Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10566
Peer-review started: March 22, 2021
First decision: April 29, 2021
Revised: May 31, 2021
Accepted: October 15, 2021
Article in press: October 15, 2021
Published online: December 6, 2021
Processing time: 252 Days and 19.6 Hours
The efficacy of endoscopic ultrasonography for the follow-up of gastric varices treated with endoscopic variceal ligation (EVL) has not been established.
To evaluate the diagnostic correlation of esophagogastroduodenoscopy (EGD) and high-frequency intraluminal ultrasound (HFIUS) for type 1 gastric varices (GOV1) after EVL and to identify the predictability for rebleeding of EGD and HFIUS.
In liver cirrhosis patients with GOV1, we performed endoscopic follow-up using EGD and HFIUS synchronously after EVL for hemorrhage from GOV1. Endoscopic grading and red color signs were analyzed using EGD, and the largest variceal cross-sectional areas were measured using HFIUS. In addition, 1-year follow-up was performed. Variceal rebleeding was defined as the presence of hematemesis, hematochezia, or melena without other evidence of bleeding on endoscopic follow-up.
In 26 patients with GOV1, variceal cross-sectional areas on HFIUS of GOV1 was poorly correlated with EGD grading of GOV1 (r = 0.36). In 17 patients who completed the 1-year follow-up, variceal cross-sectional areas on HFIUS was a good predictor of subsequent rebleeding, whereas EGD grading was not a predictor of subsequent rebleeding.
HFIUS measurement is more predictive of GOV1 rebleeding than EGD grading, so HFIUS measurement may be necessary for endoscopic follow-up after EVL in patients with GOV1.
Core Tip: Endoscopic ultrasound was an important modality in the diagnosis of varices. Recently, high-frequency intraluminal ultrasound (HFIUS) enables quantitative measurement of variceal size, so it is a more sensitive imaging modality for the estimation of the size of varices. So we examined the diagnostic correlation of esophagogastroduodenoscopy (EGD) grades and HFIUS measurements in estimating post-endoscopic variceal ligation (EVL) type 1 gastric varices (GOV1). The results suggest that HFIUS measurement is more predictive of GOV1 rebleeding than EGD grading, so HFIUS measurement may be necessary for endoscopic follow-up after EVL in patients with GOV1.
- Citation: Kim JH, Choe WH, Lee SY, Kwon SY, Sung IK, Park HS. Comparative study for predictability of type 1 gastric variceal rebleeding after endoscopic variceal ligation: High-frequency intraluminal ultrasound study. World J Clin Cases 2021; 9(34): 10566-10575
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10566.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10566
Endoscopic variceal ligation (EVL) was introduced in the 1980s as an alternative to endoscopic injection sclerotherapy[1]. Several prospective trials reported that EVL was superior to endoscopic injection sclerotherapy in that it eradicated varices more rapidly with less recurrent bleeding and fewer complications[2−7]. Thus, EVL has become the endoscopic treatment of choice for both the control of acute bleeding and the prevention of rebleeding from esophageal varices (EV)[8].
Gastric varices (GVs) are commonly categorized based on their location in the stomach and their relationship with EVs[9,10]. Type 1 gastric varices (GOV1) are the most common subtype of GV and constitute an extension of EV along the lesser curvature of the stomach[9,11]. Because they are considered a continuation of EV, current recommendations have emphasized that GOV1 should be treated as EV. In contrast, the other subtypes of GV, such as IGV1 (varices in the gastric fundus), IGV2 (ectopic varices around the pylorus), and GOV2 (varices extending along the greater curvature toward the gastric fundus), do not respond well to therapeutic modalities used for EV[11−14].
After successful control of acute bleeding with emergency EVL, endoscopic follow-up should be repeated, and residual or recurrent varices should be treated with elective EVL to prevent variceal rebleeding if indicated[12,13]. Esophagogastroduodenoscopy (EGD) is the best practical modality for the follow-up of EV after EVL[12]. However, little is known about whether it is an effective modality for post-EVL endoscopic follow-up of GOV1.
Recently, endoscopic ultrasound (EUS) has been introduced as an important modality in the diagnosis of varices[15,16]. In particular, high-frequency intraluminal ultrasound (HFIUS) has been reported to enable quantitative measurement of variceal size, so it may be a more sensitive and reproducible imaging modality than EGD for the detection of varices and the estimation of their size[17−22]. However, these data are very limited.
The aims of this study were to examine the diagnostic correlation of EGD grades and HFIUS measurements in estimating post-EVL GOV1 and to evaluate their ability to predict variceal rebleeding based on EGD findings and the cross-sectional area (CSA) of varices using HFIUS.
This study was performed at the Konkuk University Medical Center, Seoul, Korea from January 2017 to December 2018. Of the participants with liver cirrhosis with GOV1, consecutive patients who underwent EVL for GOV1 bleeding were initially selected. Patients with hepatocellular carcinoma or Child-Pugh classification C cirrhosis (Child-Pugh class score ≥ 10) were excluded. Within 2 mo after the initial EVL, a follow-up EGD was performed biweekly to reassess variceal grade, and elective EVL was performed to obliterate the residual varices. One to two months after the initial EVL, endoscopic follow-up using synchronous EGD and HFIUS was conducted on 26 patients who were enrolled in this study. Of these, 17 patients whose varices were reduced to grade 0/1 according to EGD were prospectively followed up for 1 year without additional sessions of EVL. Patients received propranolol during follow-up if red color signs (RC signs) were evident on varices and nonselective beta blockers were not contraindicated. Variceal rebleeding was defined as the presence of hematemesis, hematochezia, or melena when the source of the bleeding was endoscopically proven to be GOV1 (spurting or oozing from varices or the presence of a recent blood clot over varices). The primary end point of the study was the correlation between EGD grades and HFIUS measurements as measured by the Spearman correlation coefficient in 26 patients initially enrolled. The secondary end point was the predictabilities for variceal rebleeding of EGD grades and HFIUS measurements in 17 patients who completed the 1-year follow-up. This study was approved by the Institutional Review Board of Konkuk University Hospital and was performed in accordance with the most recent (2008) revision of the Helsinki Declaration, and informed consent was obtained directly from all enrolled patients.
EVL was performed using a pneumatic-active ligating device (Samjin, Seoul, Korea) with a 25-cm overtube or using a multiband ligator (Saeed Six Shooter; Cook Endoscopy, Winston-Salem, NC, United States). The variceal hemorrhage site was first ligated. Then, surrounding varices were ligated as much as possible. Concomitant EVs were also ligated.
Follow-up EGD was performed using a double-channel endoscope (GIF2T–240; Olympus Co. Ltd, Tokyo, Japan). During the procedure, endoscopic images were electronically recorded for subsequent review by two endoscopists (JHK and WHC). Post-EVL EGD grades of varices were classified according to the General Rules for Recording Endoscopic Findings of Esophagogastric Varices of the Japanese Research Society for Portal Hypertension: Grade 0, not visible; grade 1, small, straight; grade 2, enlarged, tortuous; grade 3, large, coil-shaped, or tumorous[23,24]. RC signs were classed as positive or negative. Positive was defined as clear evidence of a cherry-red spot, a red wale marking, or a hemocystic spot.
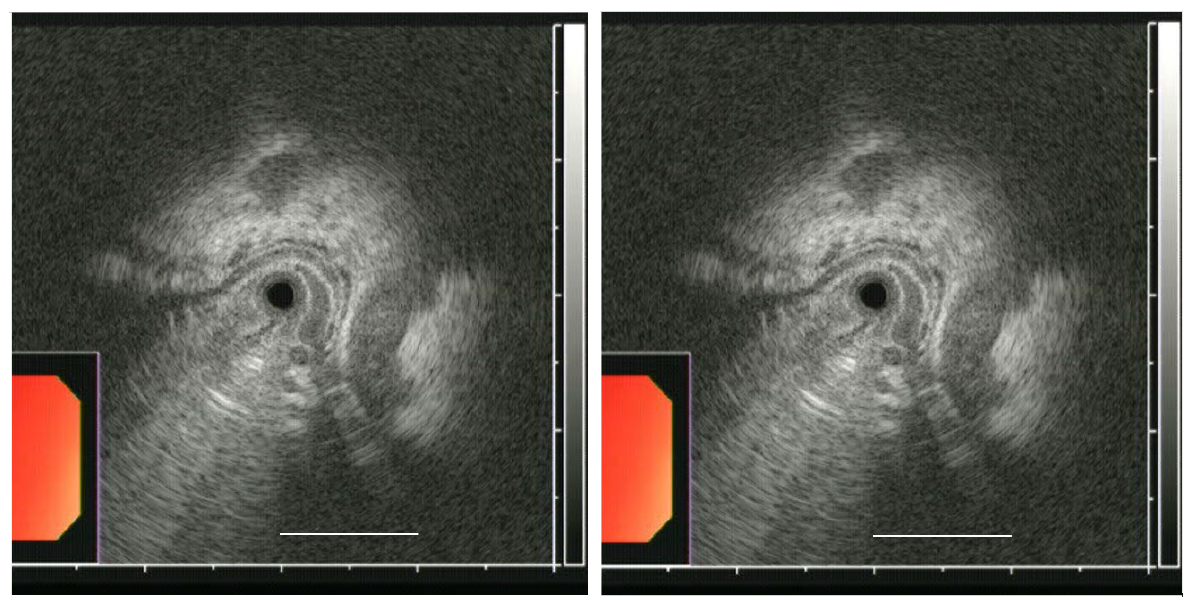
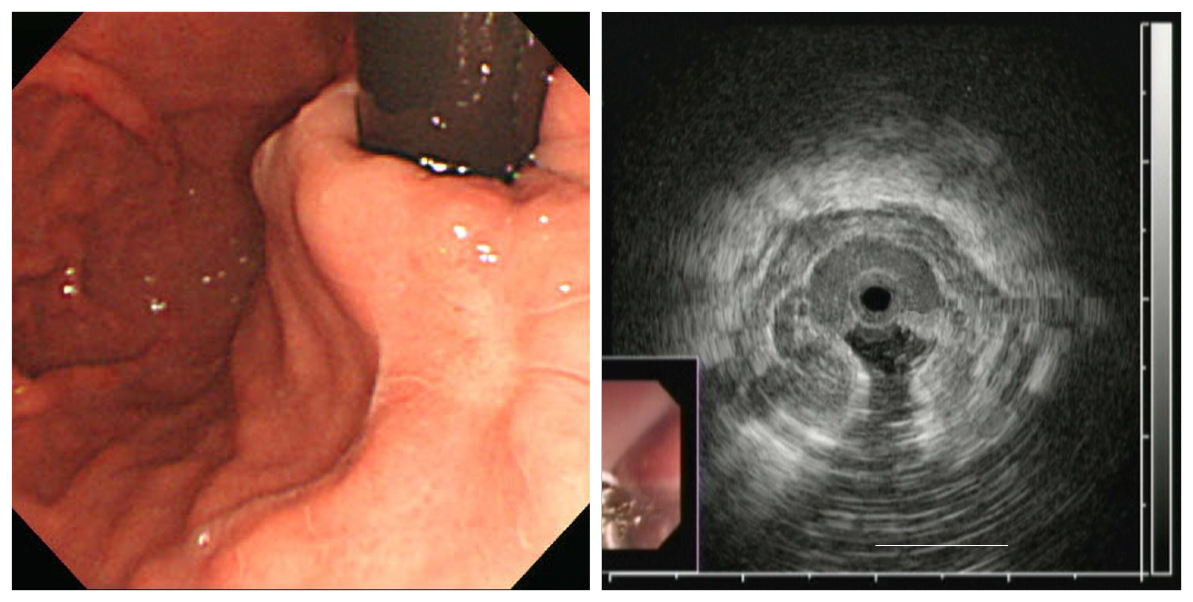
HFIUS was performed simultaneously with EGD. The HFIUS catheter assembly consisted of a 2.3-mm diameter ultrasonic miniprobe equipped with a 20-MHz transducer (UM–G20–29R, Olympus). The catheter was inserted via one of the accessory channels, and an automatic water infusion pump was attached to another channel to facilitate infusion of deaerated water. The HFIUS miniprobe, which has an axial resolution of approximately 0.1 mm and a penetration depth of 2.0 cm, was advanced to the mid-body of the stomach and was gradually withdrawn along the lesser curvature until the distal third of the esophagus was scanned. EUS images were recorded electronically for subsequent review by two examiners (JHK and WHC). The largest CSA sizes of varices using HFIUS were estimated using ImageJ software (NIH, Bethesda, MD, United States). The CSA of each varix was measured between the hypoechoic blood-filled lumen and the hyperechoic submucosa or mucosa (Figures 1 and 2).
Quantitative variables are expressed as the mean ± SD and were compared using Student’s t test. Qualitative variables were compared using the χ2 test. The correlation between EGD grade and HFIUS estimates was analyzed using the Spearman correlation coefficient. Receiver operating characteristic curves and sensitivity and specificity plots were constructed to identify predictors of variceal rebleeding. Cutoff values that resulted in the best sensitivity and specificity were identified. All P values were two-tailed, and a P value < 0.05 was considered significant.
Twenty-six patients were enrolled in this study. Table 1 shows the baseline characteristics of the patients. Varices were reduced to grade 0/1 at EGD findings within four sessions of EVL (single session in 2 patients; two sessions in 5 patients; three sessions in 7 patients; four sessions in 3 patients) in 17 patients, whereas varices were not reduced to grade 0/1 in 9 patients. Among the 17 patients who completed the 1-year follow-up, 6 patients (35%) experienced variceal rebleeding during follow-up. Patient characteristics, such as age, sex, etiology of cirrhosis, and Child-Pugh score, were not significantly associated with variceal rebleeding (Table 2).
| Characteristic | |
| Age (yr) | 52.7 ± 10.5 |
| Sex (M/F) | 22/4 |
| Alcohol/nonalcoholic | 6/20 |
| Child-Pugh score 5/6/7/8/9 | 3/3/8/7/5 |
| MELD score | 12.8 ± 3.8 |
| Hepatocellular carcinoma | 0 (0%) |
| Serum albumin (gm/dL) | 3.2 ± 0.5 |
| Serum bilirubin (mg/dL) | 2.2 ± 1.3 |
| Prothrombin time (INR) | 1.4 ± 0.2 |
| Serum creatinine | 1.1 ± 0.2 |
| Presence of ascites | 12 (46.2%) |
| Presence of hepatic encephalopathy | 1 (3.8%) |
| Platelet count (K/mm3) | 88.1 ± 39.8 |
| Hemoglobin (g/dL) | 7.8 ± 2.2 |
| Blood transfused (units) | 3.0 ± 1.5 |
| Rebleeding (+) | Rebleeding (-) | P value | |
| Age (yr) | 55.5 ± 11.8 | 51.9 ± 10.7 | 0.533 |
| Sex (M/F) | 5/9 | 1/2 | 0.728 |
| Alcohol/nonalcoholic | 2/2 | 4/9 | 0.445 |
| Child-Pugh classification A/B | 1/3 | 5/8 | 0.555 |
| MELD score | 12.8 ± 2.6 | 13.4 ± 3.1 | 0.725 |
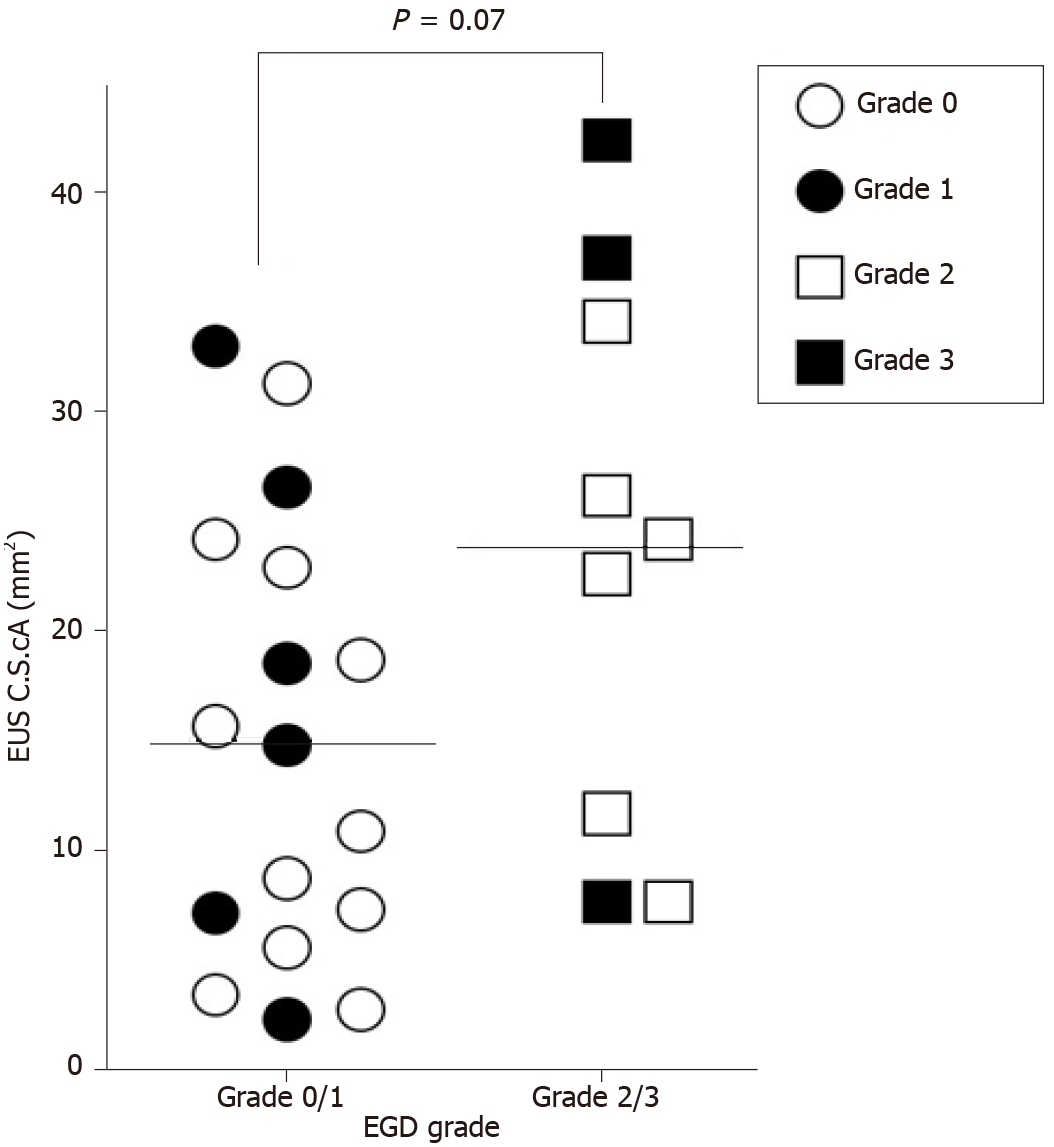
In 26 patients, EGD identified GOV1 grades 0, 1, 2, and 3 in 11, 6, 6, and 3 patients, respectively. The mean largest variceal CSA values measured using HFIUS in patients with EGD grades 0, 1, 2, and 3 were 13.9 ± 9.5 mm2, 17.2 ± 11.6 mm2, 21.0 ± 9.8 mm2, and 28.9 ± 18.7 mm2, respectively. GOV1 grades estimated using EGD were not significantly correlated with largest variceal CSA measured using EUS (correlation coefficient = 0.36, P = 0.07), and the mean largest variceal CSA of grade 2/3 GOV1 was not significantly different from that of grade 0/1 GOV1 (23.7 ± 12.7 vs 15.1 ± 10.0; P = 0.07) (Figure 3).
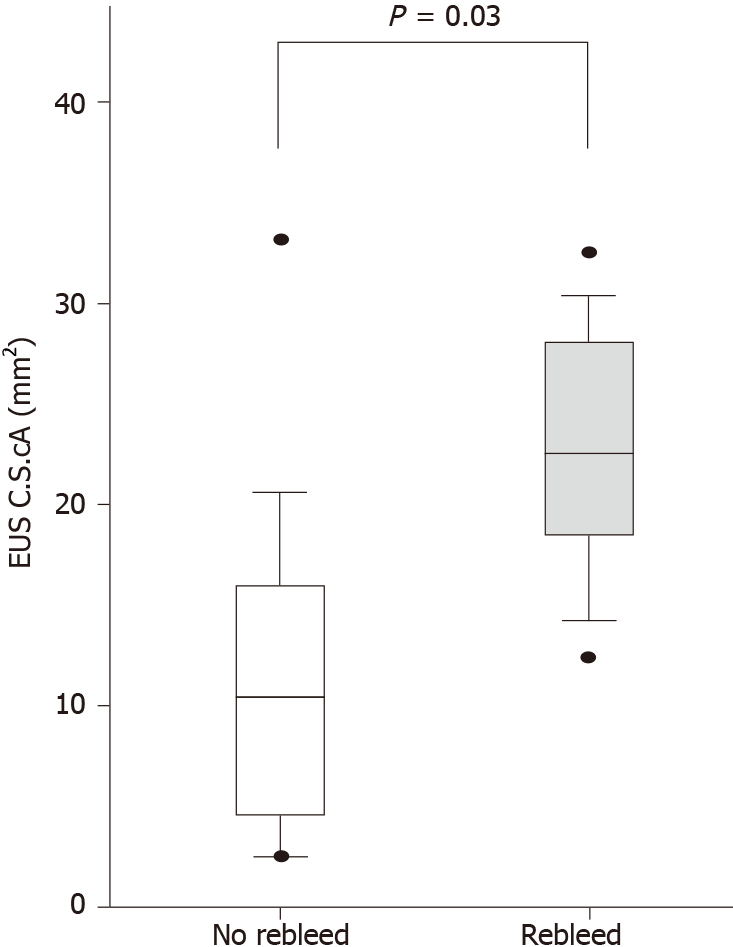
Among the 17 patients who completed the 1-year follow-up, the mean largest variceal CSA of GOV1 was significantly greater in patients who experienced rebleeding compared with patients who did not (22.2 ± 7.7 mm2 vs 11.2 ± 9.2 mm2, respectively; P = 0.03) (Figure 4). A cutoff value of 17.2 mm2 for largest variceal CSA resulted in a sensitivity and specificity for subsequent GOV1 bleeding of 83% and 82%, respectively. Rebleeding was significantly more frequent in those with largest variceal CSAs greater than the CSA cutoff value compared with those with largest variceal CSAs less than the CSA cutoff value, whereas EGD grading and RC sign were not predictive of GOV1 rebleeding (Table 3).
In this study, we initially enrolled 26 patients with liver cirrhosis with GOV1 who underwent synchronous EGD and HFIUS as endoscopic follow-up after the initial EVL and evaluated the diagnostic correlation of these two modalities. Then, in 17 patients with 1-year follow-up, we confirmed that HFIUS was a good predictor of subsequent rebleeding compared with EGD grading.
Theoretically, both GOV1 and EV bleeding can be treated with EVL given their similar pathophysiology[12]. Technically as well as theoretically, EVL exhibits an advantage with respect to controlling acute bleeding from GOV1 given its better endoscopic view and easier accessibility compared with other subtypes of GV. However, from a practical perspective, EVL does not always eradicate GOV1 because its effect is limited to superficial layers, and GV often extends into the submucosa or deeper layers[25,26]. Therefore, endoscopic follow-ups are very important for preventing GOV1 rebleeding.
EGD is less sensitive than EUS for the evaluation of GV[22,27−29]. However, the availability of EUS is limited in clinical practice, and conventional EGD is typically used as an endoscopic follow-up modality after gastric variceal ligation[24,25]. It has not been established whether EUS or EGD is more appropriate for post-EVL follow-up of GOV1. In patients with GOV1 in this study, EGD grading was not significantly correlated with post-EVL HFIUS size estimation of GOV1. Moreover, EGD findings, including grading and RC signs, did not predict post-EVL GOV1 bleeding. In contrast, HFIUS measurement of a cutoff value for the largest CSA enabled prediction of rebleeding. Therefore, we suggest that EGD grading is insufficient for post-EVL follow-up of GOV1 and that endoscopic follow-up with EUS, especially HFIUS, could be performed to estimate the accurate variceal size and predict rebleeding of GOV1.
There are some limitations to the present study. First, the EUS finding of small varices is very similar to the EUS finding of small cyst in the fundus, yet the doppler examination is able to differentiate small varices from small cysts. However, we did not perform the doppler examination in our study, so we are unable to completely distinguish small varices from small cysts. Nonetheless, the patients in this study experienced active bleeding from gastric varices before the enrollment, and bleeding from small cysts in the fundus is extremely rare. As it follows, we have ruled out the possibility of finding small cysts in the fundus even without performing the doppler examination. Second, as the sample size of this study is relatively small, the present results need to be validated in future studies.
We used 20 MHz HFIUS for EUS evaluation of post-EVL GOV1. This technique is more sensitive than conventional 7.5 MHz EUS because it enables better visualization of details at close range and accurate and quantitative measurement of variceal size[16,19−21]. In addition, the HFIUS miniprobe does not require placement of a water-filled balloon around the ultrasound transducer, which prevents compression or distortion of varices. Patient discomfort is also minimized because HFIUS can be performed as a single-step procedure during EGD. We estimated variceal size based on the largest CSA instead of the largest diameter because post-EVL varices may be deformed by overlying scar tissue.
To our knowledge, this is the first study in which HFIUS has been used to predict post-EVL GOV1. This study demonstrated that HFIUS measurement is predictive of post-EVL GOV1 rebleeding; on the other hand, EGD is insufficient. Therefore, we suggest that HFIUS could be mandatory for endoscopic follow-up of GOV1 after EVL.
After successful control of acute bleeding with emergency endoscopic variceal ligation (EVL), endoscopic follow-up should be repeated, and residual or recurrent varices should be treated with elective EVL to prevent variceal rebleeding if indicated. Recently, endoscopic ultrasound (EUS) has been introduced as an important modality in the diagnosis of varices, because EUS is more sensitive than esophagogastroduodenoscopy (EGD) for the evaluation of gastric varices.
The efficacy of endoscopic ultrasonography for the follow-up of gastric varices treated with EVL has not been established.
This study aimed to evaluate the diagnostic correlation of EGD and high-frequency intraluminal ultrasound (HFIUS) for type 1 gastric varices (GOV1) after EVL and to identify the predictability for rebleeding with EGD and HFIUS.
In liver cirrhosis patients with GOV1, we performed endoscopic follow-up using EGD and HFIUS synchronously after EVL for hemorrhage from GOV1. Endoscopic grading and red color signs were analyzed using EGD, and the largest variceal cross-sectional areas (CSAs) were measured using HFIUS. In addition, 1-year follow-up was performed. Variceal rebleeding was defined as the presence of hematemesis, hematochezia or melena without other evidence of bleeding on endoscopic follow-up.
In 26 patients with GOV1, variceal CSA on HFIUS of GOV1 was poorly correlated with EGD grading of GOV1 (r = 0.36). In 17 patients who completed the 1-year follow-up, variceal CSA on HFIUS was a good predictor of subsequent rebleeding, whereas EGD grading was not a predictor of subsequent rebleeding.
HFIUS measurement is more predictive of GOV1 rebleeding than EGD grading, so HFIUS measurement may be necessary for endoscopic follow-up after EVL in patients with GOV1.
Future work and basic research should be performed to confirm that EUS, especially HFIUS, could be performed to estimate the accurate variceal size and predict rebleeding of GOV1.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li Y S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Stiegmann GV, Goff JS, Sun JH, Davis D, Bozdech J. Endoscopic variceal ligation: an alternative to sclerotherapy. Gastrointest Endosc. 1989;35:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Stiegmann GV, Goff JS, Michaletz-Onody PA, Korula J, Lieberman D, Saeed ZA, Reveille RM, Sun JH, Lowenstein SR. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med. 1992;326:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 415] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Lo GH, Lai KH, Cheng JS, Hwu JH, Chang CF, Chen SM, Chiang HT. A prospective, randomized trial of sclerotherapy vs ligation in the management of bleeding esophageal varices. Hepatology. 1995;22:466-471. [PubMed] |
| 4. | Gimson AE, Ramage JK, Panos MZ, Hayllar K, Harrison PM, Williams R, Westaby D. Randomised trial of variceal banding ligation vs injection sclerotherapy for bleeding oesophageal varices. Lancet. 1993;342:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 186] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Laine L, el-Newihi HM, Migikovsky B, Sloane R, Garcia F. Endoscopic ligation compared with sclerotherapy for the treatment of bleeding esophageal varices. Ann Intern Med. 1993;119:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 203] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Hou MC, Lin HC, Kuo BI, Chen CH, Lee FY, Lee SD. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: a prospective randomized trial. Hepatology. 1995;21:1517-1522. [PubMed] |
| 7. | Baroncini D, Milandri GL, Borioni D, Piemontese A, Cennamo V, Billi P, Dal Monte PP, D'Imperio N. A prospective randomized trial of sclerotherapy vs ligation in the elective treatment of bleeding esophageal varices. Endoscopy. 1997;29:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int. 2017;37 Suppl 1:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Park EJ, Jang JY, Lee JE, Jeong SW, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Cho JY, Kim HS, Kim BS, Kim YJ. The risk factors for bleeding of fundal varices in patients with liver cirrhosis. Gut Liver. 2013;7:704-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Triantafyllou M, Stanley AJ. Update on gastric varices. World J Gastrointest Endosc. 2014;6:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Vine LJ, Subhani M, Acevedo JG. Update on management of gastric varices. World J Hepatol. 2019;11:250-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 12. | Ashkenazi E, Kovalev Y, Zuckerman E. Evaluation and treatment of esophageal varices in the cirrhotic patient. Isr Med Assoc J. 2013;15:109-115. [PubMed] |
| 13. | Jakab SS, Garcia-Tsao G. Evaluation and Management of Esophageal and Gastric Varices in Patients with Cirrhosis. Clin Liver Dis. 2020;24:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Hwang JH, Shergill AK, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ, Fonkalsrud L, Jue T, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 15. | Girotra M, Raghavapuram S, Abraham RR, Pahwa M, Pahwa AR, Rego RF. Management of gastric variceal bleeding: Role of endoscopy and endoscopic ultrasound. World J Hepatol. 2014;6:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Hammoud GM, Ibdah JA. Utility of endoscopic ultrasound in patients with portal hypertension. World J Gastroenterol. 2014;20:14230-14236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wang AJ, Li BM, Zheng XL, Shu X, Zhu X. Utility of endoscopic ultrasound in the diagnosis and management of esophagogastric varices. Endosc Ultrasound. 2016;5:218-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Carneiro FO, Retes FA, Matuguma SE, Albers DV, Chaves DM, Dos Santos ME, Herman P, Chaib E, Sakai P, Carneiro D'Albuquerque LA, Maluf Filho F. Role of EUS evaluation after endoscopic eradication of esophageal varices with band ligation. Gastrointest Endosc. 2016;84:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Jeong SW, Kim HS, Kim SG, Yoo JJ, Jang JY, Lee SH, Lee JS, Kim YS, Kim BS. Useful Endoscopic Ultrasonography Parameters and a Predictive Model for the Recurrence of Esophageal Varices and Bleeding after Variceal Ligation. Gut Liver. 2017;11:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Masalaite L, Valantinas J, Stanaitis J. Endoscopic ultrasound findings predict the recurrence of esophageal varices after endoscopic band ligation: a prospective cohort study. Scand J Gastroenterol. 2015;50:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Liao SC, Yang SS, Ko CW, Lien HC, Tung CF, Peng YC, Yeh HZ, Chang CS. A miniature ultrasound probe is useful in reducing rebleeding after endoscopic cyanoacrylate injection for hemorrhagic gastric varices. Scand J Gastroenterol. 2013;48:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Seno H, Konishi Y, Wada M, Fukui H, Okazaki K, Chiba T. Endoscopic ultrasonograph evaluation of vascular structures in the gastric cardia predicts esophageal variceal recurrence following endoscopic treatment. J Gastroenterol Hepatol. 2006;21:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bandoh T, Mitarai Y, Kitano S, Yoshida T, Kobayashi M. Clinical significance of esophageal variceal pressure in patients with esophageal varices. J Hepatol. 1994;21:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1822] [Article Influence: 260.3] [Reference Citation Analysis (2)] |
| 25. | Tantau M, Crisan D, Popa D, Vesa S, Tantau A. Band ligation vs. N-Butyl-2-cyanoacrylate injection in acute gastric variceal bleeding: a prospective follow-up study. Ann Hepatol. 2013;13:75-83. [PubMed] |
| 26. | Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, Lee SD. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection vs band ligation. Hepatology. 2006;43:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Liao WC, Chen PH, Hou MC, Chang CJ, Su CW, Lin HC, Lee FY. Endoscopic ultrasonography assessment of para-esophageal varices predicts efficacy of propranolol in preventing recurrence of esophageal varices. J Gastroenterol. 2015;50:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Sharma M, Singh P, Pathak A, Arya S. Diagnostic dilemma in gastric varices: Endoscopic ultrasound resolves the issue (with videos). Endosc Ultrasound. 2017;6:409-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Fung BM, Abadir AP, Eskandari A, Levy MJ, Tabibian JH. Endoscopic ultrasound in chronic liver disease. World J Hepatol. 2020;12:262-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |