Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10696
Peer-review started: June 2, 2021
First decision: June 25, 2021
Revised: July 6, 2021
Accepted: October 20, 2021
Article in press: October 20, 2021
Published online: December 6, 2021
Processing time: 181 Days and 3.3 Hours
Metal-on-metal (MoM) total hip arthroplasty (THA) has been associated with adverse reactions to metal debris, presenting clinically as pseudotumors.
This case report presents a female aged 73 year-old with MoM THA-related pseudotumor. After arthrotomy and bursectomy surgeries, histologic examinations of surgical specimens revealed a specific lymphocyte-dominant immunologic response, now known as aseptic lymphocyte-dominant vasculitis-associated lesion (ALVAL). Due to soft tissue persisting effusion after arthrotomy and bursectomy, revision surgery was then performed with ceramic-on-polyethylene THA. However, revision did not resolve the patient’s symptoms. Here we describe our application of tigecycline sclerotherapy to treat recurrent pseudotumor after revision THA and no recurrence after 24-mo follow-up.
Tigecycline sclerotherapy is safe and effective in the management of recurrent pseudotumor after revision non-MoM THA in ALVAL cases.
Core Tip: Metal-on-metal (MoM) total hip arthroplasty (THA) often associates with metal debris, presenting as pseudotumors. We here described a case with MoM THA-related pseudotumor. Revision surgery was performed; however, further histologic examinations revealed the presence of aseptic lymphocyte-dominant vasculitis-associated lesion (ALVAL) and recurrent pseudotumors formation was noted after revision THA. We locally injected an infusion of tigecycline sclerotherapy for treating the pseudotumor successfully. Our findings indicated that tigecycline sclerotherapy is safe and effective in managing recurrent pseudotumor after revision non-MoM THA in ALVAL cases.
- Citation: Lin IH, Tsai CH. Tigecycline sclerotherapy for recurrent pseudotumor in aseptic lymphocyte-dominant vasculitis-associated lesion after metal-on-metal total hip arthroplasty: A case report. World J Clin Cases 2021; 9(34): 10696-10701
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10696.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10696
Metal-on-metal (MoM) hip articulations were first introduced in the 1960s and were thought to be more favorable biologically and biomechanically than conventional metal-on-polyethylene total hip arthroplasty (THA) implants[1]. However, registry data reporting significantly higher failure rates and revision rates caused concern[2,3]. Adverse reaction to metal debris (ARMD) released as metal particles, which may result in macroscopic necrosis of the periprosthetic space, corrosive osteolysis; large, sterile hip effusions; and periprosthetic solid and cystic masses (pseudotumors), was thought to contribute to the high failure rates[4]. In addition, histologic findings of surgical specimens exhibited a specific lymphocyte-dominant immunologic response, now known as aseptic lymphocyte-dominant vasculitis-associated lesion (ALVAL)[5].
This case report describes recurrent hip joint effusion and pseudotumor formation after revision THA as a result of MoM-related ALVAL disease. Management of the recurrent pseudotumor with locally infused tigecycline (trade name, Tygecil) as a sclerosing agent is discussed.
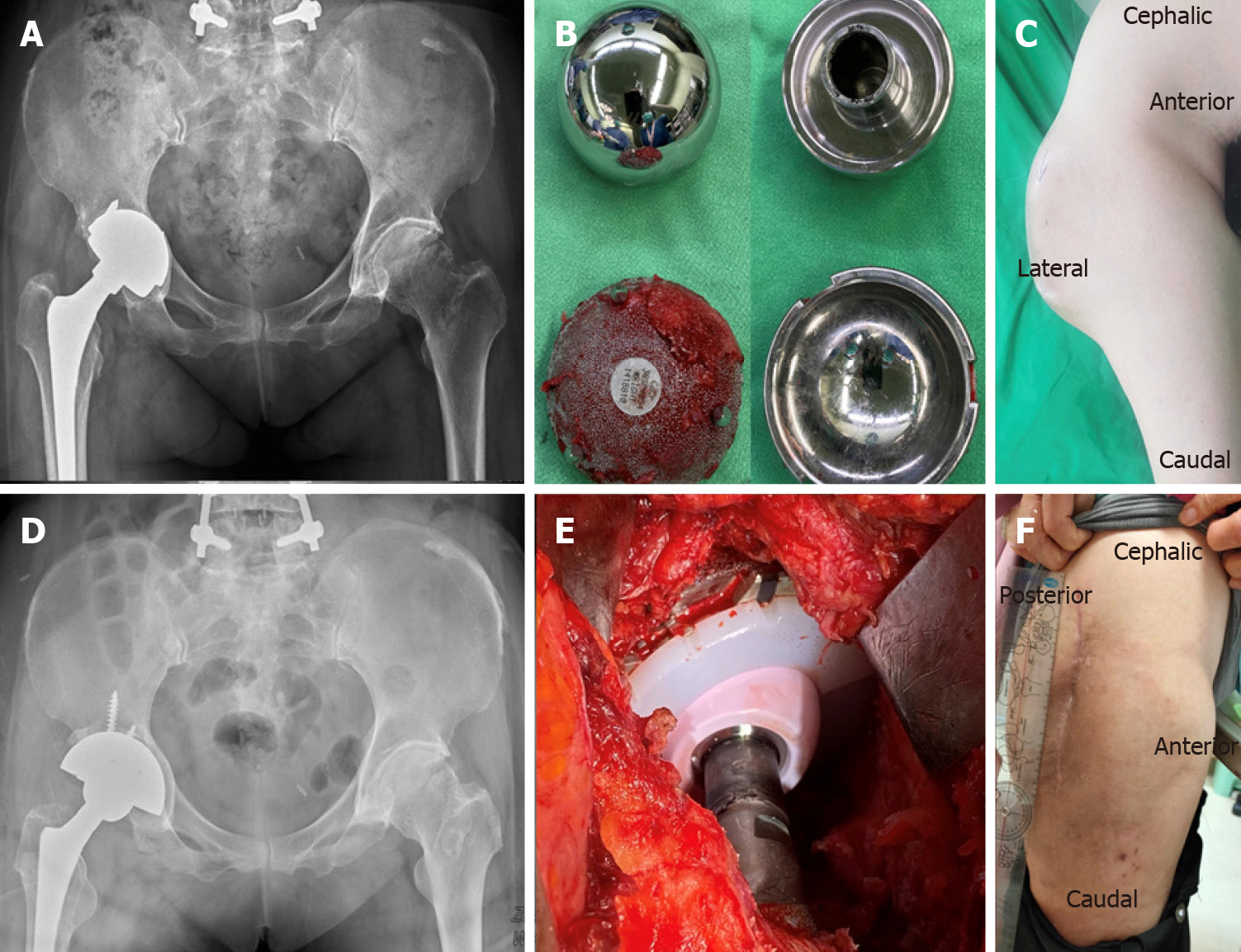
A female aged 73 years, with hypertension and right hip osteoarthritis, visited our clinic due to recurrent right hip mass after receiving MoM THA (CONSERVE® total Hip system, BFH with Spiked Shell, Wright Medical, Inc., Arlington, TN) surgery seven years ago (Figure 1A). The patient's signs were soft tissue swelling with mild tenderness, but no local heating, and no erythematous changes of the skin.
She had MoM THA surgery seven years ago, and had recurrent right hip mass.
The patient had hypertension and was under medication control.
The patient had hypertension and right hip osteoarthritis. There was no significant family medical history to note.
Physical examination revealed soft tissue swelling with mild tenderness, but no local heating, and no erythematous changes of the skin.
Laboratory examinations of serum C-reactive protein and erythrocyte sedimentation rate revealed that these were within the normal ranges, excluding the possibility of infection. Analysis of synovial fluid drainage also ruled out the likelihood of infection. Examinations of serum levels of cobalt and chromium revealed that these were within the normal ranges.
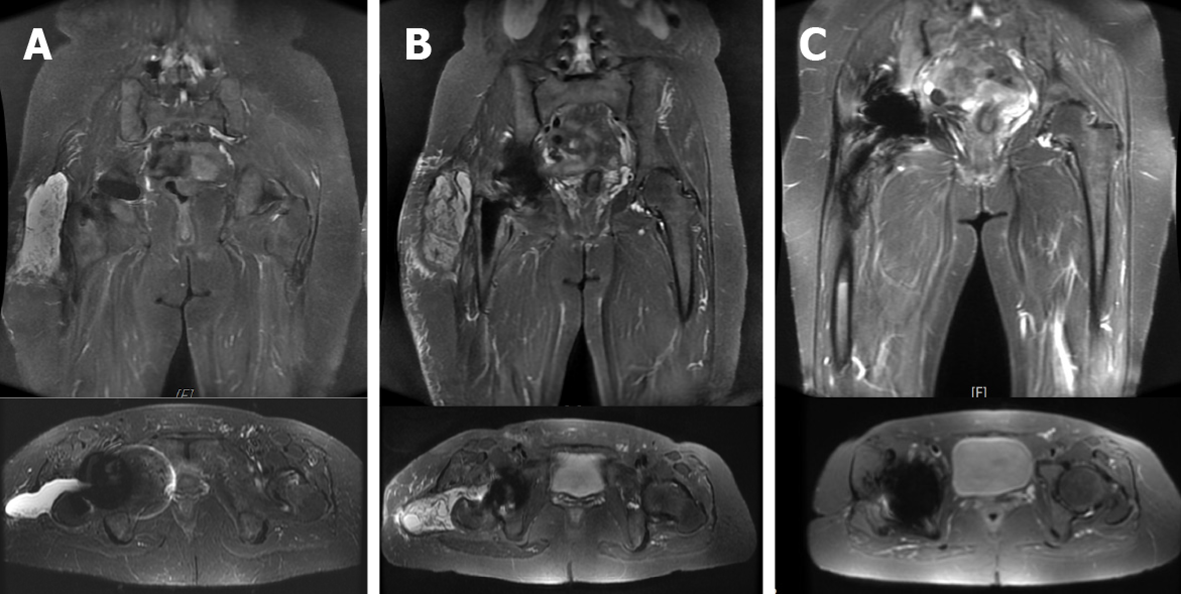
Magnetic resonance imaging (MRI) images with multiacquisition variable-resonance image combination (MAVRIC) of right hip showed joint effusion and trochanteric bursitis involving the right lateral side of the right artificial hip (Figure 2A).
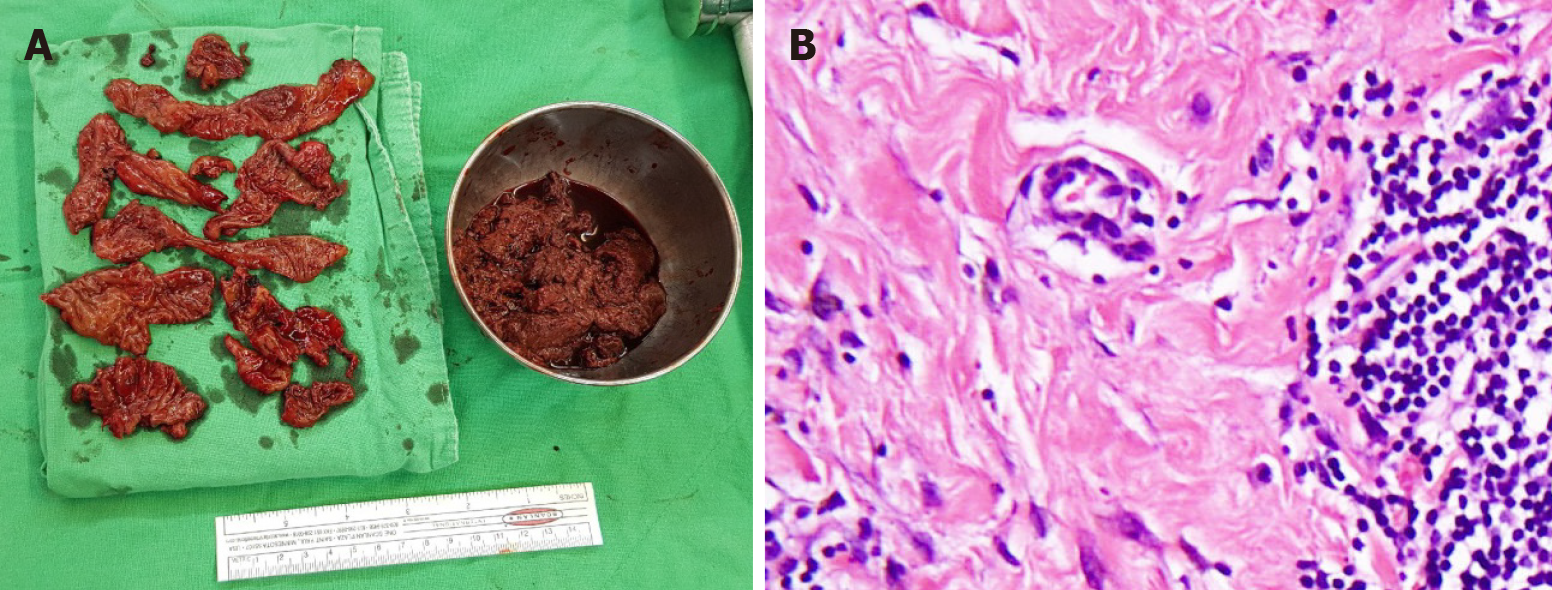
Formation of metallosis-related pseudotumor was suggested. According to the normal serum levels of cobalt and chromium, and the patient’s poor response to conservative treatment (oral nonsteroidal anti-inflammatory drug, antihistamine and local injection of steroid), surgical interventions of right hip arthrotomy and bursectomy was performed. After removing all hypertrophic bursa, necrotic periprosthetic soft tissue and pseudotumor, the synovial lining cell hyperplasia with lymphocytic cells infiltration, stromal fibroplasia, and massive fibrin exudation confirmed the histological diagnosis of ALVAL[6] (Figure 3).
We performed ceramic-on-polyethylene THA (Biolox delta Option, Biomet G7, Zimmer Biomet, Inc., Warsaw, IN) to treat persistent postoperative effusion and soft tissue swelling following revision (Figure 1D). However, persistent joint effusion (about 100 mL-daily straw fluid from drainage) was still noted for two months after revision THA. Infection was excluded after checking the drainage fluid culture and performing the microscopic tests. Repeat MRI revealed recurrent periprosthetic pseudotumor after revision THA (Figure 2B). Chemical pleurodesis treatment for pleural effusion using tetracycline (single-dose tigecycline 50 mg into the joint space and periprosthetic soft tissue) was performed one week after revision THA.
The effusion was much improved one week after the local tigecycline infusion. Following MRI scan also showed subsidence of pseudotumor, only minimal subcutaneous fibrotic scaring tissue, without effusion collection was noted (Figure 2C). No recurrent effusion or recurrent pseudotumor was found at the end of 24-mo postoperative follow-up (Figure 1F).
ALVAL is a histological diagnosis of adverse ARMD in MoM THA, consisting of related metallosis and type IV hypersensitivity reaction[5]. Severe complications were reported, including dislocation, recurrent ALVAL and re-revision requiring post-revision surgery. In this case, before receiving revision THA, chronic periprosthetic soft tissue swelling with pseudotumor formation, complicated by massive effusion, was noted. For lesion evaluation, MRI image with MAVRIC was used in the coronal plane to reduce susceptibility artifact[7]. The pseudotumor recurred even after surgical interventions of arthrotomy and bursectomy. In response, we arranged revision ceramic-on-polyethylene THA for this case. However, the pseudotumor and soft tissue effusion still recurred after revision THA within post-operative two months. Higher rates of complication, including instability, neurovascular injury, deep infection, reoperation, component loosening had been reported in revision cases for failed MoM hip implants[8]. However, for the present recurrent pseudotumor with persist effusion condition after revision THA with non-MoM component, only few cases were reported and there is no specific management guidelines available[9]. Residual metal debris within soft tissue or persisted hypersensitivity reaction maybe the cause of recurrent pseudotumor. In this case, after excluding infection conditions, local treatment with tigecycline was used as a sclerosing agent. Tigecycline is an broad spectrum antibiotic derivation of tetracycline, which has demonstrated to be an effective sclerosing agent for pleurodesis of different type pleural effusion[10]. The sclerosing agent application to the primary target as pleural mesothelial lining results in the release of several mediators like interleukin-8, transforming growth factor-beta and basic fibroblast growth factor[11]. This leads the diffuse inflammation activity in the cavity, which causes coagulation-fibrinolysis imbalance. This imbalance results in favoring the production of fibrin chain, collagen and extracellular matrix components by fibroblast. These mechanisms eventually result in space obliteration[12]. In the literature review, there was no other study reported application of tigecycline as a sclerosing agent for space obliteration other than pleural space. The reason we chose Tigecycline as sclerosing agent was the safe, accessible, and cost-effective. The sclerosing mechanism also worked in the effusion space between periarticular soft tissue by tigecycline infusion, which resulted in obliteration of recurrent pseudotumor. In the article review of safety profile of tigecycline, the gastrointestinal symptoms are the most common reported adverse effects of tigecycline (nausea 26, vomiting 18 and diarrhea 12%)[13]. Incidence of these adverse effect is reported correlating with escalating doses. In the present case, we only injected single dose of tigecycline 50 mg into the joint space and periprosthetic soft tissue. There is no systemic or local side effect occurred in this case. After locally infusion of tigecycline, the joint effusion diminished significantly within one week. During follow-up, hip MRI images also showed subsidence of pseudotumor, and no recurrent joint effusion at 24-mo after the injection (Figure 2C). There was no complication either gait imbalance in following up.
Local infusion treatment using tigecycline is safe, cost effective, and able to provide an additional therapeutic adjunct for the treatment of recurrent pseudotumor after revision non-MoM THA in ALVAL cases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu W S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Bolognesi MP, Ledford CK. Metal-on-Metal Total Hip Arthroplasty: Patient Evaluation and Treatment. J Am Acad Orthop Surg. 2015;23:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Australian Orthopaedic Association. Australian Orthopaedic Association National Joint Replacement Registry: Annual Report 2010. Available from: https://aoanjrr.sahmri.com/documents/10180/42844/Annual+Report+2010. |
| 3. | National Joint Registry Centre. National Joint Registry for England and Wales: Seventh Annual Report. Available from: https://www.njrcentre.org.uk/njrcentre/Portals/0/NJR%207th%20Annual%20Report%202010.pdf. |
| 4. | Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br. 2010;92:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 5. | Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 6. | Berstock JR, Baker RP, Bannister GC, Case CP. Histology of failed metal-on-metal hip arthroplasty; three distinct sub-types. Hip Int. 2014;24:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2014;472:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Munro JT, Masri BA, Duncan CP, Garbuz DS. High complication rate after revision of large-head metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Desai BR, Sumarriva GE, Chimento GF. Pseudotumor recurrence in a post-revision total hip arthroplasty with stem neck modularity: A case report. World J Orthop. 2020;11:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Yilmaz N, Zeybek A, Tharian B, Yilmaz UE. Efficacy of nonsurgical tigecycline pleurodesis for the management of hepatic hydrothorax in patients with liver cirrhosis. Surg Case Rep. 2015;1:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration. 2012;83:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Mierzejewski M, Korczynski P, Krenke R, Janssen JP. Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration. Respir Res. 2019;20:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Kaewpoowat Q, Ostrosky-Zeichner L. Tigecycline : a critical safety review. Expert Opin Drug Saf. 2015;14:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |