Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10585
Peer-review started: July 23, 2021
First decision: August 19, 2021
Revised: August 22, 2021
Accepted: October 14, 2021
Article in press: October 14, 2021
Published online: December 6, 2021
Processing time: 130 Days and 5.3 Hours
Prompt and effective cardiopulmonary resuscitation (CPR) can promote the recovery of spontaneous circulation to some extent and can save patients’ lives. The minimum target of cardiac resuscitation is the restoration of spontaneous circulation (ROSC). However, owing to prolonged sudden cardiac arrest, there is relatively high mortality within 24 h after cardiac resuscitation. Moreover, severe cerebral anoxia can deteriorate the prognosis of patients. Therefore, it is important to adopt an effective clinical evaluation of acute myocardial infarct (AMI) patients’ prognosis after cardiac resuscitation for the purpose of prevention and management.
To investigate early CPR effects on human myeloperoxidase (MPO), soluble ST2 (sST2), and hypersensitive C-reactive protein (hs-CRP) levels in AMI patients.
In total, 54 patients with cardiac arrest caused by AMI in our hospital were selected as the observation group, and 50 other patients with AMI were selected as the control group. The differences in serum levels of MPO, sST2, and hs-CRP between the observation group and the control group were tested, and the differences in the serum levels of MPO, sST2, and hs-CRP in ROSC and non-ROSC patients, and in patients who died and in those who survived, were analyzed.
Serum levels of MPO, sST2, hs-CRP, lactic acid, creatine kinase isoenzyme (CK-MB), and cardiac troponin I (cTnI) were significantly higher in the observation group than in the control group (P < 0.05). Serum levels of MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI in the observation group were lower after CPR than before CPR (P < 0.05). In the observation group, MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI serum levels were lower in ROSC patients than in non-ROSC patients (P < 0.05). MPO, sST2, hs-CRP, and lactic acid serum levels of patients who died in the observation group were higher than those of patients who survived (P < 0.05). The areas under receiver operating characteristic curve predicted by MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI were 0.616, 0.681, 0.705, 0.704, 0.702, and 0.656, respectively (P < 0.05). The areas under receiver operating characteristic curve for MPO, SST2, hs-CRP, and lactic acid to predict death were 0.724, 0.800, 0.689, and 0.691, respectively (P < 0.05). Logistic regression analysis showed that MPO, sST2, and hs-CRP were the influencing factors of ROSC [odds ratios = 1.667, 1.589, and 1.409, P < 0.05], while MPO, sST2, hs-CRP, and lactic acid were the influencing factors of death (odds ratios = 1.624, 1.525, 1.451, and 1.365, P < 0.05).
Serum levels of MPO, sST2, hs-CRP, and lactic acid have a certain value in predicting recovery and prognosis of patients with ROSC.
Core Tip: Acute myocardial infarction (AMI) is one of the leading causes of death. Novel cardiac markers have provided an effective method for early diagnosis of AMI. Our study mainly explored the effects of early cardiopulmonary resuscitation on serum levels in AMI patients.
- Citation: Hou M, Ren YP, Wang R, Lu LX. Early cardiopulmonary resuscitation on serum levels of myeloperoxidase, soluble ST2, and hypersensitive C-reactive protein in acute myocardial infarction patients. World J Clin Cases 2021; 9(34): 10585-10594
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10585.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10585
Acute myocardial infarction (AMI) is one of the leading causes of death. Therefore, early detection, diagnosis, and treatment are of great significance[1]. The traditional diagnosis of AMI mainly relies on examining myocardial enzyme profiles. However, elevation of markers is usually significant 4 h after AMI, which can lead to misdiagnosis and missed diagnosis of AMI[2].
In recent years, novel cardiac markers have provided an effective method for the early diagnosis of AMI[3]. AMI patients receive treatment with percutaneous coronary intervention to dredge the blocked blood vessel, significantly reducing the incidence of adverse cardiac events[4]. However, many patients eventually succumb to adverse cardiac events due to severe systemic or local cardiac inflammation. To cope with immediate cardiac arrest after AMI, timely cardiopulmonary resuscitation (CPR) is the main approach to shorten the duration of myocardial ischemia and hypoxia, thus improving the prognosis of patients[5]. Our study mainly explored and discussed the effects of early cardiopulmonary resuscitation on serum levels of human myeloperoxidase (MPO), soluble ST2 (sST2), and hypersensitive C-reactive protein (hs-CRP) in patients with cardiac arrest caused by AMI.
A total of 54 AMI patients with cardiac arrest who were managed in our hospital from January 2020 to April 2021 were selected as the observation group. The following were the inclusion criteria: (1) diagnosis of AMI based on standards of "Practical Internal Medicine"[6]; (2) meets criteria of cardiac arrest: Loss of consciousness, with or without the disappearance of great artery pulsation, no spontaneous breathing or sighing breathing; (3) time from onset to admission ≤ 6 h; and (4) informed consent obtained from the patient’s family. The following were the exclusion criteria: (1) patients with absolute contraindication to CPR; and (2) those with complications, such as malignancy, liver and kidney dysfunction, and blood system diseases. Fifty patients with AMI were selected as the control group (Table 1).
| Clinical data | Observation group (n = 54) | Control group (n = 50) | t/χ2 | P value |
| Male / female | 32/22 | 31/19 | 0.082 | 0.775 |
| Age (yr) | 56.60 ± 6.67 | 57.12 ± 7.10 | -0.385 | 0.701 |
| Body mass index (kg/m2) | 22.19 ± 2.03 | 22.03 ± 2.17 | 0.389 | 0.698 |
| Smoking | 34 (62.96) | 32 (64.00) | 0.012 | 0.913 |
| Hypertension | 33 (61.11) | 29 (58.00) | 0.104 | 0.747 |
| Diabetes | 16 (29.63) | 17 (34.00) | 0.229 | 0.632 |
| Hyperlipidemia | 20 (37.04) | 15 (30.00) | 0.576 | 0.448 |
About 10 mL of venous blood from the patients’ elbow was extracted before and after treatment. A 2500 r/min centrifuge with a centrifugation radius of 6 cm was used for 5 min to separate the supernatant. Double antibody sandwich chemiluminescent immunoassay was used to detect the levels of MPO and cardiac troponin I (cTnI). Biotinylated monoclonal MPO- and cTnI-specific antibodies were mixed with serum to form an antigen-antibody complex. Next, streptomycin magnetic beads were added for incubation. The magnetic beads were adsorbed on the electrode surface by the combination of biotin and streptavidin. Electrode voltage promoted the chemiluminescence of the complex and measured the luminescence intensity. Elecsys software was used to automatically calculate MPO contents and high-sensitive cardiac troponin T through the calibration curve. Serum levels of lactic acid, SST2, and hs-CRP were determined using an enzyme-linked immunosorbent assay kit (Shanghai Enzyme Link Industrial Co., Ltd., Shanghai, China). Biochemical indices included creatine kinase isoenzyme (CK-MB), serum creatinine (Scr), blood urea nitrogen (BUN), and the ratio of aspartate aminotransferase to alanine aminotransferase. The Japan 7170A automatic biochemical analyzer was used to detect cTnI using a rapid test kit.
The medical staff evaluated and examined the patients’ vital indicators at the scene. CPR was initiated if the patient had no vital signs. We made sure the patient was lying flat when chest compressions were performed. The palms of both hands were placed on the xiphoid process of the patient’s chest with appropriate folding methods, and the pressure applied was vertical to the weight and the strength of the body. The depth of the pressure in adult was a sternum depression of > 5 cm, and the pressure was maintained 30 times in each group with the frequency of 100 times per min. The patients’ airways were kept open, their head and neck were lifted, and any dirt in the mouth was removed. Subsequently, they were provided artificial respiration twice. The patients’ nasal cavities were closed when blowing, and the air was made sufficient to make the patients’ chest rise and fall. The ratio of chest compressions to artificial respiration was 30:2. Restoration of spontaneous circulation (ROSC) was achieved if after cardiac arrest, continuous heartbeat, and breathing resumed within 24 h after treatment.
SPSS software version 22.0 (Armonk, NY, United States) was used for all statistical analyses. Statistical significance was set at P < 0.05. Measurement data conforming to normal distribution were expressed as mean ± SD, and the t-test was used to compare groups. Enumeration data were expressed as frequency or percentage, and comparisons between groups were made using the χ2 test. The predicted value was analyzed by the receiver operating characteristic (ROC) curve. Logistic regression was used for multivariate analysis.
The serum levels of MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI in the observation group were significantly higher than those in the control group (P < 0.05). There was no significant difference in the serum levels of Scr and BUN between the two groups (P > 0.05, Table 2).
| Index | Control group (n = 54) | Control group (n = 50) | t | P value |
| MPO (ng/L) | 2.95 ± 0.89 | 2.01 ± 0.92 | 5.295 | 0.000 |
| sST-2 (μg/L) | 115.50 ± 21.10 | 96.60 ± 17.22 | 4.981 | 0.000 |
| hs-CRP (mg/L) | 3.76 ± 0.97 | 2.67 ± 0.87 | 6.015 | 0.000 |
| Lactic acid (mmol/L) | 5.77 ± 0.88 | 5.02 ± 0.92 | 4.249 | 0.000 |
| CK-MB (U/L) | 76.39 ± 8.28 | 65.50 ± 12.21 | 5.358 | 0.000 |
| cTnI (μg/L) | 4.59 ± 0.82 | 3.83 ± 0.90 | 4.506 | 0.000 |
| Scr (μmol/L) | 78.29 ± 21.12 | 74.40 ± 19.18 | 0.981 | 0.329 |
| BUN (mmol/L) | 6.70 ± 1.00 | 6.92 ± 1.04 | -1.100 | 0.274 |
| AST (U/L) | 32.20 ± 9.29 | 34.40 ± 8.15 | -1.279 | 0.204 |
| ALT (U/L) | 29.38 ± 5.60 | 30.10 ± 5.12 | -0.683 | 0.496 |
| TC (mmol/L) | 4.20 ± 0.92 | 4.10 ± 0.98 | 0.537 | 0.593 |
| TG (mmol/L) | 1.30 ± 0.32 | 1.35 ± 0.39 | -0.717 | 0.475 |
| HDL-C (mmol/L) | 1.31 ± 0.29 | 1.35 ± 0.30 | -0.691 | 0.491 |
| LDL-C (mmol/L) | 2.24 ± 0.82 | 2.44 ± 0.91 | -1.179 | 0.241 |
The serum levels of MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI in the observation group after CPR were significantly lower than those before CPR (P < 0.05) (Table 3).
| Index | Before CPR (n = 54) | After CPR (n = 54) | t | P value |
| MPO (ng/L) | 2.95 ± 0.89 | 2.30 ± 0.90 | 3.701 | 0.000 |
| sST-2 (μg/L) | 115.50 ± 21.10 | 105.54 ± 17.89 | 2.586 | 0.011 |
| hs-CRP (mg/L) | 3.76 ± 0.97 | 3.01 ± 0.95 | 3.979 | 0.000 |
| Lactic acid (mmol/L) | 5.77 ± 0.88 | 5.15 ± 0.82 | 3.709 | 0.000 |
| CK-MB (U/L) | 76.39 ± 8.28 | 70.40 ± 11.16 | 3.124 | 0.002 |
| cTnI (μg/L) | 4.59 ± 0.82 | 4.02 ± 0.97 | 3.244 | 0.002 |
The serum levels of MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI in ROSC patients of the observation group were significantly lower than those of non-ROSC patients (P < 0.05) (Table 4).
| Index | ROSC group (n = 24) | Non-ROSC group (n = 30) | t | P value |
| MPO (ng/L) | 2.71 ± 0.42 | 3.14 ± 0.47 | -3.500 | 0.001 |
| sST-2 (μg/L) | 110.20 ± 15.65 | 119.90 ± 17.05 | -2.154 | 0.036 |
| hs-CRP (mg/L) | 3.54 ± 0.72 | 3.97 ± 0.82 | -2.020 | 0.049 |
| Lactic acid (mmol/L) | 5.52 ± 0.70 | 5.98 ± 0.63 | -2.538 | 0.014 |
| CK-MB (U/L) | 74.43 ± 6.50 | 78.38 ± 7.10 | -2.108 | 0.040 |
| cTnI (μg/L) | 4.41 ± 0.70 | 4.81 ± 0.65 | -2.172 | 0.034 |
The serum levels of MPO, sST2, hs-CRP, and lactic acid in patients who died in the observation group before CPR were significantly higher than those of patients who survived (P < 0.05). However, there was no significant difference in the serum levels of CK-MB and cTnI in the observation group between those patients who died and those who survived (P > 0.05) (Table 5).
| Index | Death (n = 35) | Survival (n = 19) | t | P value |
| MPO (ng/L) | 3.11 ± 0.58 | 2.64 ± 0.68 | 2.676 | 0.010 |
| sST-2 (μg/L) | 120.02 ± 15.30 | 106.83 ± 16.10 | 2.971 | 0.004 |
| hs-CRP (mg/L) | 3.89 ± 0.59 | 3.50 ± 0.60 | 2.306 | 0.025 |
| Lactic acid (mmol/L) | 5.69 ± 0.80 | 5.19 ± 0.74 | 2.250 | 0.029 |
| CK-MB (U/L) | 74.82 ± 6.82 | 73.68 ± 7.05 | 0.580 | 0.565 |
| cTnI (μg/L) | 4.62 ± 0.78 | 4.53 ± 0.69 | 0.421 | 0.675 |
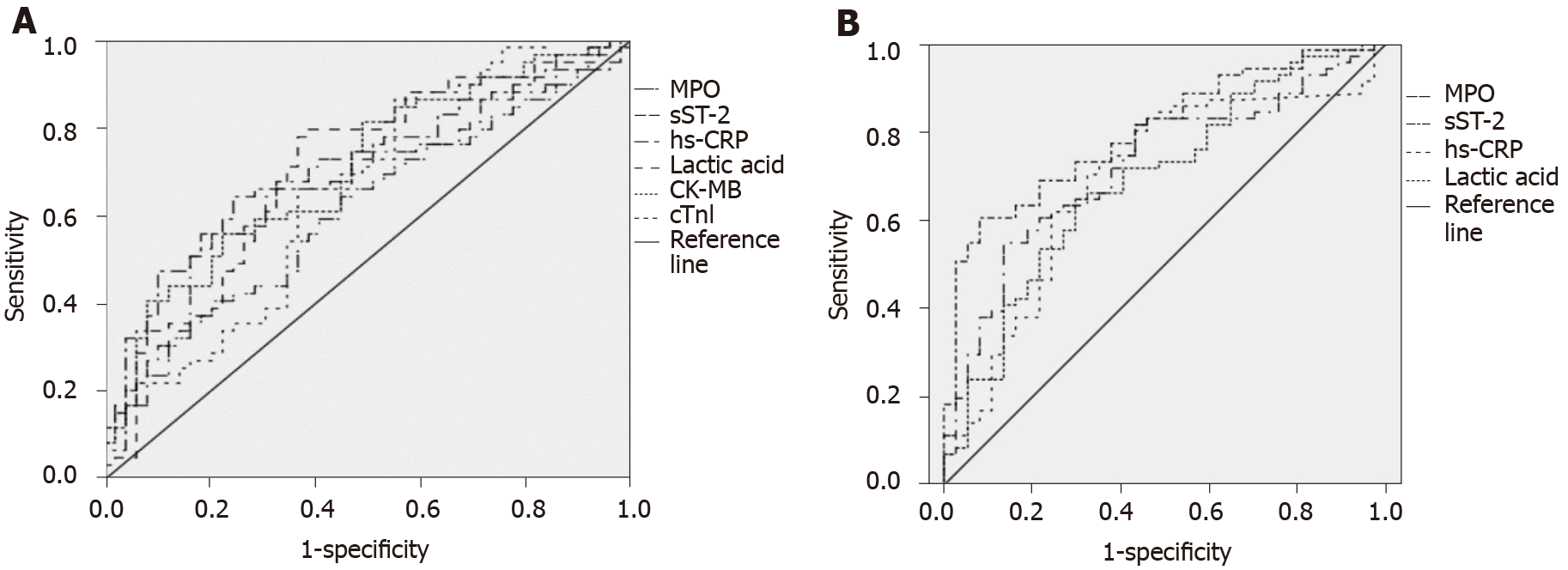
The areas under the ROC curve predicted by MPO, SST2, hs-CRP, lactic acid, CK-MB, and cTnI were 0.616, 0.681, 0.705, 0.704, 0.702, and 0.656, respectively (P < 0.05), as shown in Figure 1A, while the specific parameters are shown in Table 6. On the other hand, the areas under the ROC curve for MPO, SST2, hs-CRP, and lactic acid in predicting death were 0.724, 0.800, 0.689, and 0.691, respectively (P < 0.05), as shown in Figure 1B, while the specific parameters are shown in Table 7.
| Parameter | Area under curve | P value | Cut off value | Sensitivity (%) | Specificity (%) |
| MPO | 0.616 | 0.039 | 3.50 | 40.70 | 79.60 |
| sST-2 | 0.681 | 0.001 | 121.69 | 55.90 | 79.60 |
| hs-CRP | 0.705 | 0.000 | 3.93 | 64.40 | 75.50 |
| Lactic acid | 0.704 | 0.000 | 5.76 | 78.00 | 63.30 |
| CK-MB | 0.702 | 0.000 | 76.96 | 55.90 | 77.60 |
| cTnI | 0.656 | 0.005 | 3.98 | 86.40 | 44.90 |
| Parameter | Area under curve | P value | Cut off value | Sensitivity (%) | Specificity (%) |
| MPO | 0.724 | 0.000 | 3.36 | 54.90 | 86.50 |
| sST-2 | 0.800 | 0.000 | 114.52 | 60.60 | 91.90 |
| hs-CRP | 0.689 | 0.001 | 3.48 | 73.20 | 64.90 |
| Lactic acid | 0.691 | 0.001 | 5.39 | 64.80 | 70.30 |
Logistic regression analysis was conducted with MPO, sST2, hs-CRP, lactic acid, CK-MB, and cTnI as independent variables and ROSC (or non-ROSC) as the dependent variable. The results showed that MPO, sST2, and hs-CRP were the influencing factors of ROSC [odds ratios (OR) = 1.667, 1.589, and 1.409, respectively, P < 0.05] (Table 8). Moreover, logistic regression analysis was conducted with MPO, sST2, and hs-CRP as independent variables, and death (or survival) as the dependent variable. The results showed that MPO, sST2, hs-CRP, and lactic acid were the influencing factors for death (OR = 1.624, 1.525, 1.451, and 1.365, respectively, P < 0.05) (Table 9).
| Factor | SE | Walds | P value | OR (95%CI) | |
| MPO | 0.511 | 0.194 | 6.938 | 0.000 | 1.667 (1.140-2.438) |
| sST-2 | 0.463 | 0.135 | 11.762 | 0.000 | 1.589 (1.219-2.070) |
| hs-CRP | 0.343 | 0.112 | 9.379 | 0.000 | 1.409 (1.131-1.755) |
| Factor | SE | Walds | P value | OR (95%CI) | |
| MPO | 0.485 | 0.182 | 7.101 | 0.000 | 1.624 (1.137-2.320) |
| sST-2 | 0.422 | 0.121 | 12.163 | 0.000 | 1.525 (1.203-1.933) |
| hs-CRP | 0.372 | 0.109 | 11.648 | 0.000 | 1.451 (1.172-1.796) |
| lactic acid | 0.311 | 0.123 | 6.393 | 0.000 | 1.365 (1.072-1.737) |
Brain damage in patients with cardiac arrest is usually caused by abnormal blood flow resulting in systemic ischemia. Since the brain has high oxygen demand and sensitivity to hypoxia, cardiac arrest leads to depolarization of cell membranes and production of free radicals[7,8]. Moreover, free radicals can induce oxidative stress and neuronal damage to a certain extent. Cells will also undergo apoptosis and necrosis, and many metabolites will cross through the blood-brain barrier. Therefore, the prognostic outcome of patients can be evaluated by testing the corresponding serum markers[9-11].
MPO is a type of hemoglobin, an important inflammatory factor, and an important marker of oxidative stress, which plays a significant role in atherosclerosis[12]. Therefore, the increase in MPO will affect the activity of heme oxidase, leading to metabolic disorders of hemoglobin. This further affects the blood oxygen saturation and contributes to the deterioration of an AMI patient’s condition[13]. As a member of the interleukin-1 receptor superfamily, sST2 is mainly expressed in mast cells. In Th2 cells and fibroblasts, its role is mainly for immunomodulatory functions in various inflammatory processes[14,15].
Our study showed that serum levels of MPO and sST2 in AMI patients were significantly higher than those in the control group. Moreover, the levels of MPO and sST2 were significantly decreased after CPR, indicating that MPO and sST2 may participate in the occurrence and development of AMI. Furthermore, in vivo MPO reduces the utilization of nitric oxide in the body, promotes the oxidation of low-density lipoprotein, and accelerates the deposition of cholesterol in the blood vessel wall. These promote endothelial dysfunction, leading to the formation of unstable plaques and adverse cardiovascular events, wherein inflammation is significantly increased. In contrast, after CPR, the blood oxygen saturation, immune inflammation, MPO, and sST2 levels are significantly reduced.
Hs-CRP, an acute-phase protein synthesized by the liver, can chemically attract monocytes, induce the production of tissue factors, and promote thrombin[16]. Meanwhile, CRP is also a chemokine of fibrinogen, which enables macrophages to adhere to the endothelial surface and transplant to the intima, causing reactive T lymphocytes accumulation, enhanced platelet activity, imbalance of coagulation and fibrinolysis systems, and promotion of arterial thrombosis. All these mechanisms can lead to instability and rupture of atherosclerotic plaques, leading to acute coronary syndrome[17].
CK-MB and cTnI are the main clinical indicators of myocardial injury examination and have a certain reference value in predicting the degree of myocardial ischemia injury. The combined detection of the two can improve early diagnosis rate and degree monitoring in the treatment process[18,19]. Lactic acid is the final product of human anaerobic glycolysis. When tissues are starved of oxygen, they undergo anaerobic metabolism, resulting in elevated levels of lactic acid in the patient’s blood, which can indicate the extent of brain damage[20].
In our study, the serum levels of sST2, hs-CRP, lactic acid, CK-MB, and cTnI in the observation group were significantly higher than those in the control group (P < 0.05). These indices decreased after CPR (P < 0.05). In addition, the levels of serum MPO, hs-CRP, lactic acid, CK-MB, and cTnI in ROSC patients were significantly lower than those in non-ROSC patients (P < 0.05). The analysis suggests that the myocardium of patients with myocardial infarction has different degrees of damage, coagulation dysfunction, secondary brain injury, cardiac insufficiency, and other symptoms. Therefore, the serum levels of sST2, hs-CRP, lactic acid, CK-MB, and cTnI increased accordingly. When CPR was performed and ROSC occurred, brain injury and myocardial ischemia injury symptoms improved, myocardial contractility significantly increased, and myocardial indices significantly decreased. Thus, serum indicators have a higher value in predicting ROSC and death. Multivariate analysis results showed that MPO, sST2, and hs-CRP were the influencing factors of ROSC, and MPO, SST2, hs-CRP, and lactic acid were the influencing factors of patients’ death, and this is consistent with the findings of previous reports. Thus, these serum indicators could be used as important predictors in clinical research.
Currently, there are no clinical studies that report on changes in serum MPO, sST2, hs-CRP, lactic acid, among others in patients with AMI who had cardiac arrest and CPR. Our study suggests using these objective laboratory indicators to predict ROSC recovery and clinical prognosis of patients with AMI who had CPR.
The limitations of our study include a lack of in-depth research on the corresponding mechanism and its relatively small sample size. Therefore, further in-depth multi-center research with large samples is recommended.
The levels of serum MPO, sST2, hs-CRP, and lactic acid were significantly decreased in patients with cardiac arrest caused by AMI after CPR. Moreover, MPO, sST2, hs-CRP, and lactic acid had a certain value in predicting the recovery and prognosis of patients with ROSC.
The minimum target of cardiac resuscitation is the restoration of spontaneous circulation.
Effective clinical evaluation of the prognosis of patients with acute myocardial infarction (AMI) after cardiac resuscitation is of great significance.
This study aimed to explore the effect of cardiopulmonary resuscitation (CPR) on the levels of myeloperoxidase (MPO), soluble ST2 (sST2), and hypersensitive C-reactive protein (hs-CRP) in patients with AMI.
A total of 54 AMI patients with cardiac arrest who were managed in our hospital were selected as the observation group. Fifty patients with AMI were selected as the control group.
Serum levels of MPO, sST2, hs-CRP, lactic acid, creatine kinase isoenzyme, and troponin I were significantly higher in the observation group than in the control group (P < 0.05).
MPO, sST2, hs-CRP, and lactic acid had a certain value in predicting the recovery and prognosis of patients with restoration of spontaneous circulation.
Further in-depth multi-center research with large samples is recommended.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Emergency Medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reyher C S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Jensen MT. Resting heart rate and relation to disease and longevity: past, present and future. Scand J Clin Lab Invest. 2019;79:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, Quenot JP, Kimmoun A, Cariou A, Lassus J, Harjola VP, Meziani F, Louis G, Rossignol P, Duarte K, Girerd N, Mebazaa A, Vignon P; Collaborators. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2018;72:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Wojciechowska A, Braniewska A, Kozar-Kamińska K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med. 2017;26:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 4. | Benedek T, Popovici MM, Glogar D. Extracorporeal Life Support and New Therapeutic Strategies for Cardiac Arrest Caused by Acute Myocardial Infarction - a Critical Approach for a Critical Condition. J Crit Care Med (Targu Mures). 2016;2:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Kapur NK, Thayer KL, Zweck E. Cardiogenic Shock in the Setting of Acute Myocardial Infarction. Methodist Debakey Cardiovasc J. 2020;16:16-21. [PubMed] |
| 6. | Zeymer U. [Diagnosis and initial management of acute myocardial infarction]. MMW Fortschr Med. 2019;161:34-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Wang M, Ni Y, Liang B, Liang Z. Can Systemic Thrombolysis Improve Prognosis of Cardiac Arrest Patients During Cardiopulmonary Resuscitation? J Emerg Med. 2019;57:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D; International Liaison Committee on Resuscitation; American Heart Association; European Resuscitation Council; Australian Resuscitation Council; New Zealand Resuscitation Council; Heart and Stroke Foundation of Canada; InterAmerican Heart Foundation; Resuscitation Councils of Southern Africa; ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004;110:3385-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1109] [Cited by in RCA: 1273] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 9. | Huang Y, Gao X, Zhou X, Xie B, Zhang Y, Zhu J, Zhu S. Mitophagy in the Hippocampus Is Excessive Activated After Cardiac Arrest and Cardiopulmonary Resuscitation. Neurochem Res. 2020;45:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | He Y, Liu B, Yao P, Shao Y, Cheng Y, Zhao J, Wu J, Zhao ZW, Huang W, Christopher TA, Lopez B, Ma X, Cao Y. Adiponectin inhibits cardiac arrest/cardiopulmonary resuscitationinduced apoptosis in brain by increasing autophagy involved in AdipoR1AMPK signaling. Mol Med Rep. 2020;22:870-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Singla I, Hreybe H, Saba S. Risk of death and recurrent ventricular arrhythmias in survivors of cardiac arrest concurrent with acute myocardial infarction. Indian Pacing Electrophysiol J. 2008;8:5-13. [PubMed] |
| 12. | Zeindler J, Angehrn F, Droeser R, Däster S, Piscuoglio S, Ng CKY, Kilic E, Mechera R, Meili S, Isaak A, Weber WP, Muenst S, Soysal SD. Infiltration by myeloperoxidase-positive neutrophils is an independent prognostic factor in breast cancer. Breast Cancer Res Treat. 2019;177:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Ooi JD, Jiang JH, Eggenhuizen PJ, Chua LL, van Timmeren M, Loh KL, O’Sullivan KM, Gan PY, Zhong Y, Tsyganov K, Shochet LR, Ryan J, Stegeman CA, Fugger L, Reid HH, Rossjohn J, Heeringa P, Holdsworth SR, Peleg AY, Kitching AR. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat Commun. 2019;10:3392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Scheirlynck E, Dejgaard LA, Skjølsvik E, Lie OH, Motoc A, Hopp E, Tanaka K, Ueland T, Ribe M, Collet C, Edvardsen T, Droogmans S, Cosyns B, Haugaa KH. Increased levels of sST2 in patients with mitral annulus disjunction and ventricular arrhythmias. Open Heart. 2019;6:e001016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Zahler D, Rozenfeld KL, Stein M, Milwidsky A, Berliner S, Banai S, Arbel Y, Shacham Y. C-reactive protein velocity and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. J Nephrol. 2019;32:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Kang DO, Park Y, Seo JH, Jeong MH, Chae SC, Ahn TH, Jang WY, Kim W, Park EJ, Choi BG, Na JO, Choi CU, Kim EJ, Rha SW, Park CG, Seo HS; KAMIR-NIH Registry Investigators. Time-dependent prognostic effect of high sensitivity C-reactive protein with statin therapy in acute myocardial infarction. J Cardiol. 2019;74:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Dell’anna AM, Bini Viotti J, Beumier M, Orbegozo-Cortes D, Donadello K, Scolletta S, Vincent JL, Taccone FS. C-reactive protein levels after cardiac arrest in patients treated with therapeutic hypothermia. Resuscitation. 2014;85:932-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Oh SH, Kim YM, Kim HJ, Youn CS, Choi SP, Wee JH, Kim SH, Jeong WJ, Park KN. Implication of cardiac marker elevation in patients who resuscitated from out-of-hospital cardiac arrest. Am J Emerg Med. 2012;30:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Song L, Zhang D, Guo C, Gu Z, Wang L, Yao YS, Wang H, Zeng Z, Wang W, Yang Y, Bei W, Rong X, Guo J. The traditional Chinese medicine formula Fufang-Zhenzhu-Tiaozhi protects myocardia from injury in diabetic minipigs with coronary heart disease. Biomed Pharmacother. 2021;137:111343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Rubinfeld GD, Smilowitz NR, Berger JS, Newman JD. Association of Thrombocytopenia, Revascularization, and In-Hospital Outcomes in Patients with Acute Myocardial Infarction. Am J Med. 2019;132:942-948.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |