Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.10792
Peer-review started: May 13, 2021
First decision: July 4, 2021
Revised: July 17, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 16, 2021
Processing time: 210 Days and 21.1 Hours
Cytomegalovirus (CMV) infection is common in liver transplant (LT)_ recipients, and biliary complications occur in a large number of patients. It has been reported that CMV-DNA is more detectable in bile than in blood.
To investigate the effects of CMV infection on biliary complications by comparing the levels of CMV-DNA in the bile and blood of patients after LT.
We conducted a retrospective analysis of 57 patients who underwent LT, 10 of these patients had no biliary complications and 47 patients had biliary complications. We also compared the levels of CMV-DNA in patients’ bile and blood, which were sampled concurrently. We used RNAscope technology to identify CMV in paraffin-embedded liver sections.
CMV-DNA was not detected in bile samples and was detected in 2 blood samples from patients without biliary complications. In the 47 patients with biliary complications, CMV-DNA was detected in 22 bile samples and 8 blood samples, both bile and blood samples were positive for CMV-DNA in 6 patients. The identification rate of CMV-DNA in blood was 17.0%, and was 46.8% in bile. Moreover, tissue samples from 4 patients with biliary complications tested positive using RNAscope technology but were negative with hematoxylin and eosin staining. During the follow-up period, graft failure occurred in 13 patients with biliary complications, 8 of whom underwent retransplantation, and 3 died. CMV-DNA in bile was detected in 9 of 13 patients with graft failure.
In patients with biliary complications, the identification rate of CMV-DNA in bile was higher than that in blood. Blood CMV-DNA negative patients with biliary complications should still be monitored for CMV-related biliary tract diseases. Potential occult CMV infection may also be a contributing etiological factor in the development of graft failure.
Core Tip: For patients with biliary complications after liver transplantation, the clinical doctors should be alert to cytomegalovirus (CMV)-related biliary tract diseases even though the test of CMV-DNA in the blood is negative. The test for CMV-DNA in bile may be a novel approach for diagnosing occult CMV-related biliary disease. There has been no study on diagnosing CMV-related biliary complications after liver transplant by detecting CMV-DNA in isolated bile. Occult CMV infection in the biliary tract may be associated with biliary stenosis and a contributing factor to graft failure, leading to high mortality after surgery.
- Citation: Liu JY, Zhang JR, Sun LY, Zhu ZJ, Wei L, Qu W, Zeng ZG, Liu Y, Zhao XY. Impact of cytomegalovirus infection on biliary disease after liver transplantation - maybe an essential factor. World J Clin Cases 2021; 9(35): 10792-10804
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/10792.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.10792
Biliary complications occur in up to 40% of patients after liver transplantation (LT) and cause significant mortality[1-3]. Post-LT biliary complications include strictures (anastomotic and non-anastomotic), leaks, stones, sphincter of Oddi dysfunction, and recurrence of primary biliary diseases. Cytomegalovirus (CMV) infection is a common opportunistic infection in LT recipients[4-5] and can occur in the liver, lungs, and gut and as a systemic infection[6-7]. CMV infection in LT recipients has been associated with many complications in addition to an increased risk of graft loss and death. CMV disease in LT patients most frequently presents as CMV syndrome, with the constellation of fever, neutropenia, and/or thrombocytopenia or as CMV hepatitis, as evidenced by elevated levels in liver function tests.
In our clinical work, we have found that in some patients with biliary complications after LT, CMV-DNA in the bile was positive but was negative in blood[8]. Rauber et al[9] reported that CMV was more frequent in bile than in liver biopsy or serum. Bile is not routinely obtained in patients after LT if there are no biliary complications. We obtained the bile from the patients without biliary complications using a T-tube during surgery following the surgeon’s judgement of the biliary system. Although it is difficult to obtain bile, this phenomenon is not observed in patients without biliary complications.
This finding suggests that serum CMV-DNA may be negative in patients with CMV biliary tract disease after LT. Testing for CMV in the biliary tract may be a novel approach for diagnosing occult CMV biliary disease. The present study aimed to determine the role of CMV infection in biliary complications after LT.
From December 2012 to January 2020, 57 patients who underwent LT in Beijing Friendship Hospital were retrospectively analyzed; 10 patients did not have biliary complications and 47 patients did have biliary complications.
Patients had routine blood CMV-DNA tested every 1-2 mo according to different risk levels during the first year and had blood CMV-DNA tested every 3-6 mo according to previous CMV infection in the late postoperative period. Patients had not routinely tested CMV-DNA in body fluid such as urine and bile.
All patients received universal prophylaxis or pre-emptive therapy for CMV infection. For high-risk patients (CMV IgG D+/R-), universal prophylaxis with intravenous ganciclovir or oral valganciclovir therapy was applied immediately after surgery. Pre-emptive treatment was be used to prevent CMV infection from progre
When patients presented to the hospital with nonspecific symptoms, such as jaundice, abdominal pain, and fever, the diagnosis of biliary complications was confirmed by laboratory tests, like abnormal liver function and increased bilirubin, and imaging examination. Some of the biliary complications were proven by pathologic biopsy.
We routinely collected the bile samples weekly for patients with biliary complications by endoscopic nasobiliary drainage, percutaneous transhepatic cholangial drainage, or indwelling T-tube. For patients without complications, we obtained the bile samples by indwelling T-tube when it was removed. We analyzed the bile and blood samples for the presence of CMV-DNA.
We conducted a retrospective analysis of all patients and compared the levels of CMV-DNA in bile and blood, which were sampled concurrently. We also used RNAscope technology to identify CMV in paraffin-embedded liver sections from patients with biliary complications.
The study was conducted following the Declaration of Helsinki and was approved by local ethics committees. All patients gave informed consent.
Bile samples were analyzed for the presence of CMV by polymerase chain reaction (PCR) after DNA extraction. Nucleic acid was isolated from bile samples with a Diagnostic Kit for the Quantification of Human Cytomegalovirus DNA (PCR-fluorescence method) (Zhongshan University, Daan Gene, China). At the beginning of the experiment, 1 mL of each bile sample was transferred to a 1.5 mL centrifuge tube. The tubes were centrifuged at 12000 rpm for 5 min, and the supernatants were removed. Fifty microliters of DNA extraction buffer were added, and the samples were incubated at 100 ℃ for 10 min and centrifuged at 12000 rpm for 5 min. Then, two of each resulting filtrate was used for PCR amplification. Amplification and detection were performed on a LightCycler instrument (Roche, Shanghai, China) with a thermocycling profile of 93 ℃ for 2 min followed by 40 cycles at 93 ℃ for 5 s and 57 ℃ for 45 s.
Blood samples were analyzed for the presence of CMV by PCR after DNA extraction. Nucleic acid was isolated from blood samples with a Diagnostic Kit for the Quantification of Cytomegalovirus DNA (PCR-fluorescence method). At the beginning of the experiment, 1 mL of each blood sample was transferred to a 1.5 mL Eppendorf tube. The samples were incubated at 4 ℃ overnight, and the filtrate (serum) was transferred into a fresh 1.5 mL Eppendorf tube. Fifty microliters of serum were transferred to a 1.5 mL Eppendorf tube, and 50 mL of DNA extraction buffer was added. The samples were incubated at 100 ℃ for 10 min and centrifuged at 12000 rpm for 5 min. Then, 2 mL of each resulting filtrate was used for PCR amplification. The amplification and detection procedures were the same as those for the bile samples.
The RNAscope assay uses a novel and proprietary method of in situ hybridization to detect single RNA molecules from virtually any gene in various tissue samples, including formalin-fixed paraffin-embedded (FFPE) tissues. The sensitivity and specificity of RNAscope in situ hybridization (RISH) have been determined with a variety of viral entities, including high-risk human papillomaviruses, hepatitis E virus, and hepatitis C virus.
Analysis of FFPE tissues was conducted following the RNAscope® 2.5 HD Detection Kit (BROWN) Quick Guide for FFPE Tissues (Advanced Cell Diagnostics, Newark, CA, United States). These samples underwent deparaffinization, proteolytic digestion with enzyme denaturation, and hybridization with probes. RNAscope target RNA was retrieved by initial incubation at 98 ℃ for 15 min followed by incubation with the RNAscope enzyme at 40 ℃ for 30 min. V-CMV, peptidylpropyl isomerase B (positive control), and DapB (negative control) probes were added and allowed to hybridize for 2 h at 40 ℃. Then, AMP1 3,3’-diaminobenzidine (DAB) (40 ℃ for 30 min), AMP2 DAB (40 ℃ for 15 min), AMP3 DAB (40 ℃ for 30 min), AMP4 DAB (40 ℃ for 15 min), AMP5 DAB (room temperature for 30 min), and AMP6 DAB (room temperature for 15 min) were incubated for the noted amount of time, and the slides were washed with the BOND reagent. The slides were incubated with DAB for 15 min and then counterstained with hematoxylin for 2 min. Finally, the samples were rinsed with water, and coverslips were affixed.
All analyses were performed with SPSS 23.0 (Armonk, NY, United States). Parametric variables are expressed as the mean ± SD, whereas nonparametric variables are given as the median (interquartile range). Continuous data were compared with the nonparametric Mann-Whitney U test. Differences between actuarial estimates were analyzed with the log-rank test. Frequency differences were compared with the chi-square test. For expected frequencies less than 5, Fisher’s exact test was used. P < 0.05 was considered statistically significant.
The demographics and clinical characteristics of the 57 patients are described in Table 1, and Table 2 describes the clinical data of adult patients. The median interval between LT and the procedure for obtaining bile was 14.35 mo (range = 1.0-134.3 mo) and 0.5 mo (range = 0.2-27.5 mo) in patients with and without biliary complications, respectively. The Pediatric End-Stage Liver Disease score of children was 16.7 ± 13.5, and the Model for End-Stage Liver Disease score of adults was 14.6 ± 6.8, which had no statistically significant difference with patients without complications. The biliary reconstructions and drainage technique were not statistically different, neither was the incidence of graft rejection and hepatic artery thrombosis. The P value of biliary CMV status and cold/warm ischemia time was less than 0.05 in Table 1, which included the children and adults. However, the P value of cold/warm ischemia time was more than 0.05 in Table 2 with adults only.
| Patients with biliary complications (n = 47) | Patients without biliary complications (n = 10) | P value | |
| Age (yr) | 33.0 (3.3-51.8) | 51.6 (40.6-54.5) | 0.021 |
| Sex (M/F) | 30/17 | 10/0 | 0.059 |
| Primary disease (child) | / | ||
| Biliary atresia | 11 | 0 | |
| Metabolic disease | 6 | 0 | |
| Other | 2 | 0 | |
| PELD | 16.7 ± 13.5 | / | / |
| Primary disease (adult) | |||
| Liver failure | 3 | 0 | |
| Decompensated liver cirrhosis | 14 | 10 | |
| HCC | 9 | 0 | |
| Other | 2 | 0 | |
| MELD | 14.4 ± 6.3 | 15 ± 5.5 | 0.533 |
| Liver transplantation | 0.056 | ||
| LDLT | 10 | 0 | |
| DDLT | 34 | 10 | |
| Cross-assisted liver transplantation | 3 | 0 | 0.016 |
| Cold ischemia time (min) | 361.9 ± 244.4 | 582.1 ± 150.9 | |
| Warm ischemia time (min) | 5 (3-5) | 5 (5-7) | 0.025 |
| Biliary reconstruction (Duct-to-duct / Roux-en-Y) | 23/9 | 1/9 | 0.404 |
| Blood CMV status (P/N) | 8/39 | 8/2 | 0.822 |
| Biliary CMV status (P/N) | 22/25 | 0/10 | 0.016 |
| Rejection (Yes/No) | 29/4 | 9/1 | 0.855 |
| HAT (Yes/No) | 1/32 | 0/10 | 0.578 |
| Biliary drainage | 1 | ||
| PTCD | 30 | 0 | |
| ENBD | 12 | 0 | |
| T-tube | 5 | 10 | |
| Outcome (alive/died) | 39/8 | 10/0 | 0.365 |
| Patients with biliary complications (n = 28) | Patients without biliary complications (n = 10) | P value | |
| Age (yr) | 49.3 (37.8-57.5) | 51.6 (40.6-54.5) | 0.619 |
| Sex (M/F) | 19/9 | 10/0 | 0.079 |
| Primary disease (adult) | 0.047 | ||
| Liver failure | 3 | 0 | |
| Decompensated liver cirrhosis | 14 | 10 | |
| HCC | 9 | 0 | |
| Other | 2 | 0 | |
| MELD | 14.6 ± 6.8 | 15 ± 5.5 | 0.63 |
| Liver transplantation | 1 | ||
| LDLT | 1 | 0 | |
| DDLT | 25 | 10 | |
| Cross-assisted liver transplantation | 2 | 0 | |
| Cold ischemia time (min) | 477.9 ± 193.7 | 582.1 ± 150.9 | 0.208 |
| Warm ischemia time (min) | 5 (3.25-5) | 5 (5-7) | 0.069 |
| Biliary reconstruction (Duct-to-duct/Roux-en-Y) | 16/2 | 9/1 | 0.927 |
| Blood CMV status (P/N) | 6/22 | 2/8 | 1 |
| Biliary CMV status (P/N) | 10/18 | 0/10 | 0.038 |
| Rejection (Yes/No) | 3/16 | 1/9 | 0.667 |
| HAT (Yes/No) | 1/18 | 0/10 | 0.46 |
| Outcome (alive/died) | 25/3 | 10/0 | 0.552 |
All the patients without biliary complications had negative CMV-DNA in bile, and only 2 patients had positive blood CMV-DNA.
Of the 47 patients with biliary complications, the median age was 33.0 years (3.3-51.8 years), including 30 male patients and 17 female patients. Ten patients received living donor liver transplantation (LDLT), 34 patients received deceased donor liver transplantation (DDLT), and 3 patients received cross-assisted liver transplanta
Biliary CMV-DNA was detected in 22 of 47 patients. Table 3 shows the patients’ baseline demographics with biliary complications and compares demographic and clinical parameters based on the biliary CMV-DNA status of the patients. The detected biliary CMV-DNA levels were between 100 copies/mL and 1.5 × 106 copies/mL. The median interval between surgery and biliary CMV-DNA detection was 9.7 mo (range = 1.6-91.7 mo).
| Biliary cytomegalovirus status | Children with biliary complications | P value | Adults with biliary complications | P value | ||
| Positive (n = 12) | Negative (n = 7) | Positive (n = 10) | Negative (n = 18) | |||
| Age | 25.8 (7.9-126.3) mo | 9.0 (6.0-94.1) mo | 0.612 | 51.6 ± 8.3 yr | 44.7 ± 14.5 yr | 0.250 |
| Sex (M/F) | 6/6 | 5/2 | 0.667 | 7/3 | 12/6 | 0.856 |
| Primary disease (child) | 0.414 | / | ||||
| Biliary atresia | 7 | 4 | / | / | ||
| Metabolic disease | 4 | 2 | / | / | ||
| Other | 1 | 1 | / | / | ||
| Primary disease (adult) | / | 0.487 | ||||
| Liver failure | / | / | 1 | 2 | ||
| Decompensated liver cirrhosis | / | / | 4 | 10 | ||
| HCC | / | / | 5 | 4 | ||
| Other | / | / | 0 | 2 | ||
| Liver transplantation | 0.351 | 0.241 | ||||
| LDLT | 6 | 3 | 0 | 1 | ||
| DDLT | 6 | 3 | 10 | 15 | ||
| Cross-assisted liver transplantation | 0 | 1 | 0 | 2 | ||
| Cold ischemia time (min) | 169 (57.5-547.5) | 94 (55.5-296) | 0.429 | 445.5 (195.5-583.8) | 490.0 (402.5-672.5) | 0.604 |
| Warm ischemia time (min) | 3 (3-6) | 3.5 (1.5-5.5) | 0.788 | 5 | 5 (2-5) | 0.145 |
| Biliary reconstruction (Duct-to-duct/Roux-en-Y) | 4/3 | 3/4 | 0.710 | 6/0 | 10/2 | 0.529 |
| Laboratory parameters before bile drainage | ||||||
| Bilirubin (mol/L) | 100.4 (35.2-390.8) | 14.9 (9.5-36.9) | 0.009 | 91.0 (35.9-203.0) | 37.0 (24.1-97.9) | 0.150 |
| AST (U/L) | 287.1 (58.2-635.0) | 66.1 (54.3-123.0) | 0.118 | 115.2 (69.2-189.8) | 66.1 (36.5-146.4) | 0.292 |
| ALT (U/L) | 106.0 (71.8-445.8) | 79.0 (44.0-98.0) | 0.205 | 117.0 (71.5-151.5) | 60.5 (39.0-183.0) | 0.502 |
| ALP (U/L) | 702.0 (177.0-1096.8) | 256.0 (183.0-968.0) | 0.735 | 368.0 (168.8-908.0) | 388.5 (199.5-628.25) | 0.943 |
| GGT (U/L) | 469.5 (388.25-847.25) | 478.0 (206.0-1206.0) | 0.866 | 334.5 (160.5-731.5) | 289.5 (229.5-492.5) | 0.811 |
| Bac Inf. (biliary tract) (P/N) | 9/3 | 5/2 | 0.865 | 4/4 | 9/3 | 0.503 |
| Biliary stricture (P/N) | 12/0 | 5/2 | 0.237 | 9/1 | 18/0 | 0.761 |
| Rejection (Yes/No) | 1/7 | 0/6 | 0.755 | 2/5 | 1/11 | 0.523 |
| HAT (Yes/No) | 0/8 | 0/6 | 1.000 | 0/7 | 1/11 | 1.000 |
| Outcome (Alive/Died) | 9/3 | 5/2 | 0.865 | 8/2 | 17/1 | 0.585 |
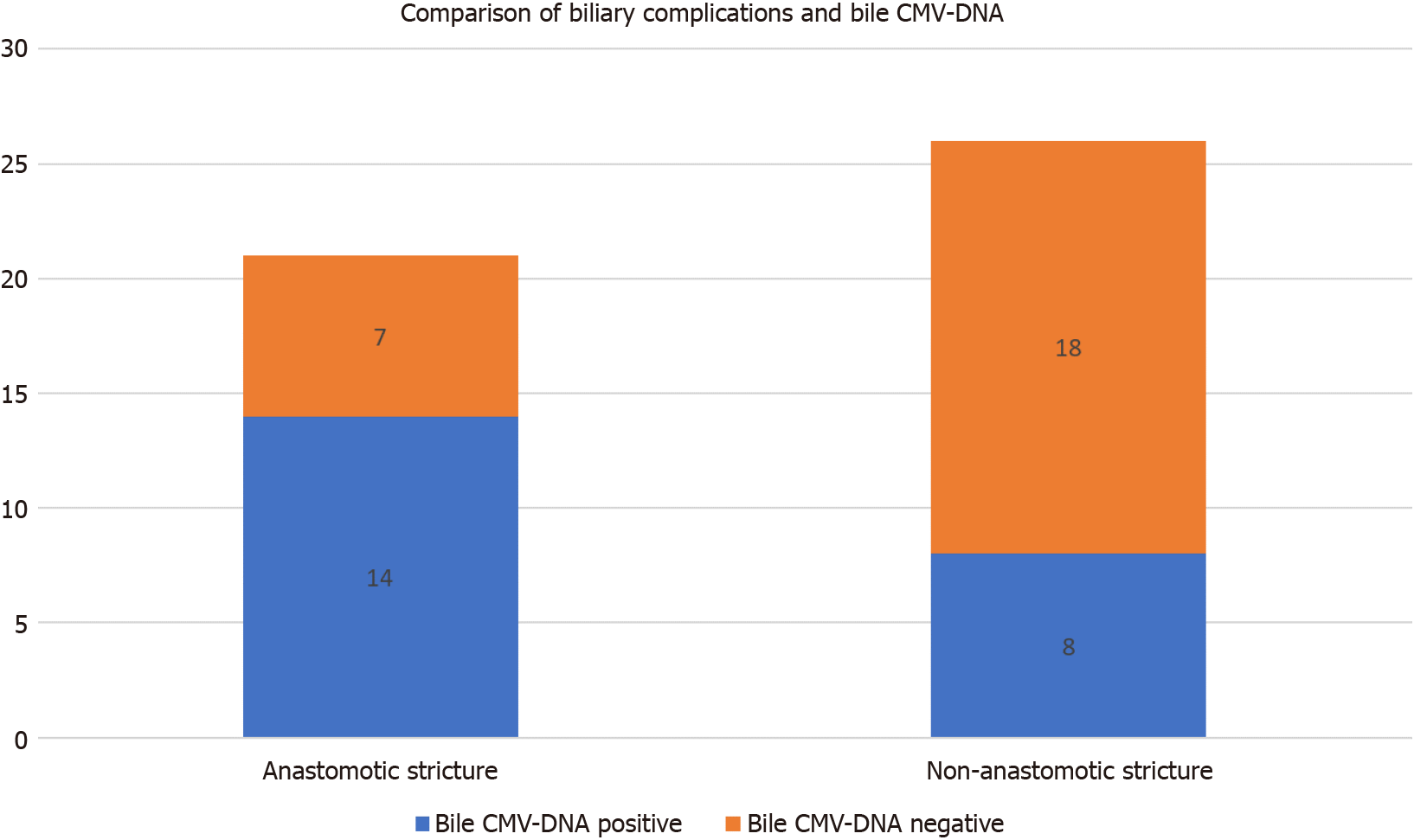
Among the 47 patients with biliary complications after LT, 21 had biliary anasto
| Biliary complications | Total | |||
| Anastomotic stricture | Non-anastomotic stricture | |||
| Bile cytomegalovirus-DNA | Positive | 14 | 8 | 22 |
| Negative | 7 | 18 | 25 | |
| Total | 21 | 26 | 47 | |
Among the 13 patients with graft failure, 9 patients had positive bile CMV-DNA. Of the 8 patients who died, 5 patients had positive bile CMV-DNA.
Of the 47 patients with biliary complications, CMV-DNA in blood was detected in 8 patients. Six patients tested positive for CMV-DNA in both blood and bile simultaneously. CMV-DNA was positive only in blood in 2 patients. Furthermore, both bile and blood were negative for CMV-DNA in 23 of 47 patients (Table 5). The positive identification rate of CMV-DNA in blood was 17.0% (8/47), and the positive identification rate in bile was 46.8% (22/47); the P value was 0.123. The difference was not statistically significant.
| Biliary CMV-DNA | Total | |||
| Positive | Negative | |||
| Blood cytomegalovirus-DNA | Positive | 6 | 2 | 8 |
| Negative | 16 | 23 | 39 | |
| Total | 22 | 25 | 47 | |
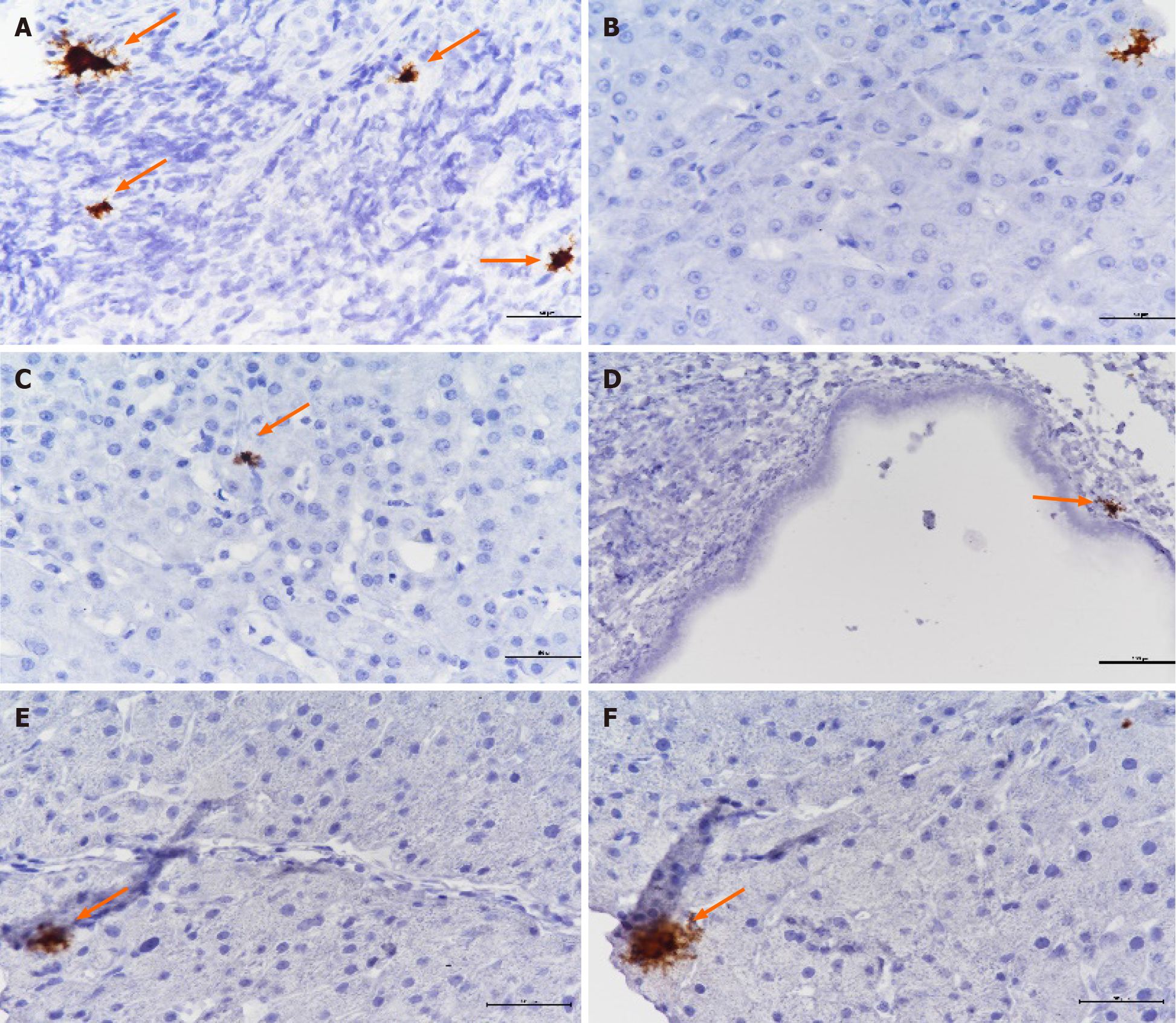
We performed hematoxylin eosin staining of liver tissue in patients after LT. Although the results were negative, pathological manifestations were identified in patients who tested positive for bile CMV-DNA. These pathological manifestations included infiltration of inflammatory cells in the portal tract area, vacuolar degeneration in the portal tract area, deletion in the portal tract area, dilation of the lumen, and epithelial disorder or degeneration in the small bile duct. However, the patients who tested negative for CMV-DNA did not exhibit these manifestations; thus, we believe these manifestations were related to CMV infection. In recent years, some studies have reported that RISH is more sensitive than immunohistochemistry (IHC) in detecting CMV. Therefore, we used RISH to test for the presence of CMV in paraffin-embedded liver sections from 8 patients who tested positive for biliary CMV-DNA. Four patients tested positive using RNAscope technology (Figure 2).
CMV infections are the primary cause of illness and death in immunocompromised patients, and CMV infection usually has no clinical symptoms[10-11]. Although pp65 antigen and CMV-DNA in serum are useful and early markers of CMV infection, it was reported that CMV detection in body fluids and tissues was more sensitive than in blood[12-14].
It is worth noting that only 10 adult patients without biliary complications were included in this study. Only the patients who presented to the hospital with biliary complications would receive endoscopic nasobiliary drainage, percutaneous trans
To reduce statistical error, we compared baseline data not only between patients with and without biliary complications but also between groups of adults. The demographics and clinical characteristics of the 57 patients are described in Table 1, and Table 2 describes the clinical data of adult patients. It is understandable that the P value related to age and cold/warm ischemia time in Table 1 was less than 0.05, but in Table 2 it was more than 0.05. LDLT is more commonly used in pediatric LT, resulting in a significant difference in cold/warm ischemia time compared with adult LT. The P value of biliary CMV status was less than 0.05, which also confirmed the relationship between biliary CMV status and biliary complications. There were no statistically significant differences in other baseline data in patients with or without biliary complications.
Table 3 shows the demographics and clinical parameters of patients with biliary complications and different biliary CMV status. In children with biliary complications, the P value of bilirubin showed that a higher bilirubin level might indicate positive biliary CMV status.
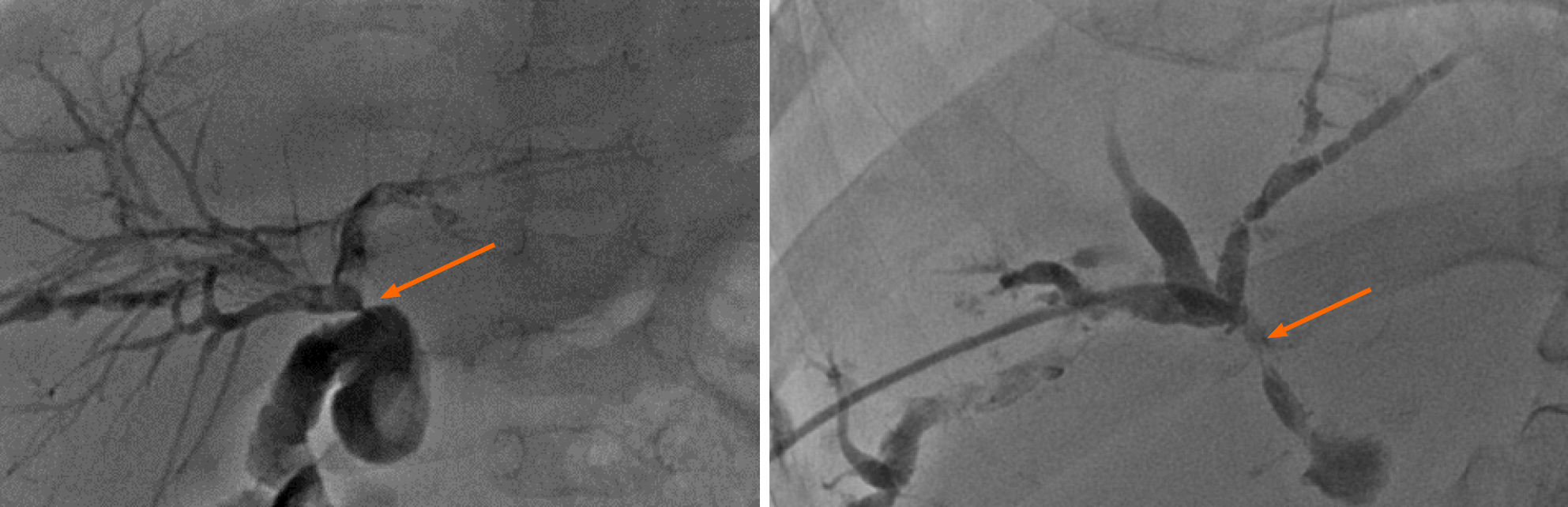
The biliary complications observed were primarily biliary strictures and obstruc
Murine models suggest that CMV latency occurs in epithelial and endothelial cells. Latent CMV in hepatic sinusoidal endothelial cells leads to its reactivation in the liver[17]. CMV infection promotes fibroblast proliferation during the bile duct’s healing process, resulting in anastomotic scarring, which leads to biliary anastomotic stenosis. In scar tissue, myofibroblasts are active. The relevant literature confirms that myo
Our research demonstrated that in 10 patients without biliary complications, none had positive CMV-DNA in bile, and 2 patients had positive CMV-DNA in blood. In patients with biliary complications, the positive rate of CMV-DNA in bile was much higher than that in blood. However, the difference between the positive rate in bile and blood of patients with biliary complications was not statistically significant (P = 0.123, See Table 5).
CMV infection may present only as viremia without clinical symptoms. In patients with biliary complications after LT, the phenomenon that CMV DNA in bile is positive, but CMV-DNA in blood is negative, may be highly suggestive of CMV biliary disease. According to a summary of these results, serum pp65 antigen and CMV-DNA negative patients with biliary complications should still be monitored for CMV-related biliary diseases. Testing for CMV in the biliary tract may be a novel approach for diagnosing occult CMV biliary diseases.
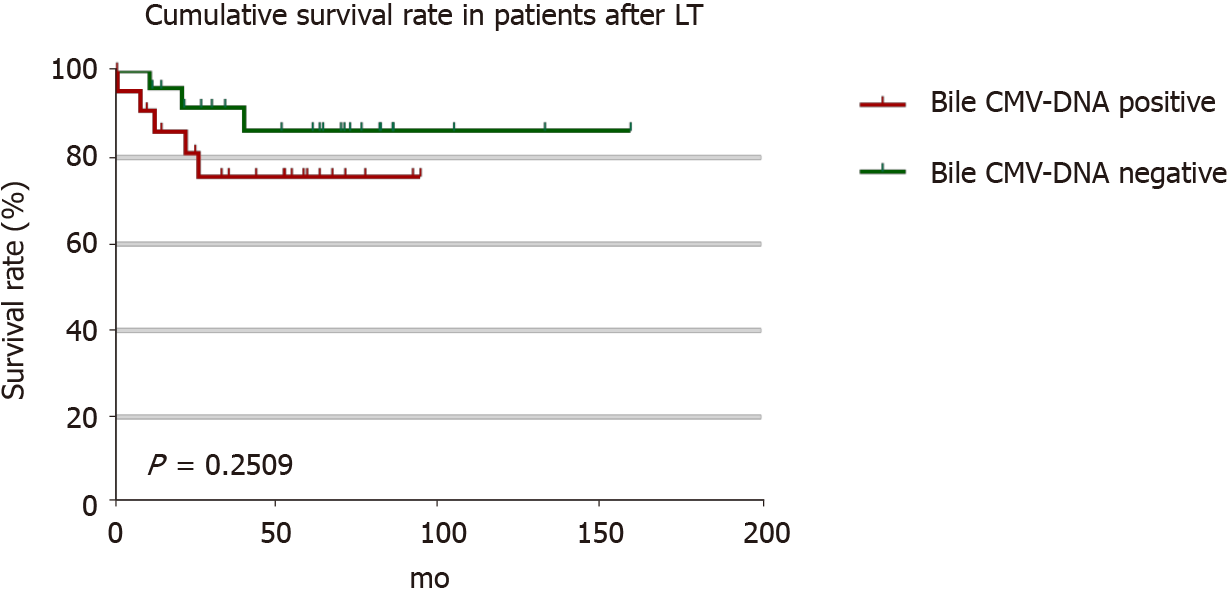
Routine detection of CMV in liver tissue is not sensitive. We used RISH to test paraffin-embedded liver sections from 8 patients with positive biliary CMV-DNA. All the tissues tested negative with CMV IHC, but the tissues of 4 patients tested positive using RISH. The results of our study suggest that RISH is more sensitive than IHC, which is consistent with previous studies[20-21]. Moreover, it also demonstrated that the identification rate of CMV-DNA in bile was higher in patients with CMV diseases.In this study, graft dysfunction occurred in 13 patients with biliary complications, 5 patients died, 8 patients underwent secondary transplantation, and 3 patients died after retransplantation. CMV-DNA in the biliary tract was detected in 9 of 13 patients with graft failure. The 1-year cumulative survival rate was 96.0%, the 3-year cumulative survival rate was 91.6%, and the 5-year cumulative survival rate was 86.2% in patients with CMV-DNA negative bile. The 1-year cumulative survival rate was 90.9% in patients with CMV-DNA positive bile, and the cumulative 3- and 5-year survival rates were 75.7% (Figure 4). Occult CMV infection is a risk factor for chronic graft failure and mortality after kidney transplantation[22-25]. Our study suggested that occult CMV infection might be related to chronic graft dysfunction and death in patients after LT. CMV infection in the biliary tract leads to increased biliary ob
Currently, prophylactic or preemptive treatment with ganciclovir or valganciclovir has partly reduced the incidence of CMV disease[27-28]. Patients in this study were given antiviral drug therapy, such as ganciclovir or valganciclovir, after CMV infec
In this study, the positive rate of CMV-DNA in bile was higher than that in the blood of patients with biliary complications after LT. Therefore, CMV-DNA negative patients with biliary tract complications should be monitored for CMV-related biliary tract diseases and tested for CMV-DNA in bile. RISH is more sensitive than traditional immunohistochemical methods to detect CMV infection in liver tissue. Furthermore, occult CMV infection may be associated with biliary anastomotic stenosis and a contributing factor in graft failure, leading to high mortality after surgery. Improving CMV prevention strategies and treatment options is a priority.
The association of cytomegalovirus (CMV) with biliary complications after liver transplant (LT) is an essential topic in clinical practice.
In clinical work, we have found that CMV-DNA in the bile and blood was inconsistent in patients with biliary complications after LT, and the positive rate of CMV-DNA in bile was higher than that in the blood.
To investigate the impact of CMV infection on biliary disease.
We conducted a retrospective analysis of the clinical data from 57 patients with or without biliary complications.
CMV detection in bile is more sensitive than in blood. RNAscope in situ hybridization is more sensitive than traditional methods to detect CMV infection in liver tissue. Biliary CMV infection is definitively associated with biliary complications and poor prognosis after LT, especially anastomotic stenosis.
Patients with negative CMV-DNA in blood should still be monitored for bile CMV-DNA. Bile CMV infection maybe a contributing etiological factor in the development of graft failure.
Current prevention strategies for CMV infection are inadequate and clinical doctors should be more vigilant of biliary CMV infection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: ILTS Anesthesia/Critical Care Committee, ILTS The Acute Liver Failure Special Interest Group.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Feier F, Kowalewski G S-Editor: Wang LL L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Kochhar G, Parungao JM, Hanouneh IA, Parsi MA. Biliary complications following liver transplantation. World J Gastroenterol. 2013;19:2841-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 186] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 2. | Nemes B, Gámán G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol. 2015;9:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Paya CV, Hermans PE, Washington JA 2nd, Smith TF, Anhalt JP, Wiesner RH, Krom RA. Incidence, distribution, and outcome of episodes of infection in 100 orthotopic liver transplantations. Mayo Clin Proc. 1989;64:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Green M, Tzakis A, Reyes J, Nour B, Todo S, Starzl TE. Infectious complications of pediatric liver transplantation under FK 506. Transplant Proc. 1991;23:3038-3039. [PubMed] |
| 6. | Mutimer D. CMV infection of transplant recipients. J Hepatol. 1996;25:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Aberg F, Mäkisalo H, Höckerstedt K, Isoniemi H. Infectious complications more than 1 year after liver transplantation: a 3-decade nationwide experience. Am J Transplant. 2011;11:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Liu Y, Sun LY, Zhu ZJ, Qu W. Novel approach for the diagnosis of occult cytomegalovirus cholangitis after pediatric liver transplantation: A case report. World J Clin Cases. 2020;8:2597-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Rauber C, Bartelheimer K, Zhou T, Rupp C, Schnitzler P, Schemmer P, Sauer P, Weiss KH, Gotthardt DN. Prevalence of human herpesviruses in biliary fluid and their association with biliary complications after liver transplantation. BMC Gastroenterol. 2019;19:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Brytting M, Xu W, Wahren B, Sundqvist VA. Cytomegalovirus DNA detection in sera from patients with active cytomegalovirus infections. J Clin Microbiol. 1992;30:1937-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Goodrum F, Caviness K, Zagallo P. Human cytomegalovirus persistence. Cell Microbiol. 2012;14:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Kullberg-Lindh C, Ascher H, Krantz M, Lindh M. Quantitative analysis of CMV DNA in children the first year after liver transplantation. Pediatr Transplant. 2003;7:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Özkarata E, Özbek ÖA, Avkan Oğuz V, Sayıner AA. Solid organ nakli alıcılarında CMV antijenemi testi ve CMV-DNA PCR sonuçlarının karşılaştırılması [Comparison of the CMV antigenemia test and CMV-DNA PCR results in solid organ transplant recipients]. Mikrobiyol Bul. 2016;50:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Hernando S, Folgueira L, Lumbreras C, San Juan R, Maldonado S, Prieto C, Babiano MJ, Delgado J, Andres A, Moreno E, Aguado JM, Otero JR. Comparison of cytomegalovirus viral load measure by real-time PCR with pp65 antigenemia for the diagnosis of cytomegalovirus disease in solid organ transplant patients. Transplant Proc. 2005;37:4094-4096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Gotthardt DN, Senft J, Sauer P, Weiss KH, Flechtenmacher C, Eckerle I, Schaefer Y, Schirmacher P, Stremmel W, Schemmer P, Schnitzler P. Occult cytomegalovirus cholangitis as a potential cause of cholestatic complications after orthotopic liver transplantation? Liver Transpl. 2013;19:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kühnapfel B, Reddehase MJ, Grzimek NK. Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol. 2009;83:8869-8884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Xu J, Geng ZM, Ma QY. Microstructural and ultrastructural changes in the healing process of bile duct trauma. Hepatobiliary Pancreat Dis Int. 2003;2:295-299. [PubMed] |
| 19. | Corrales I, Giménez E, Solano C, Amat P, de la Cámara R, Nieto J, Garcia-Noblejas A, Navarro D. Incidence and dynamics of active cytomegalovirus infection in allogeneic stem cell transplant patients according to single nucleotide polymorphisms in donor and recipient CCR5, MCP-1, IL-10, and TLR9 genes. J Med Virol. 2015;87:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Roe CJ, Siddiqui MT, Lawson D, Cohen C. RNA In Situ Hybridization for Epstein-Barr Virus and Cytomegalovirus: Comparison With In Situ Hybridization and Immunohistochemistry. Appl Immunohistochem Mol Morphol. 2019;27:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Holdhoff M, Guner G, Rodriguez FJ, Hicks JL, Zheng Q, Forman MS, Ye X, Grossman SA, Meeker AK, Heaphy CM, Eberhart CG, De Marzo AM, Arav-Boger R. Absence of Cytomegalovirus in Glioblastoma and Other High-grade Gliomas by Real-time PCR, Immunohistochemistry, and In Situ Hybridization. Clin Cancer Res. 2017;23:3150-3157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | van Ree RM, de Vries AP, Zelle DM, de Vries LV, Oterdoom LH, Gans RO, Schouten JP, Lems SP, van Son WJ, Bakker SJ. Latent cytomegalovirus infection is an independent risk factor for late graft failure in renal transplant recipients. Med Sci Monit. 2011;17:CR609-CR617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Gatault P, Halimi JM, Forconi C, Thibault G, Barbet C, Mérieau E, Gaudy-Graffin C, Marlière JF, Goudeau A, Bruyère F, Lebranchu Y, Büchler M, Baron C. CMV infection in the donor and increased kidney graft loss: impact of full HLA-I mismatch and posttransplantation CD8(+) cell reduction. Am J Transplant. 2013;13:2119-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Issa DH, Alkhouri N. Long-term management of liver transplant recipients: A review for the internist. Cleve Clin J Med. 2015;82:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wiesner RH, Menon KV. Late hepatic allograft dysfunction. Liver Transpl. 2001;7:S60-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Paya CV. Prevention of cytomegalovirus disease in recipients of solid-organ transplants. Clin Infect Dis. 2001;32:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Simon P, Sasse M, Laudi S, Petroff D, Bartels M, Kaisers UX, Bercker S. Two strategies for prevention of cytomegalovirus infections after liver transplantation. World J Gastroenterol. 2016;22:3412-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |