Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10451
Peer-review started: July 20, 2021
First decision: August 9, 2021
Revised: August 24, 2021
Accepted: October 18, 2021
Article in press: October 18, 2021
Published online: December 6, 2021
Processing time: 132 Days and 17.8 Hours
Sepsis is a major medical challenge. Magnolol is an active constituent of Houpu that improves tissue function and exerts strong anti-endotoxin and anti-inflammatory effects, but the mechanism by which it reduces intestinal inflammation in sepsis is yet unclear.
To assess the protective effect of magnolol on intestinal mucosal epithelial cells in sepsis and elucidate the underlying mechanisms.
Enzyme-linked immunosorbent assay was used to measure tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and regulated on activation, normal T-cell expressed and secreted (RANTES) levels in serum and ileal tissue in animal studies. The histopathological changes of the ileal mucosa in different groups were observed under a microscope. Cell Counting Kit-8 and cell permeability assays were used to determine the concentration of drug-containing serum that did not affect the activity of Caco2 cells but inhibited lipopolysaccharide (LPS)-induced decrease in permeability. Immunofluorescence and Western blot assays were used to detect the levels of RANTES, inhibitor of nuclear factor kappa-B kinase β (IKKβ), phosphorylated IKKβ (p-IKKβ), inhibitor of nuclear factor kappa-B kinase α (IκBα), p65, and p-p65 proteins in different groups in vitro.
In rats treated with LPS by intravenous tail injection in the presence or absence of magnolol, magnolol inhibited the expression of proinflammatory cytokines, IL-1β, IL-6, and TNF-α in a dose-dependent manner. In addition, magnolol suppressed the production of RANTES in LPS-stimulated sepsis rats. Moreover, in vitro studies suggested that magnolol inhibited the increase of p65 nucleation, thereby markedly downregulating the production of the phosphorylated form of IKKβ in LPS-treated Caco2 cells. Specifically, magnolol inhibited the translocation of the transcription factor nuclear factor-kappa B (NF-κB) from the cytosol into the nucleus and down-regulated the expression level of the chemokine RANTES in LPS-stimulated Caco2 cells.
Magnolol down-regulates RANTES levels by inhibiting the LPS/NF-κB signaling pathways, thereby suppressing IL-1β, IL-6, and TNF-α expression to alleviate the mucosal barrier dysfunction in sepsis.
Core Tip: In this study, it was found that magnolol inhibited the lipopolysaccharide-induced nuclear factor-kappa B signaling pathway in the intestinal mucosal epithelium to regulate the secretion of regulated on activation, normal T-cell expressed and secreted (RANTES) and thus reduce intestinal inflammation in sepsis. Various biological constituents, isolated from traditional Chinese medicine, show multifunctional activities. Magnolol, isolated from Magnolia, has been documented to possess a range of biological activities. The current results for the first time proved that magnolol plays a role in the treatment of sepsis by down-regulating RANTES. Thus, additional studies on its anti-inflammatory mechanism might provide novel ideas and methods for the clinical prevention and treatment of sepsis.
- Citation: Mao SH, Feng DD, Wang X, Zhi YH, Lei S, Xing X, Jiang RL, Wu JN. Magnolol protects against acute gastrointestinal injury in sepsis by down-regulating regulated on activation, normal T-cell expressed and secreted. World J Clin Cases 2021; 9(34): 10451-10463
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10451.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10451
Severe sepsis is a life-threatening syndromic response with a 27%-54.1% mortality rate[1,2]. However, the underlying mechanism is not yet understood. It is speculated that mucosal epithelial dysfunction and acute gastrointestinal injury (AGI) are the initial factors of multiple-organ failure[3], which permit translocation of intestinal bacteria and endotoxins to the bloodstream, inducing a strong systemic inflammatory response and leading to AGI[4,5]. Currently, the diagnosis of AGI is poorly understood.
Border et al[6] first reported an association between bacterial translocation and gut-origin sepsis in 1987. Since then, the gut has been shown to have a major role in the progression of systemic inflammatory response syndrome, sepsis, and multiple organ dysfunction syndrome (MODS). Several studies have reported that increased intestinal permeability in acute bowel injury results in the translocation of large amounts of intestinal bacteria, thus initiating or aggravating systematic inflammatory response syndrome[7]. The current treatment for sepsis to prevent MODS requires rapid diagnosis to allow timely treatment with antibiotics and interventions[8]. Therefore, early and effective inhibition of the production of proinflammatory factors may protect against the intestinal damage caused by excessive inflammation in addition to preventing and treating sepsis and MODS.
Houpu is a traditional Chinese medicine for phlegm and gas removal. In clinical practice, Houpu is widely utilized for treating vomiting and diarrhea, abdominal distension, and constipation. Some studies suggested that Houpu improves gastrointestinal ischemia, inhibits bacterial migration, and reduces endotoxin absorption, and could be the favored approach in adjusting immunity[9,10]. Magnolol, an active constituent of Houpu, improves tissue function, exerts strong anti-endotoxin and anti-inflammatory effects, and acts as an oxygen-free-radical scavenger[11]. However, the exact mechanism underlying the magnolol effect on intestinal inflammation is yet unclear.
A subset of CC motif chemokines and cytokines is increased in sepsis patients compared to normal controls[12]. Various interactive and dynamic chemokines involved in sepsis are used in diagnosis, prognosis, etiology, and evaluation of response to therapy[13]. Regulated on activation, normal T-cell expressed and secreted (RANTES) is a critical member of the chemokine superfamily; its specific receptor is the transmembrane G protein-coupled receptor (CCR1, CCR2, CCR3, CCR4, and CCR5). CCR1 and CCR5 are high-affinity receptors, while CCR4 is a low-affinity receptor[14]. RANTES and its receptors affect chemotaxis or stimulation of T lymphocytes, monocytes, eosinophils, and basophils, especially in lymphocyte CD4+/CD45RO+ memory type, to induce T cell activation and proliferation, regulate Th cell and cytotoxic cell immune response, and stimulate eosinophils, basophils, and degranulation[15]. Ajuebor et al[16] found that RANTES was significantly expressed in Sprague-Dawley (SD) rats with chronic colitis. The same study suggested that the administration of the receptor antagonist reduced the colon tissue damage along with the number of mononuclear cells, mast cells, and neutrophils in the lesion. Furthermore, Kucuk et al[17] treated colitis with Met-RANTES and found less damage and bacterial translocation. Previous studies have shown that the promoters of RANTES genes contain the binding sites for the transcription factor nuclear factor-kappa B (NF-κB)[18].
NF-Κb is a nuclear transcription factor in cells involved in the inflammatory and immune responses of the body and regulates cell apoptosis, stress response, and NF-κB overactivation. NF-κB has been associated with several human diseases, such as rheumatoid arthritis and inflammatory changes in the heart and brain diseases. NF-κB is a major transcription factor in the inflammatory response, and its accumulation in the nucleus influences transcription by binding to the promoter of the RANTES gene, thereby inducing its production in large quantities[19]. Magnolol significantly suppresses the expression of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), phosphorylated extracellular regulated protein kinases (p-ERK), phosphorylated C-jun N-terminal kinase (p-JNK), and p-p38 in lipopolysaccharide (LPS)-induced mouse uterine epithelial cells (MUECs), which is associated with the inhibition of Toll-like receptor 4 (TLR4)-regulated NF-κB signaling[20]. Therefore, whether magnolol can inhibit the LPS/NF-κB signaling pathway in the intestinal mucosal epithelium to regulate the secretion of RANTES and thus reduce intestinal inflammation in sepsis needs to be further investigated.
All animal studies (including mouse euthanasia) were carried out in compliance with the regulations and guidelines of Zhejiang Chinese Medical University institutional animal care and according to the Institutional Animal Care and Use Committee (IACUC) guidelines. Healthy male SD rats (n = 55), weighing 200 ± 20 g, were obtained from the Experimental Animal Center of Zhejiang Chinese Medical University, China (certification number SCXK [Hu] 2017-0005) and housed for 2 wk under normal conditions (certification number SYXK [Zhe] 2013-0184) at 20 ± 1 °C and 50%-60% humidity, under 12:12-h light/dark cycle, with a ventilation rate of 8-15 times/h.
Using a random number table, the rats were divided into five groups (n = 11/group): Control group (Control), severe sepsis group (Model), low-dose magnolol (Tongtian Biologicals, Shanghai, China) group (LM, 5 μg/kg), middle-dose magnolol group (MM, 10 μg/kg), and high-dose magnolol group (HM, 20 μg/kg).
A rat model of severe sepsis was established by intravenous injection of LPS via the tail vein. Briefly, all rats were deprived of food but had free access to water for 12 h before surgery. The Control rats received 15 μg/kg normal saline plus 4 mg/kg normal saline. The Model animals received 15 μg/kg normal saline plus 4 mg/kg LPS. The rats in the LM, MM, and HM groups received 5 μg/kg magnolol plus 4 mg/kg LPS, 10 μg/kg magnolol plus 4 mg/kg LPS, and 20 μg/kg magnolol plus 4 mg/kg of LPS, respectively. Blood was collected from each group after 6, 12, and 24 h. After 24 h, the animals were euthanized using an intraperitoneal injection of chloral hydrate, and their mesenteric lymph nodes, liver, spleen, and terminal ileum tissues were collected under aseptic conditions.
TNF-α, IL-1β, and IL-6 levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Medical Equipment Co., Ltd (80-2), Shanghai, China).
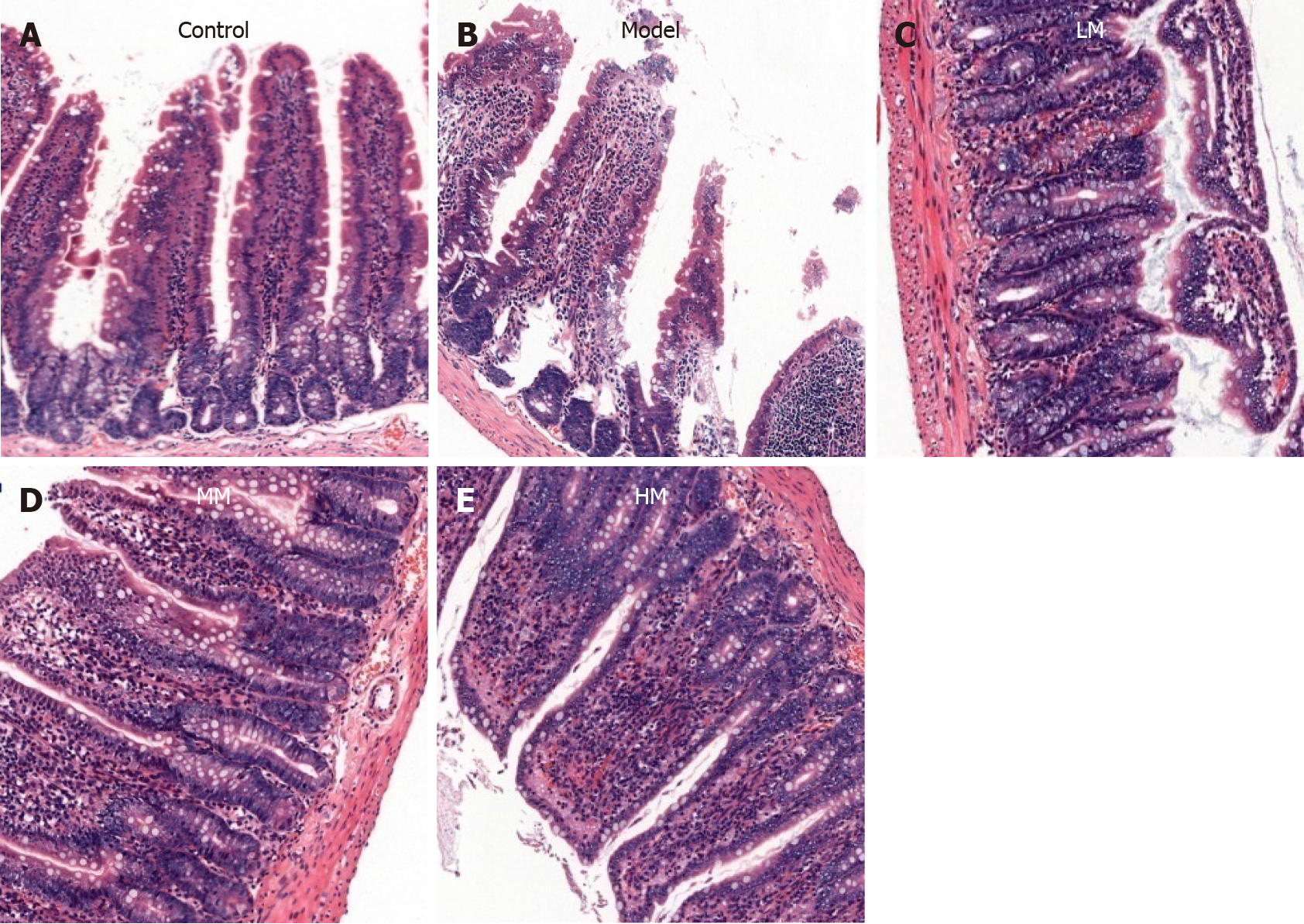
The paraffin sections of the rat terminal ileum were stained with hematoxylin and eosin and observed under an XZT-302 microscope (Shanghai Yuguang Detection Equipment Co., Ltd, Shanghai, China) at × 100 magnification to assess the histopathological changes.
Peripheral blood samples and serum were isolated. Serum RANTES levels were measured using ELISA kits following the manufacturer’s instructions (Shanghai Medical Equipment Co., Ltd (80-2), Shanghai, China).
Caco2 cells were cultured in RPMI-1640 medium containing 20% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 U/mL streptomycin (Gibco, Waltham, United States). The cells were incubated (Thermo Scientific, Waltham, United States) at 5% CO2, 37 °C, and saturated humidity. The medium was changed every 2-3 d until the cells reached 80% confluency. Under a microscope (Olympus, Tokyo, Japan), Caco2 cells were observed to be adherent like paving stones.
Subsequently, cells were divided into four groups: Group A (Solvent), treated with solvent only; group B (Magnolol), treated with different concentrations of magnolol (2 μmol/L, 5 μmol/L, and 10 μmol/L); group C (LPS), treated with solvent and LPS (100 μg/mL); group D (Magnolol plus LPS), treated with magnolol (2, 5, and 10 μmol/L) and LPS (100 μg/mL). Groups B/C/D were pretreated with magnolol or solvent for 8 h, followed by LPS for 24 h.
After 24 h of culture, cells were digested with trypsin-EDTA (Solarbio, Beijing, China) and collected by centrifugation at 1000 rpm for 5 min (Eppendorf, Hamburg, Germany) and resuspended in complete fresh medium (GIBCO, Waltham, United States). The cell suspension was inoculated in a 96-well plate and incubated at 37 °C and 5% CO2. Then, 10 μL of cell counting kit-8 (CCK-8) (Biyuntian, C0037, Shanghai, China) was added to each well and incubated at 37 °C for another 2 h. The absorbance was measured at 450 nm using a microplate reader (Thermo Scientific, Waltham, United States).
Caco2 cells in the logarithmic phase were inoculated in 24-well Transwell (Corning, 3415, Shanghai, China) plate. Epithelial cells were inoculated in the upper chamber. The filtration membrane embedded in the chamber was a polycarbonate membrane, on which the cells grew to form a monolayer. After the epithelial cells were overgrown, phenol-free red minimum essential medium (MEM) (GIBCO, Waltham, United States) containing fluorescein isothiocyanate isomer (FITC)-labeled glucan (10 kDa, 100 μg/mL) (Santa Cruz, Sc-263323), magnolol, and LPS was added to the upper chamber, and phenol-free red MEM containing glucan, magnolol, and LPS was added to the lower chamber. After treatment for 12 h, 100 μL of the lower chamber medium was placed in 96-well plates, and the fluorescence intensity of FITC was detected by an enzyme marker (excitation wavelength of 490 nm and emission wavelength of 520 nm) (Thermo Scientific, Waltham, United States).
According to the CCK-8 and cell permeability detection, we found that 10 μmol/L of magnolol had a maximal inhibitory effect on increased permeability of Caco2 cells after LPS induction. Caco2 cells were divided into four groups again and treated with the appropriate concentrations of magnolol. RANTES levels were measured using ELISA kits, according to the manufacturer’s instructions (Shanghai Medical Equipment Co., Ltd (80-2), Shanghai, China).
Caco2 cells were lysed in 200 mL of radio-immunoprecipitation assay (RIPA) (Beyotime Biotechnology, Shanghai, China) buffer and homogenized. The protein concentration was determined using the bicinchoninic acid method. The cells were then washed with ice cold phosphate buffered saline (PBS) (Sinopharm Chemical Reagent, Shanghai, China) and re-centrifuged at 5500 rpm for 5 min at 4 °C. The cell pellets were re-suspended in a lysis buffer [100 mmol/L phenylmethylsulfonylfluoride (PMSF) (Aladdin, Shanghai, China), 10 μL phosphatase inhibitor (Beyotime Biotechnology, Shanghai, China)] for 30 min. The lysate was centrifuged at 12000 rpm for 5 min at 4 °C before the supernatant was collected. We performed the Bradford assay (Biorad, Hercules, CA, United States) and UV spectrophotometry (Bio-wave II; Biochrom WPA, Cambridge, United Kingdom) to equalize loading protein. Equal amounts of protein (40 μg) were transferred to vinylidene fluoride membranes (Milipore, Billerica, MA, United States). The membranes were incubated with blocking buffer [5% non-fat dry-milk in TBS containing 0.1% Tween-20 (TBST)] for 2 h at room temperature. The membranes were probed with anti-RANTES (1:2000; AF5151, Affinity Biosciences, United States), anti-IKKβ (1:2000; AF6009, Affinity Biosciences, United States), anti-p-IKKβ (1:2000; AF3010, Affinity Biosciences, United States), anti-IKBα (1:2000; AF5002, Abcam, United Kingdom), anti-p65 (1:2000; ab16502, Affinity Biosciences, United States), and anti-p-p65 (1:2000; AF2006, Affinity Biosciences, United States) polyclonal antibodies overnight at 4 °C. Subsequently, the membranes were incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibody (1:50000, BA1054, Dr. DE Biological Engineering Co. Ltd, Wuhan, China) at 37 °C for 2 h. GAPDH (AB-P-R 001, Xianzhi Biology Co. Ltd, Hangzhou, China) was used as the internal control and detected on the membrane. The signals were detected on an X-ray imaging system (6535876, Ruike Medical Equipment Co., Ltd, Xiamen, China), and the gray value of the immunoreactive bands was analyzed using BandScan.
Cells were dehydrated, cleared, waxed, embedded, sliced, and incubated with primary antibody (Abcam, United States) and secondary antibody (San Eagle, Wuhan, China). Subsequently, the cells were stained with 4,6-guanidine-2-phenyl indole (DAPI) and observed under a confocal microscope (Perkin Elmer & Olympus, Tokyo, Japan).
Data were analyzed using IBM SPSS Statistics version 22. Measurement data following a Gaussian distribution are expressed as the mean ± SD. The comparison between groups was conducted using the LSD method (minimum significance method) in one-way ANOVA or t-tests as appropriate. P values < 0.05 indicated statistical significance.
During the 24-h postoperative observation period, no rats died in the Control group. Two rats each died in the Model group and the low-dose LM group, and one rat each died in the MM and the HM groups, corresponding to the death rates of 0%, 18.18%, 18.18%, 9.09%, and 9.09%, respectively, which did not differ significantly between the groups.
The rats in the Control group behaved normally, were sensitive, and had glossy hair, normal stool, and no hyperemia or edema in the intestine. Within 6 h post-intervention, the other four groups of mice exhibited a series of abnormalities, such as piloerection, shortness of breath, listlessness, loss of appetite, diarrhea, loss of interest in the surrounding environment, the disappearance of self-cleaning behavior, and increased secretion from the eyes. The symptoms of the HM, MM, and LM groups were milder compared to those of the Model group. After 12 h, the HM, MM, and LM groups showed signs of mental improvement and self-cleaning behaviors, while the Model group did not.
Compared to the Control group, the levels of serum and ileal TNF-α, IL-1β, and IL-6 were significantly higher those of the Model group (P < 0.01; Tables 1 and 2), while they were decreased slightly in the LM, MM, and HM group (P < 0.05; Tables 1 and 2).
| Group | RANTES (ng/mL) | TNF-α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
| Control (n = 11) | 0.65 ± 0.25 | 124.91 ± 11.29 | 185.87 ± 24.97 | 183.69 ± 43.02 |
| Model (n = 9) | 5.17 ± 0.70a | 256.89 ± 19.55a | 383.01 ± 41.85a | 380.99 ± 44.11a |
| LM (n = 9) | 4.07 ± 0.67a,b | 167.39 ± 20.92a,b | 318.83 ± 74.06a,b | 308.21 ± 73.22a,b |
| MM (n = 10) | 3.13 ± 0.45a,b,c | 143.87 ± 15.22a,b,c | 256.79 ± 25.37a,b,c | 246.14 ± 41.73a,b,c |
| HM (n = 10) | 2.52 ± 0.28a,b,c,d | 144.49 ± 18.02a,b,c | 225.07 ± 17.46a,b,c,d | 215.18 ± 12.49a,b,c,d |
| Group | RANTES (g/mL) | TNF-α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
| Control (n = 11) | 3.40 ± 1.45 | 347.04 ± 60.66 | 240.4 ± 37.08 | 195.11 ± 28.60 |
| Model (n = 9) | 29.29 ± 4.07a | 1015.73 ± 90.96a | 453.52 ± 54.82a | 436.01 ± 46.29 |
| LM (n = 9) | 22.47 ± 3.72a,b | 720.82 ± 88.16b | 330.89 ± 49.06a,b | 337.66 ± 73.79b |
| MM (n = 10) | 17.39 ± 2.59a,b,c | 487.48 ± 115.29a,b | 301.43 ± 44.51a,b | 271.25 ± 36.82b,c |
| HM (n = 10) | 14.10 ± 1.59a,b,c,d | 428.79 ± 48.71a,b,c | 294.27 ± 68.2a,b | 237.75 ± 16.05b,c,d |
In the Control group, the ileal mucosal villi were arranged in an orderly manner, and the structure of the surrounding blood vessels was normal. In the Model group, the ileal mucosal villi were damaged. The intestinal mucosal epithelial cells were degenerated and wiped off, the subepithelial capillaries exhibited dilation and congestion, and inflammatory cells were diffused in the lamina propria. Compared to the Model group, the morphology of the ileal mucosal villi in the three magnolol groups was improved, although slight damage to the ileal mucosal villi, local necrosis, and some bleeding were observed. A pronounced improvement in ileal mucosal morphology was observed in the HM group (Figure 1A-E).
Compared to the Control group, the RANTES release in serum was significantly increased in the Model group (P < 0.05, Table 1). Magnolol decreased the RANTES release in the LM, MM, and HM groups compared to the Model group (P < 0.05, Table 1). Moreover, the RANTES expression levels in ileal tissue were significantly decreased in the LM, MM, and HM groups compared to the Model group (P < 0.05, Table 2).
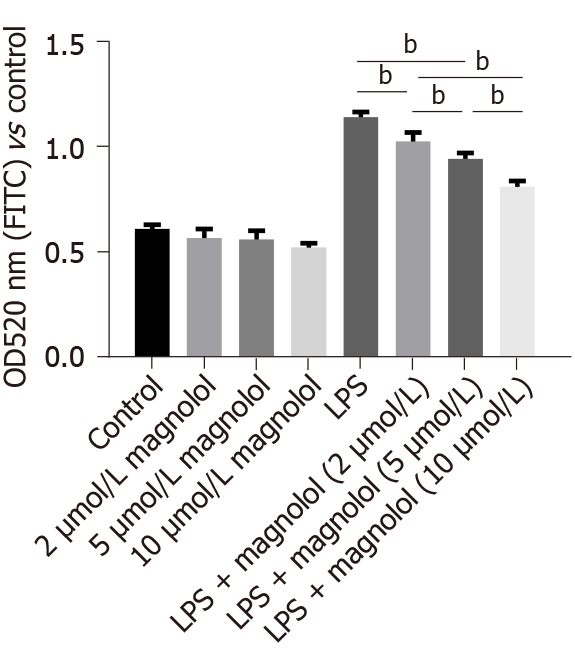
CCK-8 cells were used to detect cell permeability. The relative absorbance value of Caco2 cells treated with solvent was 1.0. No significant change was detected in cell permeability after adding different concentrations of magnolol (Figure 2). Conversely, the permeability of Caco2 cells increased significantly in the LPS-treated Caco2 cells (P < 0.01, Figure 2). However, compared to the LPS-treated Caco2 cells, the permeability decreased significantly when Caco2 cells were treated with magnolol before LPS treatment (P < 0.01, Figure 2).
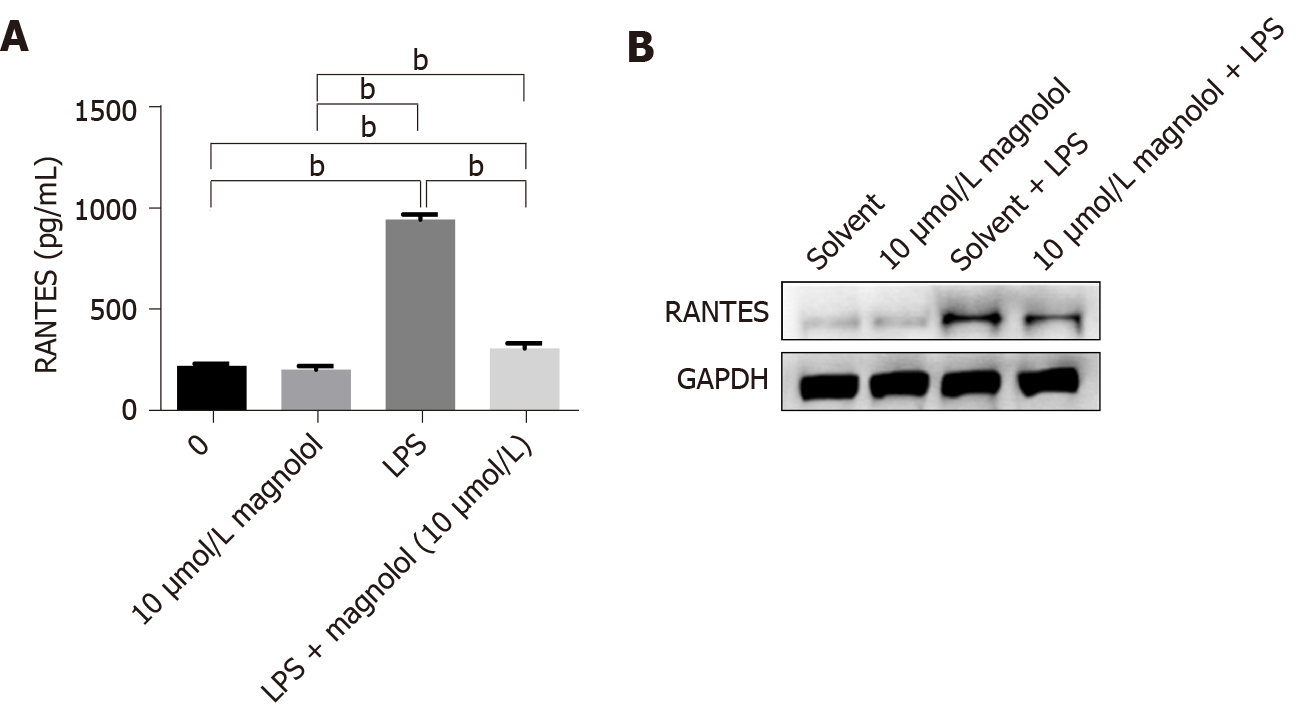
The RANTES expression in Caco2 cells in each group was determined by ELISA and Western blot analysis (Figure 3A and B). The expression of RANTES in Caco2 cells without LPS treatment was low, and magnolol had little effect on the expression of RANTES. On the other hand, LPS increased the level of RANTES in Caco2 cells, which was significantly inhibited by magnolol (P < 0.01, Figure 3A).
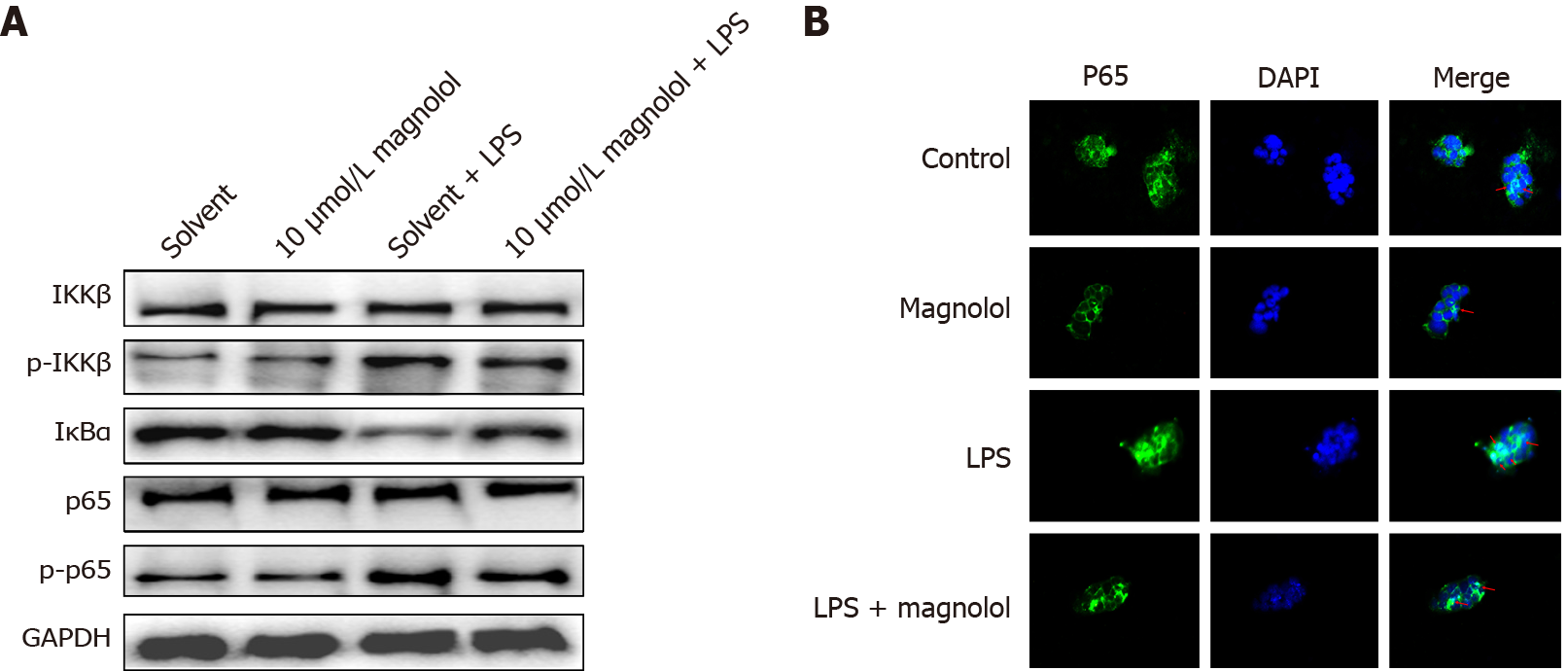
No significant difference was observed in the expression of inhibitor of nuclear factor kappa-B kinase (IKK) and p65 in different groups (Figure 4A). LPS increased the phosphorylated level of inhibitor of nuclear factor kappa-B kinase β (IKKβ) and p65 in Caco2 cells and decreased the expression of inhibitor of nuclear factor kappa-B kinase α (IκBα). Magnolol inhibited the increased phosphorylation of IKK and p65 in LPS-treated Caco2 cells and reversed the down-regulation of IκBα. Compared to the Solvent group of Caco2 cells, the p65 distribution in the nucleus of Caco2 cells treated with 10 μmol/L of magnolol did not change significantly (P > 0.05), while LPS enhanced the translocation of p65 to the nucleus. Compared to the LPS-treated Caco2 cells, 10 μmol/L of magnolol reduced the nuclear p65 abundance in Caco2 cells (Figure 4B).
The intestinal tract is the most commonly involved organ in sepsis. According to statistics, up to 60% of sepsis cases are accompanied by gastrointestinal tract dysfunction[21]. The intestinal tract is also the second-largest immune organ of the body. The gut has long been considered the “motor” of the systemic inflammatory response[22]. The common factors, TNF-α, IL-1β, IL-6, and IL-1β, are mainly secreted by activated mononuclear macrophages and partially by other nucleated cells[23]. IL-6 is secreted by mononuclear macrophages, fibroblasts, and endothelial cells. The primary function of the intestinal tract is to promote the acute phase protein synthesis and participate in inflammation. In the hemorrhagic shock model, IL-6 gene knockout protected the rats from intestinal barrier dysfunction[24]. Therefore, IL-6 has been suggested as a common inflammatory indicator of intestinal barrier dysfunction[25]. TNF-α is mainly secreted by activated mononuclear macrophages, and its main function is to heat and dilate blood vessels, and promote the chemotactic adhesion of neutrophils[26]. In the sepsis model, the increase in serum and tissue TNF-α has been closely related to mortality[27]. TNF-α is released at the initial stage of inflammation. It promotes the secretion of IL-1β and IL-6 and also stimulates the expression of adhesion molecules on the surface of intestinal microvascular endothelial cells and neutrophils, accelerating neutrophil aggregation and releasing the reactive oxygen and proteolytic enzymes. These phenomena disrupt the intestinal mucosal epithelium and accelerate apoptosis of intestinal mucosal epithelial cells[28].
In this study, we quantitatively detected the differences in the expression levels of inflammatory factors in each group, such as IL-1β, IL-6, and TNF- α. In the severe sepsis group, the expression levels of all the three inflammatory factors were significantly increased, which were consistent with previous studies[29]. After magnolol administration, the release of inflammatory cytokines was reduced in a dose-dependent manner, and the apoptosis of LPS-induced ileal epithelial cells was pronounced. Therefore, we speculated that magnolol prevents the aggravation of inflammation and the damage of intestinal mucosal tissues.
RANTES, a member of the chemotactic cytokine CC subgroup, also known as CCL5, is secreted by epithelial cells[25,30]. RANTES plays a critical role in chemotactic and stimulating T cells, accumulating a large number of T cells in the inflammatory lesions, activating T cells to produce inflammatory factors, and aggravating the tissue damage. Several studies have shown that RANTES is highly superficial in the intestinal mucosal tissues of patients with ulcerative colitis[31]. Also, its high expression is an indicator of sepsis in premature infants and neonates[32,33]. In the current study, RANTES was significantly increased in both serum and ileal tissue after LPS treatment as well as inflammatory factors. This phenomenon proved that the RANTES has a positive role in inflammation-induced LPS models. However, the serum secretion was consistent with the decreased expression of RANTES in ileal tissue after administration of magnolol, which further indicated its role in resisting the inflammatory response in sepsis.
The mechanisms by which magnolol regulates RANTES to reduce damage in intestinal mucosal epithelial cells are not well-understood. It has been hypothesized that the NF-κB signaling pathway is closely related to the production of various cytokines, chemokines, and adhesion factors in oxidative stress and inflammatory response. In normal cells, NF-κB is retained in the cytoplasm due to binding to its inhibitory protein IκB and kinase IKKα/β. When inflammation occurs, phosphorylated IκBs and IKKα/β may activate the NF-κB signaling pathway, thus leading to nucleation of p65 and p50 and promoting transcriptional activation of the relevant target genes[34]. When NF-κB is dislodged from the complex, it translocates rapidly from the cytoplasm to the nucleus and aggregates in the nucleus to induce RANTES by binding to its promoter[19]. Several studies have shown that patients with inflammatory bowel disease exhibit high expression of p65 in the nucleus of cells in intestinal mucosal tissue. Abnormal expression of intracellular p65 was also detected in the dextran sodium sulfate (DSS)-induced colitis model. Simultaneously, PDTC, an inhibitor of NF-κB, significantly upregulates the expression of the dense protein in DSS-induced colon cancer rats, restores the normal intestinal mucosal permeability, and reduced the inflammatory response[35]. The current results revealed that after magnolol treatment, the permeability of Caco2 cells induced by LPS, the phosphorylation of IKKβ, and the nucleation of p65 was decreased, and the expression of RANTES was down-regulated. The abnormal activation of the NF-κB signaling pathway was closely correlated with the expression of RANTES during inflammation, and magnolol inhibited the up-regulation of RANTES expression in the NF-κB signaling pathway.
We found that in our study, inhibition of serum TNF-α levels by magnolol was only partial, i.e., 50%-60% inhibition vs Model group and there may be enough circulating TNF-α for host defense. It appears that magnolol has more than a single anti-inflammatory effect in the treatment of sepsis. Some studies have reported that drugs with anti-inflammatory actions, such as antioxidative agents, could regulate immunity dysfunction with apparent safety in sepsis[36,37]. As we know, pathogen-associated molecular patterns (PAMPs) can lead to the initiation of innate immune and inflammatory responses. TLRs are a group of evolutionarily conserved and membrane-bound pattern recognition receptors that recognize various PAMPs including microbial nucleic acids, lipids, proteins, lipoproteins, and glycoproteins[38,39]. We have found that magnolol could down-regulate RANTES levels by inhibiting the TLR4-regulated NF-κB signaling pathways. Taken together, these findings suggest that magnolol may have an immunomodulating role in sepsis. We will test this idea in subsequent experiments.
Overall, magnolol significantly alleviates inflammatory response and pathological changes in the intestinal tissue and the levels of RANTES expression in rats with sepsis. The abnormal activation of the NF-κB signaling pathway is closely correlated with the expression of RANTES during inflammation, and magnolol inhibits the up-regulation of RANTES expression via the NF-κB signaling pathway. Thus, this study provided the pharmacological proof for use of magnolol and suggested that it is a potential agent in the prevention and treatment of sepsis. Further studies should be conducted to clarify the underlying mechanism(s) of the anti-inflammatory and immunomodulating role of magnolol in sepsis.
Sepsis is a major medical challenge, and finding specific targets and effective drugs is a scientific concern. Currently, various biological constituents are isolated from traditional Chinese medicine and have been confirmed to possess multifunctional activities.
Magnolol is an active constituent of Houpu, which improves tissue function and exerts strong anti-endotoxin and anti-inflammatory effects. Thus, we aimed to identify the role of magnolol in the treatment of sepsis.
To assess the protective effect of magnolol on intestinal mucosal epithelial cells in sepsis and elucidate the underlying mechanisms.
We carried out animal studies and cell studies in vitro, respectively. In animal studies, enzyme-linked immunosorbent assay was used to measure the differentially expressed inflammatory factors and regulated on activation, normal T-cell expressed and secreted (RANTES) levels in serum and ileal tissue. In the in vitro experiments, Cell Counting Kit-8 and cell permeability assays were employed to determine the concentration of drug-containing serum that did not affect the activity of Caco2 cells but inhibited lipopolysaccharide (LPS)-induced permeability reduction. Immunofluorescence and Western blot assays were used to detect the protein levels of RANTES, inhibitor of nuclear factor kappa-B kinase β (IKKβ), phosphorylated IKKβ (p-IKKβ), inhibitor of nuclear factor kappa-B kinase α (IκBα), p65, and p-p65 in different groups.
In animal studies, magnolol inhibited the expression of proinflammatory cytokines tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6 in a dose-dependent manner and suppressed the production of RANTES in sepsis rats. In the in vitro studies, magnolol inhibited the increase of p65 nucleation and down-regulated the production of the phosphorylated form of IKKβ in LPS-treated Caco2 cells. Moreover, magnolol inhibited the translocation of the transcription factor nuclear factor-kappa B (NF-κB) from the cytosol into the nucleus and downregulated the expression level of the chemokine RANTES in LPS-stimulated Caco2 cells.
Magnolol downregulates RANTES levels by inhibiting the LPS/NF-κB signaling pathway, resulting in the suppression of IL-1β, IL-6, and TNF-α expression that in turn, alleviates the mucosal barrier dysfunction in sepsis.
This study, for the first time, proved that magnolol plays a role in the treatment of sepsis by down-regulating RANTES, and further studies on the anti-inflammatory mechanism might provide an in-depth insight into novel methods for the clinical prevention and treatment of sepsis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Gao CC
| 1. | Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 679] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 2. | Perner A, Cecconi M, Cronhjort M, Darmon M, Jakob SM, Pettilä V, van der Horst ICC. Expert statement for the management of hypovolemia in sepsis. Intensive Care Med. 2018;44:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 16968] [Article Influence: 1885.3] [Reference Citation Analysis (2)] |
| 4. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 5. | Gao Y, Han F, Huang X, Rong Y, Yi H, Wang Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J Anim Sci. 2013;91:5614-5625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Border JR, Hassett J, LaDuca J, Seibel R, Steinberg S, Mills B, Losi P, Border D. The gut origin septic states in blunt multiple trauma (ISS = 40) in the ICU. Ann Surg. 1987;206:427-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 296] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Seoane L, Winterbottom F, Nash T, Behrhorst J, Chacko E, Shum L, Pavlov A, Briski D, Thibeau S, Bergeron D, Rafael T, Sundell E. Using quality improvement principles to improve the care of patients with severe sepsis and septic shock. Ochsner J. 2013;13:359-366. [PubMed] |
| 9. | Sun XG, Fan Q, Wang QR. [Effect of dachengqi decoction on expressions of TLR4 and TNF-alpha in the lung and the large intestine of mice with endotoxemia]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31:244-248. [PubMed] |
| 10. | Pan LY, Chen YF, Li HC, Bi LM, Sun WJ, Sun GF, Zhang XF, Xu K, Feng DX. Dachengqi Decoction Attenuates Intestinal Vascular Endothelial Injury in Severe Acute Pancreatitis in Vitro and in Vivo. Cell Physiol Biochem. 2017;44:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Tang F. [Advance in latest studies on pharmacological effects of magnolol]. Zhongguo Zhong Yao Za Zhi. 2012;37:3526-3530. [PubMed] |
| 12. | Jekarl DW, Kim JY, Ha JH, Lee S, Yoo J, Kim M, Kim Y. Diagnosis and Prognosis of Sepsis Based on Use of Cytokines, Chemokines, and Growth Factors. Dis Markers. 2019;2019:1089107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 539] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 14. | Shouman B, Badr R. Regulated on activation, normal T cell expressed and secreted and tumor necrosis factor-alpha in septic neonates. J Perinatol. 2010;30:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Song A, Nikolcheva T, Krensky AM. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol Rev. 2000;177:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Kucuk C, Sozuer E, Gursoy S, Canoz O, Artis T, Akcan A, Akyildiz H, Muhtaroglu S. Treatment with Met-RANTES decreases bacterial translocation in experimental colitis. Am J Surg. 2006;191:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Dai C, Wen X, He W, Liu Y. Inhibition of proinflammatory RANTES expression by TGF-beta1 is mediated by glycogen synthase kinase-3beta-dependent beta-catenin signaling. J Biol Chem. 2011;286:7052-7059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Khunchai S, Junking M, Suttitheptumrong A, Kooptiwut S, Haegeman G, Limjindaporn T, Yenchitsomanus PT. NF-κB is required for dengue virus NS5-induced RANTES expression. Virus Res. 2015;197:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Luo J, Xu Y, Zhang M, Gao L, Fang C, Zhou C. Magnolol inhibits LPS-induced inflammatory response in uterine epithelial cells : magnolol inhibits LPS-induced inflammatory response. Inflammation. 2013;36:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Suliburk J, Helmer K, Moore F, Mercer D. The gut in systemic inflammatory response syndrome and sepsis. Enzyme systems fighting multiple organ failure. Eur Surg Res. 2008;40:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock. 2016;46:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 23. | Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123:4695-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621-G629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Nakada TA, Boyd JH, Russell JA, Aguirre-Hernández R, Wilkinson MD, Thair SA, Nakada E, McConechy MK, Fjell CD, Walley KR. VPS13D Gene Variant Is Associated with Altered IL-6 Production and Mortality in Septic Shock. J Innate Immun. 2015;7:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Gil M, Kim YK, Hong SB, Lee KJ. Naringin Decreases TNF-α and HMGB1 Release from LPS-Stimulated Macrophages and Improves Survival in a CLP-Induced Sepsis Mice. PLoS One. 2016;11:e0164186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Arslan E, Yavuz M, Dalay C. The relationship between tumor necrosis factor (TNF)-alpha and survival following granulocyte-colony stimulating factor (G-CSF) administration in burn sepsis. Burns. 2000;26:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 29. | Hirano Y, Aziz M, Wang P. Role of reverse transendothelial migration of neutrophils in inflammation. Biol Chem. 2016;397:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018-1025. [PubMed] |
| 31. | Rosbottom A, Knight PA, McLachlan G, Thornton EM, Wright SW, Miller HR, Scudamore CL. Chemokine and cytokine expression in murine intestinal epithelium following Nippostrongylus brasiliensis infection. Parasite Immunol. 2002;24:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Kasztelewicz B, Piotrowska E, Tołłoczko J, Borszewska-Kornacka MK, Gregorek H, Dzierżanowska-Fangrat K. Assessment of interleukin-17A, C5a and RANTES for early diagnosis of neonatal sepsis - a preliminary study. Cent Eur J Immunol. 2016;41:376-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Ng PC, Li K, Leung TF, Wong RP, Li G, Chui KM, Wong E, Cheng FW, Fok TF. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol. 2011;12:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 35. | Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, de la Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24:1401-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Yang S, Shen L, Jin Y, Liu J, Gao J, Li D. Effect of Dachengqi decoction on NF-kappaB p65 expression in lung of rats with partial intestinal obstruction and the underlying mechanism. J Huazhong Univ Sci Technolog Med Sci. 2010;30:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Ma H, Kou J, Zhu D, Yan Y, Yu B. Liu-Shen-Wan, a traditional Chinese medicine, improves survival in sepsis induced by cecal ligation and puncture via reducing TNF-alpha levels, MDA content and enhancing macrophage phagocytosis. Int Immunopharmacol. 2006;6:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8061] [Cited by in RCA: 8771] [Article Influence: 461.6] [Reference Citation Analysis (0)] |
| 39. | Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2423] [Cited by in RCA: 2728] [Article Influence: 194.9] [Reference Citation Analysis (0)] |