Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10604
Peer-review started: March 21, 2021
First decision: April 29, 2021
Revised: August 20, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: December 6, 2021
Processing time: 251 Days and 22.9 Hours
Knee joint pain and stiffness are the two main symptoms of knee osteoarthritis (OA) and thus restrict a patient’s activities, such as walking and walking up and downstairs. The lower body positive pressure (LBPP) treadmill as one of the emerging body weight support system devices brings new hope for exercise-related rehabilitation for knee OA patients.
To investigate the biomechanical effects and the subjective clinical assessment of LBPP treadmill walking exercise when compared with conventional therapy in mild to moderate knee OA patients.
Eighteen patients with mild-to-moderate knee OA were recruited in this randomized controlled trial (RCT) study. The eligible knee OA patients were randomly assigned to two groups: LBPP and control groups. The patients in the LBPP group performed an LBPP walking training program for 30 min/session per day, 6 d per week for 2 wk whereas the patients in the control group performed walking on the ground for the same amount. All patients underwent clinical assessments and three-dimensional gait analysis at pre- and 2-wk post-treatment.
The Western Ontario and McMaster Universities Arthritis Index and visual analog scale scores in both the LBPP group and control group were found to decrease significantly at the post-treatment point than the pre-treatment point (LBPP: 70.25 ± 13.93 vs 40.50 ± 11.86; 3.88 ± 0.99 vs 1.63 ± 0.52; control: 69.20 ± 8.88 vs 48.10 ± 8.67; 3.80 ± 0.79 vs 2.60 ± 0.70, P < 0.001). Moreover, compared with the control group, the LBPP group showed more improvements in walking speed (P = 0.007), stride length (P = 0.037), and knee range of motion (P = 0.048) during walking, which represented more improvement in walking ability.
The results of our RCT study showed that the LBPP group has a greater effect on improving gait parameters than the conventional group, although there was no significant advantage in clinical assessment. This finding indicates that LBPP treadmill walking training might be an effective approach for alleviating pain symptoms and improving lower extremity locomotion in mild to moderate knee OA patients.
Core Tip: To our best knowledge, this is the first randomized controlled trial focusing on lower body positive pressure (LBPP) training in knee osteoarthritis rehabilitation. Both subjective clinical assessment and objective biomechanical assessments were used in this study. The LBPP group showed more improvements in pain relief and gait improvement than the conventional group. LBPP treadmill exercise training could be considered an effective approach for patients with knee osteoarthritis.
- Citation: Chen HX, Zhan YX, Ou HN, You YY, Li WY, Jiang SS, Zheng MF, Zhang LZ, Chen K, Chen QX. Effects of lower body positive pressure treadmill on functional improvement in knee osteoarthritis: A randomized clinical trial study. World J Clin Cases 2021; 9(34): 10604-10615
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10604.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10604
Osteoarthritis (OA), the most common rheumatic disease, primarily affects the articular cartilage and the subchondral bone of a synovial joint and eventually results in joint failure[1]. Knee joint pain and stiffness are the two main symptoms of knee OA and thus restrict a patient’s activities, such as walking and walking up and down stairs. Usually, pain limits the patient’s ability to move, and the reduction in lower extremity activity will exacerbate the decline of joint muscle function thus producing a vicious cycle. Ways to help the patient recover lower limb joint activity to the maxi
As one of the emerging body weight support system devices, a lower body positive pressure (LBPP) treadmill brings new hope for exercise-related rehabilitation for knee OA patients[7]. The system uses a sealed inflatable positive pressure chamber at a waist-high level to achieve accurate weight support of the lower extremities (20%-100% in 1% increments) to reduce knee pressure and pain related to the pathology of osteophyte formation and joint cavity stenosis[8,9]. The clinical effect of LBPP in knee OA clinical rehabilitation was affirmed[10-12], but the relative mechanism of biomechanical influence on the rehabilitation of lower limb function in knee OA patients is not yet clear. Moreover, to our best knowledge, there are no randomized controlled trials (RCT) focusing on LBPP training effects on knee OA rehabilitation, and only several studies use subjective clinical assessments[13]. Thus, the purpose of our RCT study was to investigate the biomechanical effects and the subjective clinical assessment of LBPP treadmill walking exercise when compared with conventional therapy in mild to moderate knee OA patients. We hypothesized that both LBPP training and conventional training could improve the clinical symptoms and gait parameters of knee OA, but the LBPP group might have a more significant effect.
All the subjects were recruited from the Department of Rehabilitation Medicine at the Fifth Affiliated Hospital of Guangzhou Medical University from July 2017 to July 2019. The inclusion criteria consisted of knee OA diagnosis based on the diagnostic criteria of the American College of Rheumatology in 2001: (1) Subjects aged 50-years-old to 75-years-old; (2) Conformed with Kellgren-Lawrence Grade II or III[14] of knee joint OA; (3) Without plans to take any pain killers during this inpatient point; (4) Without cognitive and comprehension impairment following a mini-mental state examination > 26 points; and (5) Signed informed consent. The exclusion criteria consisted of several parameters: (1) Unstable vital signs (i.e. high blood pressure and tachycardia), combined with heart, brain, blood vessel, spirit, liver, kidney, and other serious diseases that affect daily activities; (2) Combined with knee joint tuberculosis, tumors, and other diseases that affect knee function; (3) Combined with rheumatism, gout, severe osteoporosis, and other diseases that affect joint pain and lower limb mobility; (4) Cannot tolerate the experiment; and/or (5) Refused to sign the informed consent. In the cases of bilateral knee OA, the more symptomatic knee was designated as the affected knee. The participant was instructed to base their responses on only the affected knee for the purpose of pre- and post-assessments[15].
This study was designed as a prospective, single-center, randomized pilot study. This study was registered at the China Clinical Trial Registration Centre (No. ChiCTR 1800017677) and approved by the Medical Ethics Association of the Fifth Affiliated Hospital of Guangzhou Medical University (No. KY01-2018-10-18).
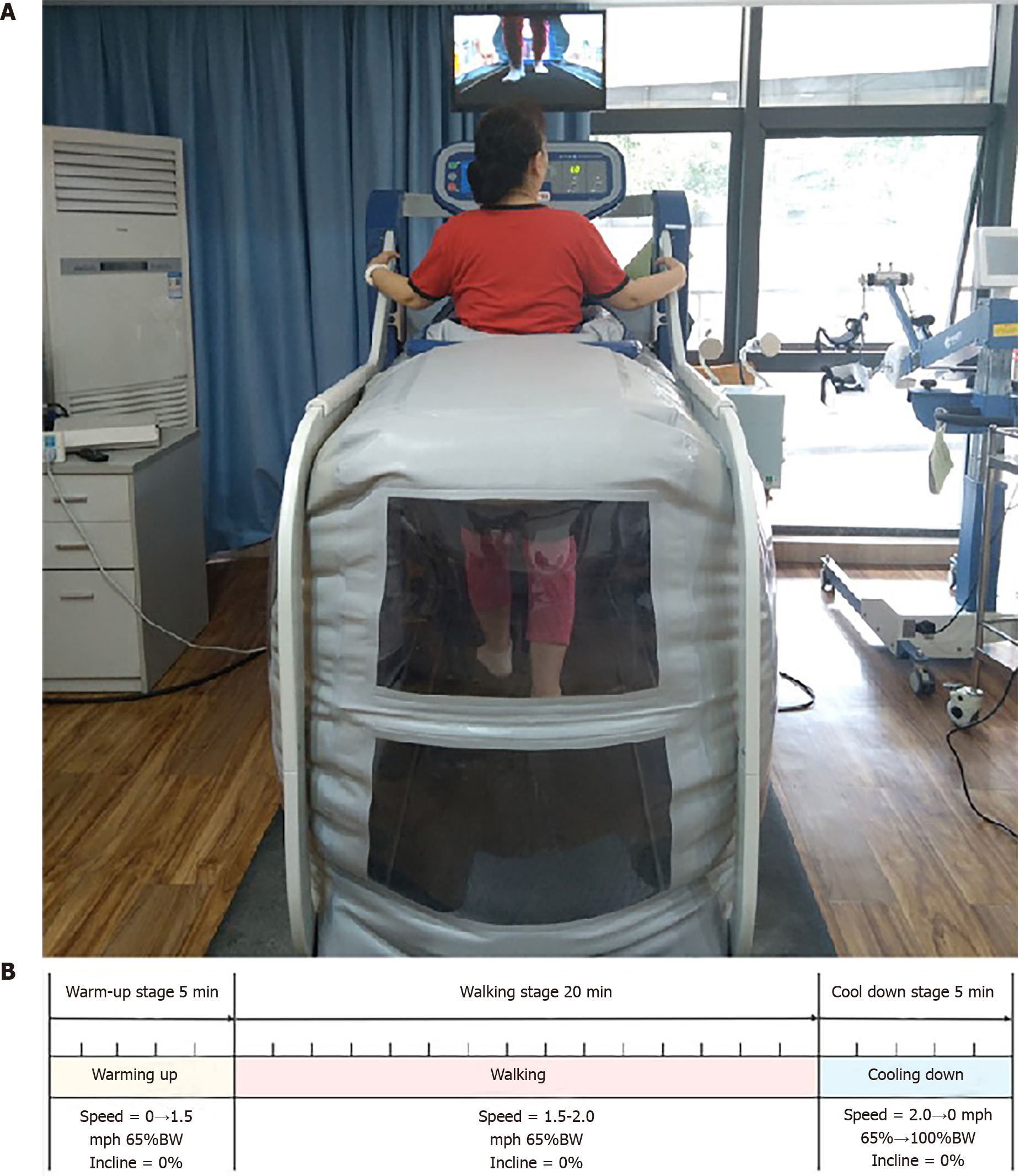
The eligible knee OA patients were randomly assigned to two groups based on the computer-generated random numbers list (LBPP group and control group). The entire training session was supervised by a trained physiotherapist for guidance and safety. The patients in the LBPP group (experimental treatment group) performed an LBPP walking training program (provided using AlterG M320 Antigravity Treadmill, California, USA) for 30 min/session/day, 6 d/week for 2 wk, which was based on our previous study[11]. The LBPP walking protocol included 20 min walking stage (speed = 1.5–2.0 mph, BW = 65%, Incline = 0%) and 10 min warm-up and cooling down stage at the beginning and ending of the session (Figure 1B). The patients were allowed to use handrails during LBPP treadmill training to help them keep their balance. Before the LBPP walking training, the physiotherapist checked the patient’s blood pressure (BP) and heart rate (HR) using an electronic blood pressure monitor (Omron-U10L, Omron Healthcare Co., Ltd., China) to make sure the patient was in optimum condition for exercise (60 bpm ≤ HR ≤ 120 bpm and 90/60 mmHg ≤ BP ≤ 160/100 mmHg). When the patient stood into the LBPP treadmill, the calculation would be run automatically before training started. And during the LBPP walking training, the safety lanyard supplied with the LBPP treadmill should be attached to the patient’s clothing for emergency stops during the training process in case the patient falls or does not feel well (Figure 1A). The patients in the control group (conventional treatment group) performed walking on the indoor ground at a self-selected speed for 30 min/session per day, 6 d per week for 2 wk. The control group (conventional treatment group) performed walking on the indoor ground at a self-selected speed for 30 min/session per day, 6 d per week for 2 wk. Each walking session consisted of 5-min walking and 5-min seated rest for 3 cycles. Moreover, during walking, the physical therapist guided the patient to keep the range of motion of the knee joint at 0-15° to make the heel fully contact the ground. The patients were allowed to use any assistive device (i.e. canes, crutches, and walkers) during walking to help them keep balance. Each group (LBPP group and control group) understood that they would complete 12 sessions for the total amount using the same venue. Meanwhile, both groups maintained the same amount of conventional physiotherapy and manual therapy daily based on the clinical guidelines of knee OA[2].
All patients underwent clinical assessments and gait analysis at pre- and 2-wk post-treatment time points. The assessment method was based on our previous study[11]. Lower limb function and mobility for knee OA patients were measured by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) with five items for pain, two items for stiffness, and 17 functional items[16]. Total scores range from 0 (complete disability) to 100 (no disability). Subjective pain was evaluated using the visual analog scale (VAS) ranging from 0 (no pain) to 10 (severe pain). The active knee joint range-of-motion (AROM) was measured using a handheld 2-arm goniometer[17]. Independence in activities of daily living (ADL) was assessed by modified Barthel index[18] and total scores range from 0 (complete dependent) to 100 (complete independent). Gait analysis was performed using the BTS Smart DX 7000 (Bioengineering Technology System, Milan, Italy). Twenty-two spherical markers were positioned bilaterally on the patient’s anatomical landmarks based on the Davis protocol[19]. The patients were instructed to stand straight for 30 sec, which represented the standing phase, and then walk with a self-selected speed along the walkway five times as the walking phase. Temporo-spatial parameters and knee flexion/extension trajectory in the gait cycle were recorded and included in statistical analysis. Calibration of the gait analysis system was performed by the designated lab member every week to make sure data acquisition was accurate. The primary outcomes were gait parameters, which were used to evaluate gait performance/ walking ability. The second outcomes were clinical assessment scales, which were used to represent symptom improvement.
To calculate the statistical power, we set the standardized difference of the primary outcome (gait velocity) to be equal to 0.2, and the dropout rate to be equal to 20%[20]. The sample size for each group should be more than nine.
All statistical analyses were conducted using IBM SPSS 25.0 software. Parametric data were presented as means ± SD if normally distributed or median if not. Counting and grade data were presented as ratios. Baseline characteristics and post-treatment outcome measures between groups were compared using independent t-tests. Pre- and post-treatment outcome measures within the group were compared using the paired-t-test. Measurement data that do not conform to the normal distribution and the uniformity of variance were compared using the nonparametric rank-sum Mann-Whitney U test. The test level was statistically significant at P < 0.05.
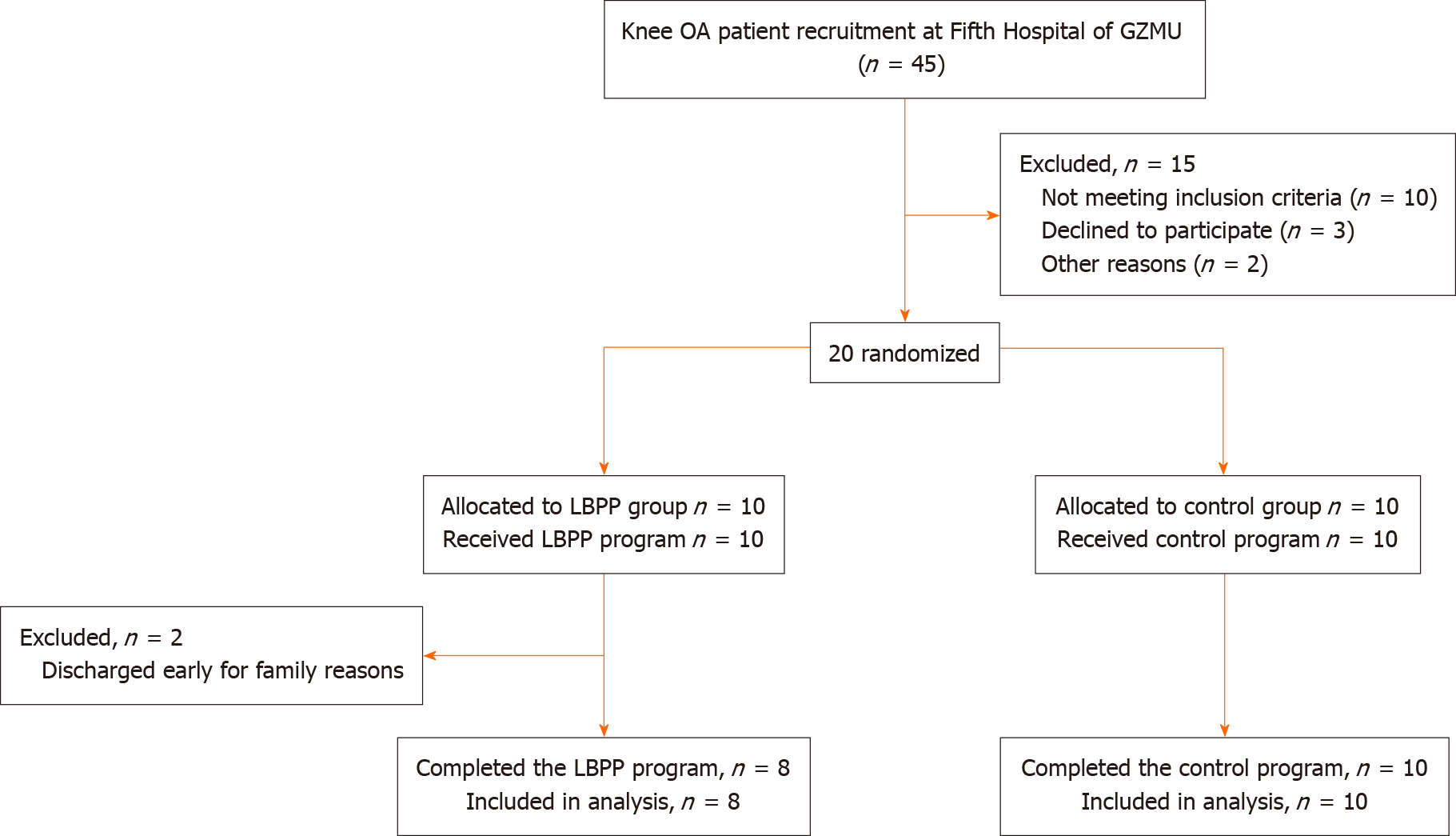
A total of twenty patients, of whom ten were allocated into the LBPP group and ten were allocated into the control group, were screened for this study. Two patients in the LBPP group were discharged early for family reasons and nobody was dropped out because of intolerance for the whole process (Figure 2). The basic characteristics of the patients in each group are displayed in Table 1. The mean age of each group was 59.63 ± 8.40-years-old in the LBPP group vs 58.30 ± 8.54-years-old in the control group. The body mass index (BMI) was 23.24 ± 0.85 in the LBPP group vs 23.02 ± 1.37 in the control group. The baseline Kellgren-Lawrence grade was 2.25 ± 0.45 in the LBPP group vs 2.30 ± 0.48 in the control group. No significant differences in age, BMI, gender ratio, and Kellgren-Lawrence grade at baseline between the two groups were noted.
| Characteristic | LBPP group (n = 8) | Conventional group (n = 10) | P value |
| Age (yr) | 59.63 ± 8.40 | 58.30 ± 8.54 | 0.746 |
| Sex (male/female) | 3/7 | 2/6 | 1.000 |
| Height (cm) | 162.25 ± 5.23 | 162.80 ± 5.12 | 0.825 |
| Weight (kg) | 61.25 ± 4.57 | 61.11 ± 6.07 | 0.958 |
| BMI (kg/m2) | 23.24 ± 0.85 | 23.02 ± 1.37 | 0.178 |
| Duration of symptoms (mo) | 68.13 ± 14.60 | 63.40 ± 15.00 | 0.983 |
| Affected knee (left/right) | 3/5 | 4/6 | 1.000 |
| Kellgren-Lawrence Grade (Grade 2/Grade 3) | 6/8 | 7/3 | 0.897 |
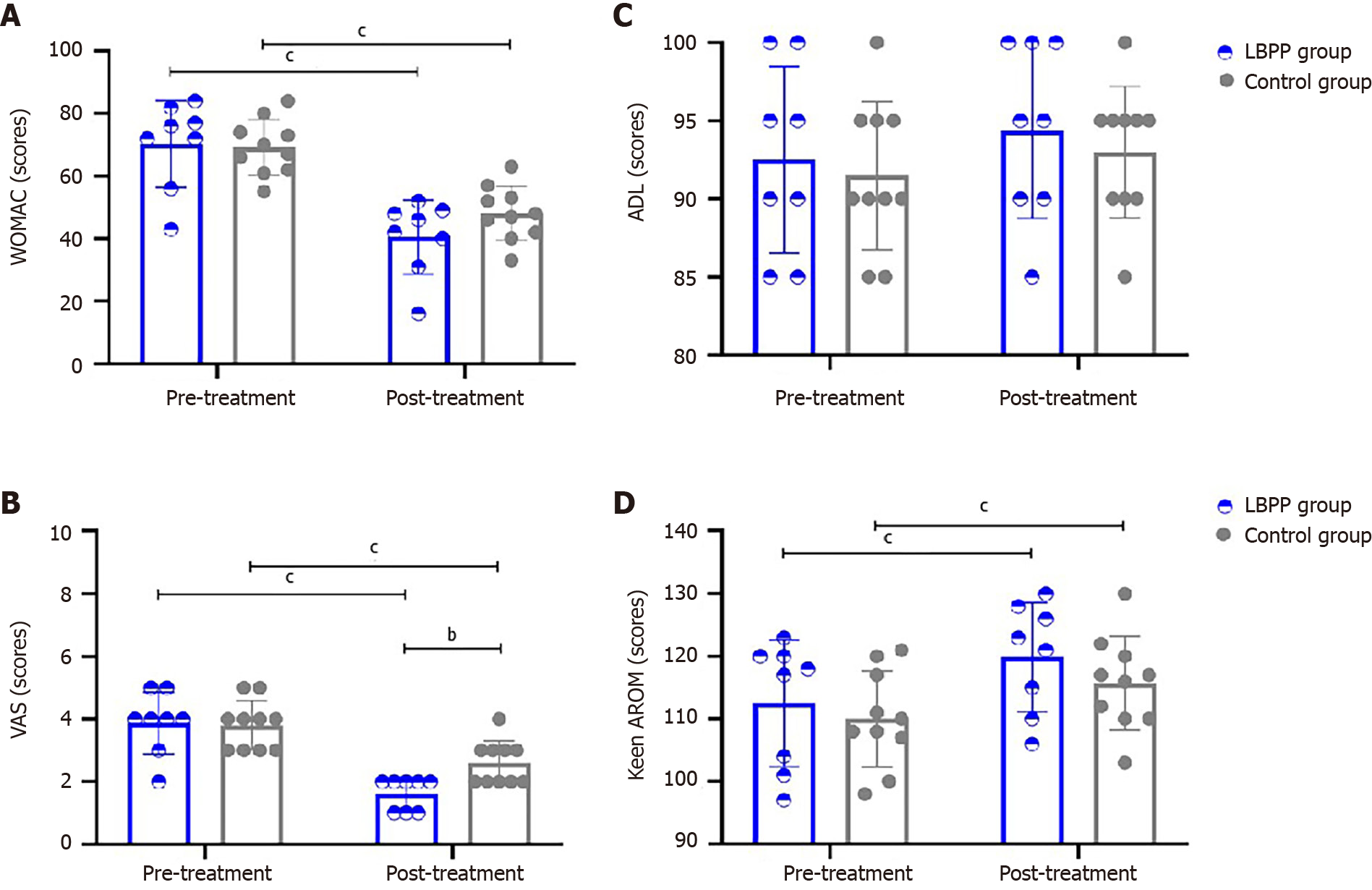
The comparison results of clinical assessments are shown within-group (pre-treatment vs post-treatment) or between two groups (LBPP group vs control group) in Table 2 and Figure 3. No significant differences in WOMAC, VAS, Knee AROM flex-extension, or ADL at baseline (pre-treatment timepoint) between the two groups were noted. For comparisons within-group between pre- and post-treatment, WOMAC scores of patients in both groups were found to decrease significantly in the post-treatment timepoint (LBPP group: 70.25 ± 13.93 vs 40.50 ± 11.86, P < 0.001; control group: 69.20 ± 8.88 vs 48.10 ± 8.67, P < 0.001), VAS scores of the patients in both groups were found to have decreased significantly in the post-treatment point (LBPP group: 3.88 ± 0.99 vs 1.63 ± 0.52, P < 0.001; control group: 3.80 ± 0.79 vs 2.60 ± 0.70, P < 0.001), and knee AROM flex-extension of the patients in both groups were found to have increased significantly in the post-treatment point (LBPP group: 112.50 ± 10.13 vs 119.88 ± 8.71, P < 0.001; control group: 110.00 ± 7.69 vs 115.70 ± 7.50, P < 0.001). For comparisons of post-treatment parameters among groups between the LBPP group and the control group, VAS scores were significantly decreased more in the LBPP group than the control group (1.63 ± 0.52 in the LBPP group vs 2.60 ± 0.70 in the control group, P < 0.01).
| LBPP group | Conventional group | P1 value | P2 value | P3 value | P4 value | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |||||
| WOMAC (scores) | 70.25 ± 13.93 | 40.50 ± 11.86 | 69.20 ± 8.88 | 48.10 ± 8.67 | < 0.001 | < 0.001 | 0.848 | 0.135 |
| VAS (scores) | 3.88 ± 0.99 | 1.63 ± 0.52 | 3.80 ± 0.79 | 2.60 ± 0.70 | < 0.001 | < 0.001 | 0.860 | 0.005 |
| Knee AROM (degree) | 112.50 ± 10.13 | 119.88 ± 8.71 | 110.00 ± 7.69 | 115.70 ± 7.50 | < 0.001 | < 0.001 | 0.559 | 0.290 |
| ADL (scores) | 92.50 ± 5.98 | 94.38 ± 5.63 | 91.50 ± 4.74 | 93.00 ± 4.22 | 0.197 | 0.081 | 0.697 | 0.561 |
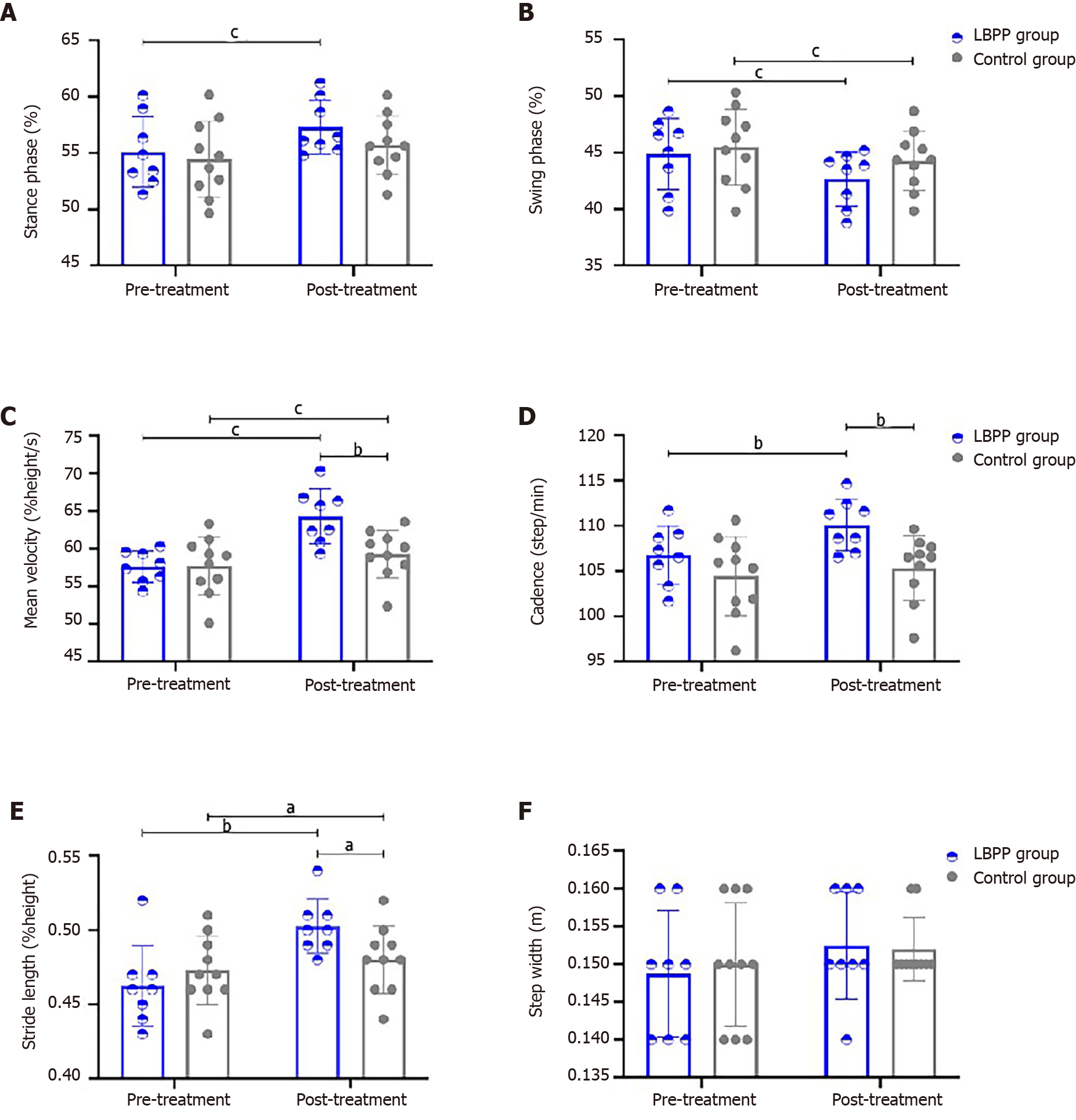
The comparison results of gait spatial-temporal parameters are shown within-group (pre- vs post-treatment) or between two groups (LBPP vs control group) in Table 3 and Figure 4. No significant differences in stance phase, swing phase, mean velocity, cadence, stride length, step width, and knee flex-extension during walking at baseline (pre-treatment timepoint) between the two groups were noted. For comparisons within group between pre- and post-treatment, stance phase (%) in LBPP group was found increased significantly at the post-treatment timepoint (55.11 ± 3.13 vs 57.31 ± 2.39, P < 0.001), swing phase (%) in both groups were found decreased significantly at the post-treatment timepoint (LBPP group: 44.89 ± 3.13 vs 42.67 ± 2.39, P < 0.001; control group: 45.52 ± 3.35 vs 44.28 ± 2.60, P < 0.001), mean velocity (%height/s) in both groups were found increased significantly at the post-treatment timepoint (LBPP group: 57.64 ± 2.10 vs 64.28 ± 3.64, P < 0.001; control group: 57.72 ± 3.84 vs 59.33 ± 3.17, P < 0.001), cadence (steps/min) in LBPP group was found increased significantly at the post-treatment timepoint (106.76 ± 3.22 vs 110.10 ± 2.84, P = 0.002), stride length (m) in both groups were found increased significantly at the post-treatment timepoint (LBPP group: 0.46 ± 0.03 vs 0.50 ± 0.18, P < 0.001; control group: 0.47 ± 0.02 vs 0.48 ± 0.23, P = 0.025), and knee flex-extension (degrees) in LBPP group was found increased significantly at the post-treatment timepoint (66.14 ± 5.43 vs 72.34 ± 5.38, P = 0.004). For comparisons of post-treatment parameters among groups between LBPP group and control group, mean velocity was significantly increased more in LBPP group than control group (64.28 ± 3.64 in LBPP group vs 59.33 ± 3.17 in control group, P = 0.007), cadence was significantly increased more in LBPP group than control group (110.10 ± 2.84 in LBPP group vs 105.36 ± 3.58 in the control group, P = 0.008), stride length was significantly increased more in LBPP group than control group (0.50 ± 0.18 in LBPP group vs 0.48 ± 0.23 in control group, P = 0.037), and knee flex-extension was significantly increased more in LBPP group than control group (72.34 ± 5.38 in LBPP group vs 66.89 ± 5.33 in control group, P = 0.048).
| LBPP group | Control group | P1 value | P2 value | P3 value | P4 value | ||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||||||
| Stance phase (%) | 55.11 ± 3.13 | 57.31 ± 2.39 | 54.49 ± 3.35 | 55.72 ± 2.60 | 0.001 | 0.096 | 0.690 | 0.201 | |
| Swing phase (%) | 44.89 ± 3.13 | 42.69 ± 2.39 | 45.52 ± 3.35 | 44.28 ± 2.60 | < 0.001 | < 0.001 | 0.690 | 0.197 | |
| Mean velocity(%height/s) | 57.64 ± 2.10 | 64.28 ± 3.64 | 57.72 ± 3.84 | 59.33 ± 3.17 | < 0.001 | < 0.001 | 0.958 | 0.007 | |
| Cadence (steps/min) | 106.76 ± 3.22 | 110.10 ± 2.84 | 104.46 ± 4.35 | 105.36 ± 3.58 | 0.002 | 0.141 | 0.233 | 0.008 | |
| Stride length (m) | 0.46 ± 0.03 | 0.50 ± 0.18 | 0.47 ± 0.02 | 0.48 ± 0.23 | 0.001 | 0.025 | 0.399 | 0.037 | |
| Step width (m) | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.00 | 0.080 | 0.343 | 0.753 | 0.854 | |
| Knee flex-extension (Degree) | 66.14 ± 5.43 | 72.34 ± 5.38 | 66.40 ± 5.26 | 66.89 ± 5.33 | 0.004 | 0.400 | 0.919 | 0.048 | |
Our study was designed as an RCT with the aim of studying an LBPP treadmill exercising training program for mild to moderate knee OA patients. We found patients in the LBPP group demonstrated more pain alleviation and improved walking ability after the intervention compared with patients in the conventionally treated group although both groups showed some improvement in clinical parameters (WOMAC, VAS, and knee active ROM) and gait parameters after their interventions. Moreover, more improvements in the LBPP group when compared with the control group with respect to walking speed, stride length, and knee ROM during walking represented improved walking ability. In addition, aquatic therapy research has proved the short-term benefits for patients with knee OA, but the high requirement of equipment limited the application.
Exercise improves the symptoms of knee OA, a finding which confirmed that pain relief was obtained with exercise[1]. However, due to the lack of relevant RCT-derived LBPP data in previous studies, it is difficult to determine whether the rehabilitative effects of LBPP for knee OA originate from the exercise effect itself or whether it has its own advantages over conventional exercise training. Thus, our study addresses this gap. In our RCT study, the LBPP group and groups both presented post-treatment improvements in WOMAC, VAS, and knee active ROM scores. Moreover, the LBPP group presented more advantages with respect to VAS relief when compared with the control group, which could be related to biomechanical changes caused by the weight support brought about by the LBPP positive pressure inflation chamber. In addition, the LBPP and control groups did not present improvements in ADL, which may be due to the “ceiling effect” of recruited patients in our study, that is to say, the knee OA patients included in this study had relatively high daily pre-treatment life activities.
The patients in both groups demonstrated improved gait function in mean velocity and stride length, whereas only the patients in the LBPP group demonstrated gait pattern changes after LBPP intervention (stance phase increased, and swing phase decreased). Previous studies presented the standing phase of affected side increased might be related to the pain released. Meanwhile, the LBPP treadmill proved useful for reducing pressure across the entire foot in normal subjects while running[21]. Previous studies have also indicated that the effects of LBPP cause an increase in the reduction of the peak joint force on the knee joint[11]. Based on this finding, we have reason to infer that the knee pressure on the mechanical chain would be reduced accordingly, which might be crucial for knee OA patients undergoing rehabilitation.
The LBPP group also demonstrated an improvement in knee flex-extension during walking both within-group (pre-and post-treatment comparisons) and among the two groups (compared with the control group). Active knee ROM improvements might be more associated with stiffness and pain alleviation, which is also consistent with the clinical assessment findings in our study. Previous research has suggested that physical activity within a certain range most likely promotes the growth and maintenance of knee cartilage, ligaments, and bones in addition to strengthening muscles in order to appropriately distribute loads across the joint[22,23]. Meanwhile, the improvement in knee joint mobility might be related to the restoration of lower limb muscle strength after exercise[1]. Our previous study also found that after LBPP training, lower extremity muscle activity increased[10]. Increased muscle strength may cause a decrease in joint loading rates or localized stress in the articular cartilage, thereby playing an important role in pain reduction and physical function improvement of knee OA[8]. Although previous studies on aquatic therapy proved the short-term benefits for patients with knee OA on the similar improved functional aspects, the high requirement of equipment limited the application.
Finally, we need to point out that all of the recruited patients in both groups were satisfied with the program and showed no side effects associated with the training program, which further supports the application of LBPP training in KOA rehabilitation from a clinical perspective. Our study results from the LBPP assistant intervention for knee OA patients may also reduce the burden of the physical therapist and increase cost-effectiveness than conventional training.
There are several limitations to this RCT study. Our LBPP protocol was a 2-wk intensive inpatient training program focusing on the small sample size of inpatients due to health care policies in China, restrictions of the inclusion criteria (such as unilateral knee joint symptoms as the chief complaint and without the use of any pain killers during this study) and three-dimensional gait analysis application. Future studies should recruit more patients with the aim of observing the long-term effects of LBPP on knee OA and exploring changes at the anatomical level in addition to a personalized weight-support LBPP program for each patient.
The result of our RCT study showed that the LBPP group has a greater effect on improving gait parameters than the conventional group, although there was no significant advantage in clinical assessment. This finding indicates that LBPP treadmill exercise training could be considered an effective approach for alleviating pain symptoms and improving lower extremity locomotion in mild to moderate knee OA patients.
Knee joint pain and stiffness are the two main symptoms of knee osteoarthritis (OA) and thus restrict a patient’s activities, such as walking and walking up and downstairs.
The lower body positive pressure (LBPP) treadmill as one of the emerging body weight support system devices brings new hope for exercise-related rehabilitation for knee OA patients.
The purpose of this study was to investigate the biomechanical effects and the subjective clinical assessment of LBPP tread mill walking exercise when compared with conventional therapy in mild to moderate knee OA patients.
The eligible 18 knee OA patients were randomly assigned to two groups: LBPP and control groups. All patients underwent clinical assessments and three-dimensional gait analysis at pre- and 2-wk post-treatment.
The Western Ontario and McMaster Universities Arthritis Index and visual analog scale scores in both the LBPP group and control group were found to decrease significantly at the post-treatment point than the pre-treatment point. Moreover, compared with the control group, the LBPP group showed more improvements in walking speed, stride length, and knee range of motion during walking, which represented more improvement in walking ability.
The results showed that the LBPP group has a greater effect on improving gait parameters than the conventional group, although there was no significant advantage in clinical assessment.
This finding indicates that LBPP treadmill walking training might be an effective approach for alleviating pain symptoms and improving lower extremity locomotion in mild to moderate knee OA patients.
The author thanks graduate students Yi-Sha Liu, Shao-Ming Xu, Dong-Qin Huang, Pei-Xi Lian, and Yuan-Lu Zhang (Guangzhou Medical University) for data collection. The author also thanks Dr. Qiang Lin and Dr. Jun-Jie Liang for methodology guidance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Rehabilitation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Osailan A S-Editor: Wang JL L-Editor: Filipodia P-Editor: Guo X
| 1. | Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49:1554-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 2. | Page CJ, Hinman RS, Bennell KL. Physiotherapy management of knee osteoarthritis. Int J Rheum Dis. 2011;14:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Basedow M, Williams H, Shanahan EM, Runciman WB, Esterman A. Australian GP management of osteoarthritis following the release of the RACGP guideline for the non-surgical management of hip and knee osteoarthritis. BMC Res Notes. 2015;8:536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, Doherty M, Geenen R, Hammond A, Kjeken I, Lohmander LS, Lund H, Mallen CD, Nava T, Oliver S, Pavelka K, Pitsillidou I, da Silva JA, de la Torre J, Zanoli G, Vliet Vlieland TP; European League Against Rheumatism (EULAR). EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 898] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 5. | Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1960] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 6. | Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1092] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 7. | Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Relat Res. 2005;213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Bennell KL, Wrigley TV, Hunt MA, Lim BW, Hinman RS. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2013;39:145-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 806] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 10. | Liang J, Guo Y, Zheng Y, Lang S, Chen H, You Y, O'Young B, Ou H, Lin Q. The Lower Body Positive Pressure Treadmill for Knee Osteoarthritis Rehabilitation. J Vis Exp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Liang J, Lang S, Zheng Y, Wang Y, Chen H, Yang J, Luo Z, Lin Q, Ou H. The effect of anti-gravity treadmill training for knee osteoarthritis rehabilitation on joint pain, gait, and EMG: Case report. Medicine (Baltimore). 2019;98:e15386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Peeler J, Leiter J, MacDonald P. Effect of Body Weight-Supported Exercise on Symptoms of Knee Osteoarthritis: A Follow-up Investigation. Clin J Sport Med. 2020;30:e178-e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Takacs J, Anderson JE, Leiter JR, MacDonald PB, Peeler JD. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kohn MD, Sassoon AA, Fernando ND. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 838] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 15. | Maly MR, Costigan PA, Olney SJ. Determinants of self efficacy for physical tasks in people with knee osteoarthritis. Arthritis Rheum. 2006;55:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-1840. [PubMed] |
| 17. | Lavernia C, D'Apuzzo M, Rossi MD, Lee D. Accuracy of knee range of motion assessment after total knee arthroplasty. J Arthroplasty. 2008;23:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575-587. |
| 20. | Heo M, Leon AC. Sample size requirements to detect an intervention by time interaction in longitudinal cluster randomized clinical trials. Stat Med. 2009;28:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Hodges-Long L, Cross K, Magrum E, Feger M, Hertel J. The effect of body weight reduction using a lower body positive pressure treadmill on plantar pressure measures while running. Phys Ther Sport. 2020;43:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Abbasi J. Can Exercise Prevent Knee Osteoarthritis? JAMA. 2017;318:2169-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 23. | Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |