.5%, specificity = 62.1%) for the diagnosis of DE. The AUC of the combination of both markers was 0.773 with improved sensitivity (67.7%) and specificity (78.9%) at a cutoff of 3.09 (g/L) and 126 (g/L), respectively (Table 4, Figure 2). The combination of both markers as a dual marker index significantly improved the diagnostic accuracy.
This study examined the predictive values of hemostatic parameters and inflammatory indices for DE. Our data suggest that the combination of Fg and HB levels could be used as a reliable predictor of DE. To the best of our knowledge, this is the first report that evaluated hemostatic parameters and inflammatory indices for the prediction of DE. Fg is a known coagulation factor associated with hypercoagulation. As an acute-phase reaction and a hemostatic parameter, Fg has an important role in coagulation, inflammation, and the maintenance of hemostasis[22-23]. Fg is also a marker of inflammation and a major determinant of thrombosis and hemorheology[24]. In this study, we found elevated Fg levels in DE patients, which is consistent with previous findings[15-16].
Increasing evidence shows that pathophysiological changes in endometriosis have features in common with those observed during tissue injury and repair (TIAR)[3,25-26]. TIAR process may contribute to the development of endometriosis. The coagulation and fibrinolytic systems have important roles in TIAR[27]. An increase in plasma Fg is more likely caused by recurrent bleeding in the ectopic implantation of the endometrium and the impairment of the fibrinolytic system in endometriosis. Elevated Fg levels may reflect hemorheological disorders, a potential hypercoagulable status, and subclinical systemic inflammation in endometriosis.
In this study, a shortened TT was found in women with DE, which is in line with previous studies[16-17]. TT reflects anticoagulation, and a shortened TT indicates hypo-fibrinolysis. In this study, an inverse relationship between TT and DE was initially detected by multivariate analysis. After adjusting for confounding factors, the association was no longer significant. Moreover, no differences in APTT and TT were found between the DE and the control groups.
Decreased plasma HB levels were identified in women with DE in this study, which is in line with the findings in women with OMA[28-29]. Moreover, our results revealed an inverse relationship between plasma HB levels and the presence of DE. An inverse association between severity of endometriosis and plasma HB levels was reported in another study[30]. The exact cause of low plasma HB levels in patients with endometriosis is not clear. It may be associated with erythrocyte regulation of iron metabolism disorders or chronic systemic inflammation[31-32]. Low plasma HB levels may be associated with hypoxia, which has been reported to facilitating endometriosis development[33]. Further studies are required to investigate how HB contributes to the development of DE.
Serum CA-125 antigen is the most frequently used biomarker in the diagnosis of endometriosis in clinic practice[11,34-35]. Santulli et al[36] reported that serum CA-125 antigen was significantly associated with the severity and the penetration depth of DE; but it is not widely used in the diagnosis of DE. In this study, we found that either plasma Fg levels alone or plasma HB levels were not powerful enough to predict DE. A good predictive value for DE was obtained when plasma Fg levels were combined with HB levels. The AUC of the combination was 0.773, and the specificity was 78.9% at cutoffs of 3.09 g/L and 126 g/L. Ding et al[37] investigated the predictive role of Fg for endometriosis and found that the combination of Fg and serum CA-125 had good predictive power for OMA. They showed that Fg had potential predictive value for endometriosis, which is consistent with our results. Our model for predicting DE with the use of plasma Fg and HB may have clinical implications. Using this model, patients suspected of DE should undergo a thorough preoperative assessment through pelvic examination and pelvic imaging to detect DE nodules. Nonetheless, further studies for optimized predictive tools for DE are warranted.
Endometriosis is associated with an inflammatory response. In this study, increased CRP levels were found in the DE group, and there the difference in NLR between women with or without DE was not significant. The results of the value of inflammatory indices such as CRP and NLR in endometriosis in previous studies are not consistent[29,37-38]. This inconsistency may be associated with a different course, subtypes, and sample sizes used in those studies. In this study, multivariate analysis did not identify any association between CRP and NLR and the presence of DE.
Low HB levels could be caused by other bleeding disorders such as adenomyosis and uterine fibroids. In this study, 13 patients in the DE group had suspected adenomyosis with no uterine fibroids. Nonetheless, they did not complain of abnormal menstrual bleeding, and the HB levels were still within the normal range. Thus, the decreased HB levels of the DE group could not be attributed to concomitant adenomyosis. In addition, the coexistence of adenomyosis and endometriosis is well known[39]. We could not exclude the women with both DE and adenomyosis from the DE group in this study.
Our study has several limitations. First, the size of the DE group was relatively small. The low incidence of DE and strict criteria imposed in this study limited the enrollment. Additionally, this is the first study that evaluated the predictive role of hemostatic parameters for DE, and one of the aims of this study was to inspire future larger investigations. Second, as we did not include cases with only SUP or OMA subtypes, the results may not be applicable for all patients with endometriosis. Finally, there was no ideal control group for studying plasma Fg levels in DE. Our control group consisted of women with surgery for benign gynecological conditions, which permitted a thorough assessment of DE. However, some of the conditions, such as tubal infertility or ovarian cysts, might be associated with altered plasma levels.
In addition to the limitations, the following strengths should also be pointed out. First, the results of Fg/HB and the presence of DE were consistent in both univariate and multivariate analysis. Second, we for adjusted those confounding factors to eliminate possible effects on coagulation function and inflammatory response, which could make the predictive value of Fg and HB more reliable. In addition, these effects were not investigated in the previous relevant studies on endometriosis.
The study findings support the routine combination of Fg and HB as an essential part of the preoperative assessment of patients with suspected DE. The model can be adopted for use in clinical practice. Furthermore, this study suggests that an altered coagulation system may have key involvement in the development of endometriosis. The results also suggested that patients with DE may have a potential hypercoagulable state. Further studies are required to determine the anticoagulant therapy for these patients.
A combination of Fg and HB could be used in routine clinical practice as a reliable predictor of DE before surgical intervention. Future studies with larger samples are needed to verify the findings and to investigate how Fg and HB contribute to the development of endometriosis, particularly DE.
Deep endometriosis (DE) is the most aggressive subtype of the disease. The diagnosis of DE is challenging. No biomarkers have been identified for discriminating women with DE from those without DE.
Developing new approaches for predicting DE before surgery is of crucial importance. It is unclear whether DE could be identified by routine hematological evaluation before surgery.
To evaluate the role of blood hemostatic parameters and inflammatory indices in the prediction of DE before surgical intervention.
A case-control study investigated the value of routine plasma hemostatic parameters and inflammatory indices in women with DE and without endometriosis. Univariate analysis and multivariate analysis following adjustment for potential confounding factors were performed. Receiver operating characteristic curves were generated, and the areas under the curve was calculated to assess the predictive values of the selected parameters.
Elevated plasma fibrinogen (Fg) and decreased hemoglobin (HB) levels were found in women with DE compared with controls. Plasma Fg and HB levels were significantly associated with DE after adjusting for potential confounding factors. The diagnostic value of Fg or HB alone for DE detection before surgical intervention was limited, but the combination of Fg and HB had good predictive value for DE.
It suggested that the combination of Fg and HB levels could be used as a reliable predictor of DE. Based on the model, a thorough assessment is recommended for suspected patients with DE.
Further studies are required to investigate how Fg and HB contribute to the development of endometriosis, particularly DE.
We thank Li M from Haining Center for Disease Control and Prevention of Zhejiang Province, China for reviewing the statistical methods used in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: Chinese Medical Doctor Association, No. Z038000369.
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Apiratwarakul K S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Xing YX
| 1. | Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D'Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 699] [Article Influence: 53.8] [Reference Citation Analysis (1)] |
| 2. | Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL, Rombauts L, Giudice LC; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 3. | Gordts S, Koninckx P, Brosens I. Pathogenesis of deep endometriosis. Fertil Steril. 2017;108:872-885.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, Singh SS, Taylor HS. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220:354.e1-354.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 5. | Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Poupon C, Owen C, Arfi A, Cohen J, Bendifallah S, Daraï E. Nomogram predicting the likelihood of complications after surgery for deep endometriosis without bowel involvement. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Ghai V, Jan H, Shakir F, Haines P, Kent A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J Obstet Gynaecol. 2020;40:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Roman H, Bubenheim M, Huet E, Bridoux V, Zacharopoulou C, Daraï E, Collinet P, Tuech JJ. Conservative surgery vs colorectal resection in deep endometriosis infiltrating the rectum: a randomized trial. Hum Reprod. 2018;33:47-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Nnoaham KE, Hummelshoj L, Kennedy SH, Jenkinson C, Zondervan KT; World Endometriosis Research Foundation Women's Health Symptom Survey Consortium. Developing symptom-based predictive models of endometriosis as a clinical screening tool: results from a multicenter study. Fertil Steril. 2012;98:692-701.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N, Hull ML. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;CD012179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Oliveira MAP, Raymundo TS, Soares LC, Pereira TRD, Demôro AVE. How to Use CA-125 More Effectively in the Diagnosis of Deep Endometriosis. Biomed Res Int. 2017;2017:9857196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Guerriero S, Ajossa S, Minguez JA, Jurado M, Mais V, Melis GB, Alcazar JL. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Laux-Biehlmann A, d'Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol Sci. 2015;36:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Wu Q, Ding D, Liu X, Guo SW. Evidence for a Hypercoagulable State in Women With Ovarian Endometriomas. Reprod Sci. 2015;22:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Ding D, Liu X, Guo SW. Further Evidence for Hypercoagulability in Women With Ovarian Endometriomas. Reprod Sci. 2018;25:1540-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Viganò P, Ottolina J, Sarais V, Rebonato G, Somigliana E, Candiani M. Coagulation Status in Women With Endometriosis. Reprod Sci. 2018;25:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Alotaibi FT, Peng B, Klausen C, Lee AF, Abdelkareem AO, Orr NL, Noga H, Bedaiwy MA, Yong PJ. Plasminogen activator inhibitor-1 (PAI-1) expression in endometriosis. PLoS One. 2019;14:e0219064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Braza-Boïls A, Marí-Alexandre J, Gilabert J, Sánchez-Izquierdo D, España F, Estellés A, Gilabert-Estellés J. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbón M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front Endocrinol (Lausanne). 2019;10:935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Zhou J, Chern BSM, Barton-Smith P, Phoon JWL, Tan TY, Viardot-Foucault V, Ku CW, Tan HH, Chan JKY, Lee YH. Peritoneal Fluid Cytokines Reveal New Insights of Endometriosis Subphenotypes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020;105:284-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 23. | Olumuyiwa-Akeredolu OO, Page MJ, Soma P, Pretorius E. Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 341] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 25. | Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280:529-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 26. | Capobianco A, Cottone L, Monno A, Manfredi AA, Rovere-Querini P. The peritoneum: healing, immunity, and diseases. J Pathol. 2017;243:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Opneja A, Kapoor S, Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. 2019;179:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 28. | Moini A, Ghanaat M, Hosseini R, Rastad H, Hosseini L. Evaluating hematological parameters in women with endometriosis. J Obstet Gynaecol. 2021;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Tokmak A, Yildirim G, Öztaş E, Akar S, Erkenekli K, Gülşen P, Yilmaz N, Uğur M. Use of Neutrophil-to-Lymphocyte Ratio Combined With CA-125 to Distinguish Endometriomas From Other Benign Ovarian Cysts. Reprod Sci. 2016;23:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Kim SK, Park JY, Jee BC, Suh CS, Kim SH. Association of the neutrophil-to-lymphocyte ratio and CA 125 with the endometriosis score. Clin Exp Reprod Med. 2014;41:151-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Defrère S, González-Ramos R, Lousse JC, Colette S, Donnez O, Donnez J, Van Langendonckt A. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol Histopathol. 2011;26:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 32. | González-Ramos R, Defrère S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Wu MH, Hsiao KY, Tsai SJ. Hypoxia: The force of endometriosis. J Obstet Gynaecol Res. 2019;45:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | O DF, Fassbender A, Van Bree R, Laenen A, Peterse DP, Vanhie A, Waelkens E, D'Hooghe TM. Technical Verification and Assessment of Independent Validation of Biomarker Models for Endometriosis. Biomed Res Int. 2019;2019:3673060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Baggio S, Zecchin A, Pomini P, Zanconato G, Genna M, Motton M, Montemezzi S, Franchi M. The Role of Computed Tomography Colonography in Detecting Bowel Involvement in Women With Deep Infiltrating Endometriosis: Comparison With Clinical History, Serum Ca125, and Transvaginal Sonography. J Comput Assist Tomogr. 2016;40:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Santulli P, Streuli I, Melonio I, Marcellin L, M'Baye M, Bititi A, Borghese B, Lafay Pillet MC, Chapron C. Increased serum cancer antigen-125 is a marker for severity of deep endometriosis. J Minim Invasive Gynecol. 2015;22:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Ding S, Lin Q, Zhu T, Li T, Zhu L, Wang J, Zhang X. Is there a correlation between inflammatory markers and coagulation parameters in women with advanced ovarian endometriosis? BMC Womens Health. 2019;19:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Thubert T, Santulli P, Marcellin L, Menard S, M'Baye M, Streuli I, Borghese B, de Ziegler D, Chapron C. Measurement of hs-CRP is irrelevant to diagnose and stage endometriosis: prospective study of 834 patients. Am J Obstet Gynecol. 2014;210:533.e1-533.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Maruyama S, Imanaka S, Nagayasu M, Kimura M, Kobayashi H. Relationship between adenomyosis and endometriosis; Different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol. 2020;253:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |