Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9662
Peer-review started: June 2, 2021
First decision: June 24, 2021
Revised: June 28, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 6, 2021
Processing time: 153 Days and 22.3 Hours
Hepatic tuberculosis (TB) is uncommon clinically. Because of a lack of specific signs, characteristic symptoms and clinical manifestations and because patho
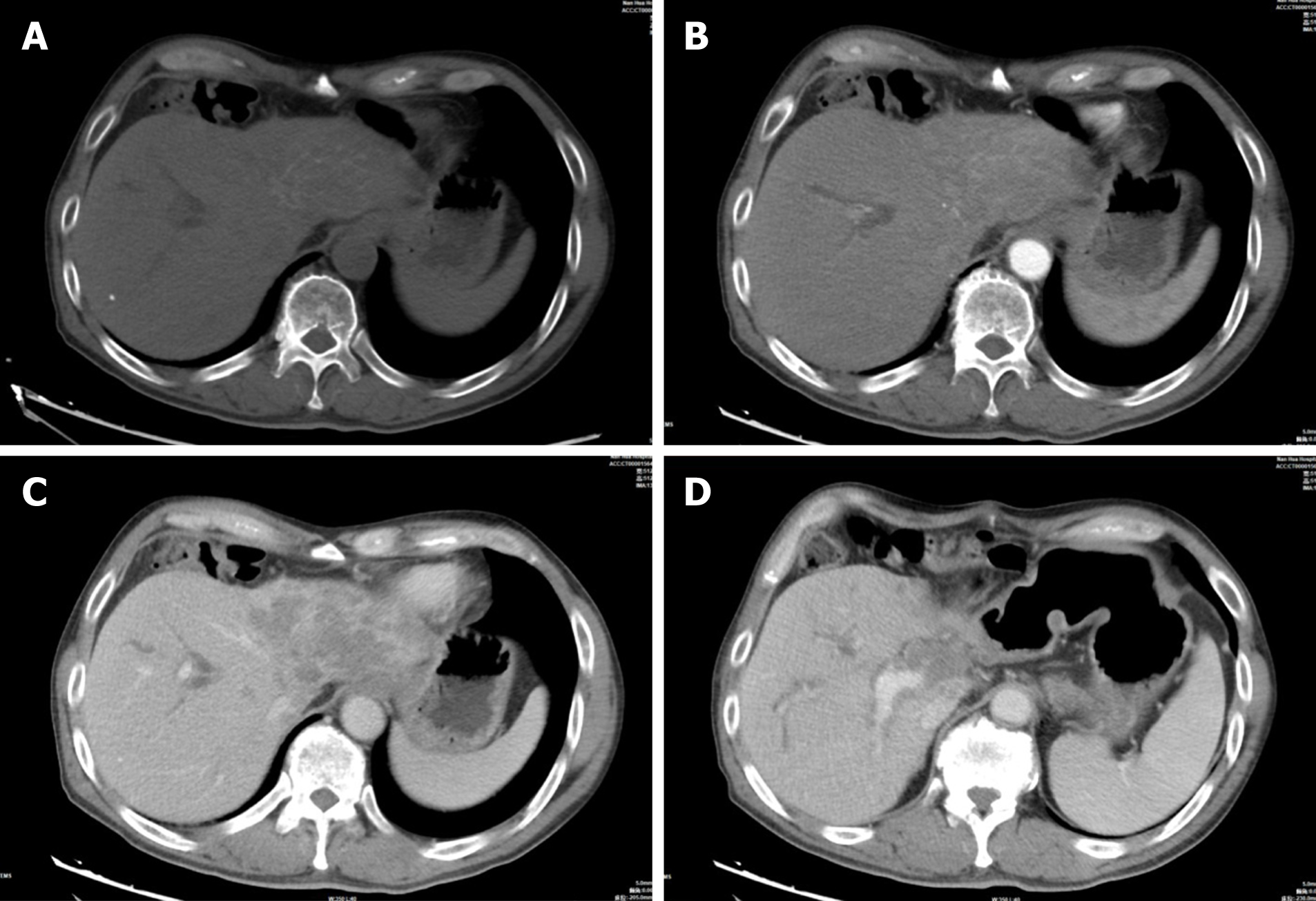
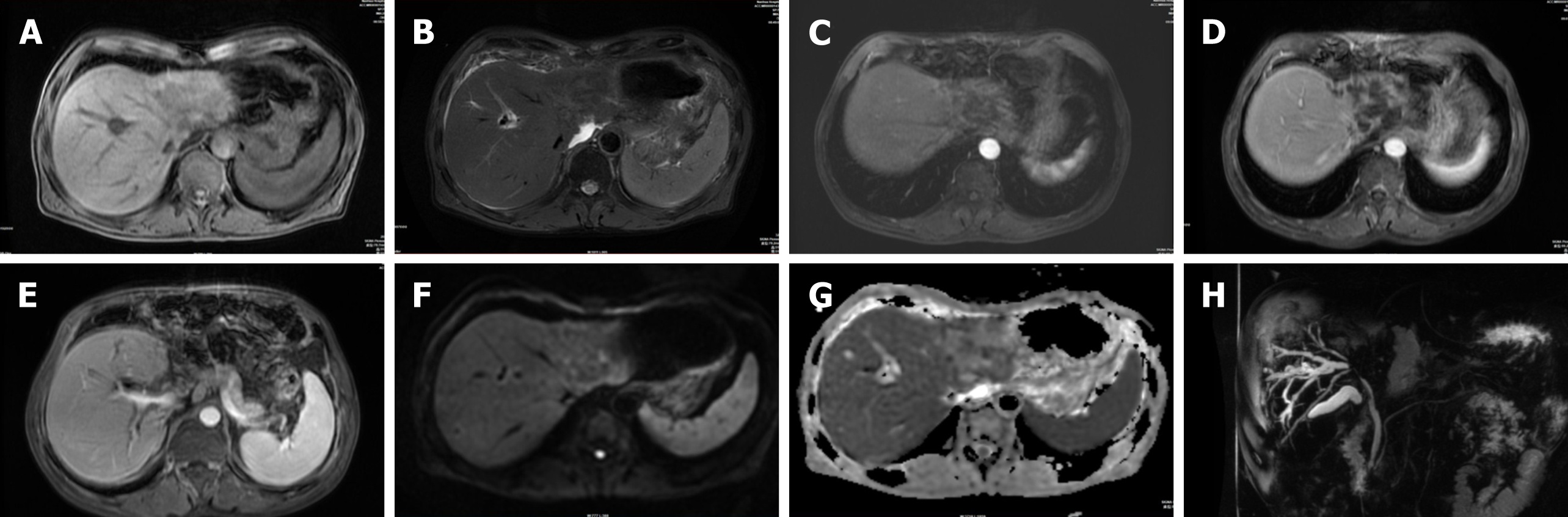
A 62-year-old Chinese man presented with jaundice for 1 wk and no abnormal laboratory tests other than elevated bilirubin, aminotransferases and C-reactive protein. Computed tomography (CT) of the abdomen showed a mass in the left lobe of the liver and hepatic hilum with striped calcified foci. Mild enhancement was visible at the edges, along with extensive intrahepatic biliary ductal dilatation in the right lobe of the liver. In the arterial phase of both CT and magnetic resonance imaging, the main trunk and right branch of the portal artery were partially visualized. Magnetic resonance cholangiopancreatography (MRCP) indicated that the left lobe of the liver and most of the bile ducts in the hilum were not visible. Pathological examination revealed coagulative necrosis, and granulomatous nodules were seen around areas of necrosis; therefore, TB was considered.
Hepatic tuberculosis is easily misdiagnosed or missed on imaging. Percutaneous puncture biopsy is the most useful tool for definitive diagnosis.
Core Tip: Hepatic tuberculosis (TB) is rare in clinical practice and can also be easily missed or misdiagnosed. The clinical symptoms of hepatic TB reported in this case were atypical and a series of investigations were completed in our hospital, including computed-tomography-enhanced scans, magnetic resonance imaging multiparametric scans and pathological examination. We have also conducted a series of discussions on the case, which provide more reference for the diagnosis of hepatic TB in the future.
- Citation: Li W, Tang YF, Yang XF, Huang XY. Misidentification of hepatic tuberculosis as cholangiocarcinoma: A case report. World J Clin Cases 2021; 9(31): 9662-9669
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9662.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9662
Tuberculosis (TB) can affect any system or organ throughout the body[1]. TB infection of the liver, also known as hepatic TB, is a manifestation of extrapulmonary infection with Mycobacterium tuberculosis[2]. Hepatic TB is uncommon clinically, and the main clinical manifestations include fever, cyanosis, jaundice, shortness of breath, cough, pulmonary moist rales, hepatomegaly, splenomegaly and abdominal distention[3]. Because of a lack of specific signs, characteristic symptoms and clinical manifestations and because pathological samples are difficult to acquire, hepatic TB is easily missed or misdiagnosed. The radiological manifestations and clinical features of hepatic TB can be mistaken for malignant lesions, leading to unnecessary surgical intervention and erroneous clinical diagnosis[4]. Among the cases of hepatic TB reported in the literature, the miliary form was common (79% of cases), with local hepatic TB acc
A 62-year-old Chinese man presented with jaundice for 1 wk.
The patient complained of yellowing of the sclera, darkened urine, fatigue, loss of appetite without significant weight loss, abdominal pain and bloating for 1 wk.
The patient had a history of lower limb deformity due to trauma at an early age, head and lower limb fracture caused by a car accident 4 years ago and surgically treated; details unknown.
The patient’s family members have no history of specific genetic or infectious diseases.
Physical examination showed double lower limb deformities. The patient’s tem
The laboratory test findings were not specific (Table 1). Biochemical tests showed that total bilirubin (380.6 μmol/L), direct bilirubin (347.9 μmol/L) and indirect bilirubin (30.9 μmol/L) were elevated, with normal electrolyte profiles and renal function. Elevated glutamic oxaloacetic transaminase and glutamic pyruvic transaminase levels indicated impaired liver function. Tumor markers such as α-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 199 were negative, and there was no evidence of hepatitis A, B, C or E virus infection.
| Patient’s results | Normal range | |
| Neutrophil (%) | 79.3 | 50.0–70.0 |
| Lymphocyte (%) | 9.40 | 20.0–40.0 |
| Prothrombin time activity (%) | 102 | 70–130 |
| Fibrinogen (g/L) | 4.05 | 2.0–4.0 |
| ESR (mm/h) | 19 | 0–10 |
| Total bilirubin (µmol/L) | 380.6 | 3.4–17.1 |
| Direct bilirubin (µmol/L) | 347.9 | 0–8 |
| Indirect bilirubin (µmol/L) | 30.9 | 0–20 |
| ALT (U/L) | 120 | 9–50 |
| AST (U/L) | 132 | 15–40 |
| C-reactive protein (mg/L) | 31.72 | 0–6 |
| AFP (IU/mL) | 1.77 | 0–20 |
| CEA (ng/mL) | 2.68 | 0–5 |
| CA199 (U/mL) | 18.86 | 0–34 |
Abdominal CT showed a large patchy hypodense shadow in the left lobe of the liver and hepatic hilum with striped calcified foci (Figure 1A). This mass measured 9.2 cm × 6.0 cm × 5.8 cm, and mild enhancement was visible at the edges with extensive intrahepatic biliary ductal dilatation in the right lobe of the liver (Figure 1B, C). Chest CT showed only a few areas of fibrosis and hard nodules, with no obvious signs of active TB. We considered hepatic schistosomiasis or intrahepatic cholangiocarcinoma based on the CT findings. Abdominal MRI demonstrated that the left lobe and caudate lobe of the liver were atrophied, with a lesion measuring 8.7 cm × 6.8 cm × 5.2 cm. A slightly hypointense signal on T1-weighted and mixed hyperintense and hypointense signals on T2-weighted fat suppressed images were observed (Figure 2A, B), with mild restricted diffusion (Figure 2F) and a low apparent diffusion coefficient value of 0.764 × 103 mm2/s (Figure 2G). The left branch of the portal vein and the left hepatic vein could not be visualized in the portal venous phase (Figure 1C, 2D). In the arterial phase of both CT and MRI scans, the main trunk of the portal vein and the right branch of the portal vein were partially visualized (Figure 1D, 2E). The bile ducts in the hilar region and most of the intrahepatic bile ducts in the left lobe of the liver could not be observed, and the above intrahepatic bile ducts showed varying degrees of dilatation on magnetic resonance cholangiopancreatography (MRCP) (Figure 2H). This was the main clue that led to our misdiagnosis of cholangiocarcinoma. Enlarged lymph nodes were seen in the retroperitoneum, and the larger lymph node was approximately 1.3 cm × 1.2 cm, with uniform strengthening after enhancement.
Intraoperative exploration of the abdominal cavity showed multiple space-occupying masses of variable size in the left liver that protruded from the surface of the liver by approximately 1 cm and were yellowish-white in color and hard in texture; there were multiple scattered small nodules in the hepatic round ligament, small omental sac and other sites, and these nodules were yellowish-white in color. Two yellow nodules were partially removed from the left liver by an ultrasonic knife and then sent for rapid frozen biopsy. Liver tissue was seen, with focal visible necrosis and granulomatous nodules around the necrosis, which were considered to indicate a microbial infection (Figure 3). The nodules in the lesser omentum and the liver nodules were again sampled and sent for rapid analysis: necrosis and granulomatous nodule formation were seen, microbial infection was considered, and TB was not excluded. The left lobe of the liver, the lymph nodes of the duodenal ligament and the omentum were granulomatous.
The final pathological diagnosis after biopsy in this case was hepatic TB.
The patient underwent laparoscopic biliary tissue biopsy, resection of the left half of the liver and portal bile duct and internal bile-intestinal drainage of the right portal bile duct.
The patient was then transferred to the intensive care unit for treatment and was subsequently discharged 1 wk after the operation.
The clinical manifestations of TB are mainly related to the character, range and type of the lesion and the reactivity of the organism. Levine classified TB as follows: (1) miliary TB; (2) pulmonary TB with hepatic involvement; (3) primary liver TB; (4) focal tuberculoma or abscess; and (5) TB cholangitis. Miliary TB of the liver is more common than other types of hepatic TB[6], and it has been reported to occur in 50%–80% of all patients with terminal pulmonary TB. During the healing stage of hepatic TB, CT scans might present diffuse hepatic calcifications (approximately 50% of these cases)[7]. In our case of hepatic TB, there was a central nonenhancing hypodense lesion due to caseous necrosis, with enhancement at the edges in contrast to the surrounding granulation tissue. Streaks of calcification were visible on CT, indicating that the imaging characteristics of hepatic TB may be multilayered lesions of various densities and demonstrating that lesions of different pathological stages can coexist in liver TB, including tuberculous granuloma, liquefied necrosis, fibrosis and calcification[8]. The heterogeneous, hypodense nodule (central caseous necrosis) with peripheral enhancement on CT after the administration of an intravenous contrast agent (surrou
MRI manifestations are associated with nodules at different pathological stages. In the early and middle stages of sarcoidosis with or without caseous or liquefied necrosis, the lesions exhibit a low T1-weighted and a high T2-weighted signal. Similar lesions with low T1-weighted and T2-weighted signals correspond to the fibrous stage of TB and might have slight or no peripheral enhancement. Early- or intermediate-stage tuberculous granulomas and fibroproliferative lesions appear as hypointense areas on CT and with various signal intensities on T2-weighted imaging, which is the major advantage of MRI for the diagnosis of hepatic TB[12]. Thick peripheral enhancement and/or enhanced irregular thickened septa with restricted diffusivity on diffusion-weighted imaging can be observed according to the protein content (thickened septa and debris) after the use of contrast media[13]. These characteristics are basically consistent with the cases we have reported.
Hepatic TB is commonly imaged with CT, and the pathological manifestations vary in different periods, so this disease needs to be differentiated from metastases, cholangiocarcinoma, bacterial liver abscess, and hepatic schistosomiasis. The misdiagnosis of hepatic TB as metastasis has also been reported in the literature, and sometimes it is hard to distinguish between hepatic TB and metastasis because of the simultaneous involvement of the liver and spleen[14]. Patients with metastases usually have a history of a primary tumor, and these metastases are not limited to the liver but may include other organs or enlarged lymph nodes.
The misdiagnosis of hepatic TB as intrahepatic cholangiocarcinoma is not uncommon[4,6,15,16]. Of the lesions misdiagnosed as intrahepatic cholangiocarcinoma, only one was located in the porta hepatis, and the rest were located in the left lobe of the liver; all of these lesions caused symptoms of biliary obstruction, including the cases we reported. These misdiagnosed cases typically presented on CT images as mild enhancement and central, hypodense lesions due to caseation necrosis, with mild enhancement at the edges corresponding to the surrounding granulation tissue. MRI of hepatic TB generally shows a hypointense nodule with a hypointense rim on T1-weighted imaging. T2-weighted imaging presents a hypointense, isointense or hyperintense nodule with a less intense margin. These signs are not truly distinguishable from those of cholangiocarcinoma. However, the difference between the case we reported and typical cholangiocarcinoma is that CT usually shows stripes of calcification in hepatic TB, while cholangiocarcinoma rarely exhibits a high number of calcifications; thus, we speculate that the presence of such stripes of calcification may be an important point in differentiating hepatic TB from cholangiocarcinoma.
Simple plain ultrasound cannot differentiate between bacterial and tubercular liver abscesses[17]. Abscesses mostly occur in elderly male patients and patients with diabetes mellitus[18]. A high incidence of TB can be found in regions where HIV is most prevalent, and HIV-induced immunosuppression results in the reactivation of latent TB[19]. Klebsiella pneumoniae is one of the microorganisms most commonly isolated from pyogenic liver abscesses in Asians, and 89.7% of patients who have liver abscesses due to K. pneumoniae may present with internal gas bubbles[20]. According to the imaging findings, the patient in this case had a hepatic venous obstruction, which needed to be differentiated from hepatic schistosomiasis, as many of the clinical and pathological characteristics of hepatic TB and hepatic schistosomiasis are similar. Pathologically, the patient had periportal inflammation and granuloma formation, which are most commonly found in schistosomiasis[21]. Ultimately, a needle biopsy is needed to identify the disease. Bacteriological surveillance in patients with extrapulmonary TB is difficult and not always feasible. Ziehl–Neelsen staining is positive in only 40% of cases[22]. According to the literature, the sensitivity of polymerase chain reaction for TB is 82%[23], and M. tuberculosis infection has also been rapidly identified by analyzing liver tissue by next-generation sequencing[2]. The Xpert Mtb/Rif test is a nucleic acid amplification test that provides a rapid diagnosis of the M. tuberculosis complex, and the Xpert Mtb/Rif test has been known to detect some extrapulmonary forms of TB[24].
Anti-TB therapy is the key to treating primary hepatic TB. Owing to the increasing incidence of drug-resistant TB, quadruple therapy (isoniazid, rifampicin, pyrazinamide and ethambutol) is recommended[25]. Recent publications advocate that treatment options for almost all forms of drug-sensitive TB in adults include an initial 2-mo period of isoniazid, rifampin, pyrazinamide, and ethambutol, followed by 4 mo of isoniazid and rifampin[22,26,27]. This regimen cures more than 90% of patients with TB[22]. The optimal duration of time to treat hepatic TB is controversial, but 6–12 mo seems to be effective for most patients[5]. Patients generally do not require any further surgical intervention after the completion of anti-TB therapy, but surgical treatment of hepatic TB is usually required in cases of TB-related biliary compression leading to jaundice, portal hypertension, or biliary bleeding, or when the diagnosis is uncertain[28]. In conclusion, there are insufficient controlled data to recommend the use of corticosteroids in all cases of hepatic TB, either in isolated hepatic TB or in complications of cornified/spread TB. Glucocorticoids may have a role in the treatment of hepatic TB that does not respond appropriately to standard anti-TB therapy[29].
Hepatic TB is rare and lacks typical symptoms, signs and laboratory findings. Diagnosing hepatic TB with imaging is also difficult due to its diverse and nonspecific imaging features, which can easily lead to misdiagnosis and underdiagnosis. For hepatic masses with calcified foci visible on CT, biliary obstruction with the progressive loss of bile ducts on MRCP, and mildly enhanced edges after enhan
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma V S-Editor: Chang KL L-Editor: Kerr C P-Editor: Zhang YL
| 1. | Evans RP, Mourad MM, Dvorkin L, Bramhall SR. Hepatic and Intra-abdominal Tuberculosis: 2016 Update. Curr Infect Dis Rep. 2016;18:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Ai JW, Li Y, Cheng Q, Cui P, Wu HL, Xu B, Zhang WH. Diagnosis of local hepatic tuberculosis through next-generation sequencing: Smarter, faster and better. Clin Res Hepatol Gastroenterol. 2018;42:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Li C, Liu L, Tao Y. Diagnosis and treatment of congenital tuberculosis: a systematic review of 92 cases. Orphanet J Rare Dis. 2019;14:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Al Umairi R, Al Abri A, Kamona A. Tuberculosis (TB) of the Porta Hepatis Presenting with Obstructive Jaundice Mimicking a Malignant Biliary Tumor: A Case Report and Review of the Literature. Case Rep Radiol. 2018;2018:5318197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Hickey AJ, Gounder L, Moosa MY, Drain PK. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. 2015;15:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Park JI. Primary hepatic tuberculosis mimicking intrahepatic cholangiocarcinoma: report of two cases. Ann Surg Treat Res. 2015;89:98-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Mortelé KJ, Segatto E, Ros PR. The infected liver: radiologic-pathologic correlation. Radiographics. 2004;24:937-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | HERSCH C. Tuberculosis of the liver. A study of 200 cases. S Afr Med J. 1964;38:857-863. [PubMed] |
| 9. | Ba TT, Cambier E, Tinton N, Lam HD, Navez B. Pseudo-tumoral hepatic tuberculosis: a report of two cases. Acta Chir Belg. 2010;110:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Essop AR, Posen JA, Hodkinson JH, Segal I. Tuberculosis hepatitis: a clinical review of 96 cases. Q J Med. 1984;53:465-477. [PubMed] |
| 11. | Schininà V, Albarello F, Cristofaro M, Di Stefano F, Fusco N, Cuzzi G, Arend SM, Goletti D, Busi Rizzi E. Diagnostic imaging of hepatic tuberculosis: case series. Int J Tuberc Lung Dis. 2018;22:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Yu RS, Zhang SZ, Wu JJ, Li RF. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J Gastroenterol. 2004;10:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 13. | Bächler P, Baladron MJ, Menias C, Beddings I, Loch R, Zalaquett E, Vargas M, Connolly S, Bhalla S, Huete Á. Multimodality Imaging of Liver Infections: Differential Diagnosis and Potential Pitfalls. Radiographics. 2016;36:1001-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Keri VC, Jorwal P, Kodan P, Biswas A. Tuberculosis masquerading as metastasis in liver: a rare and an unusual presentation. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Prabhudesai R, Lawande D, Gondal G, Keny S. Primary hepatic tuberculosis masquerading as intrahepatic cholangiocarcinoma. Indian J Tuberc. 2019;66:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Maguire C, Sivabalan P, Jhamb S, Palamuthusingam P. Hepatic tuberculosis masquerading as cholangiocarcinoma: an unusual differential for a liver mass. J Surg Case Rep. 2020;2020:rjaa247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Garg M, Khan Y, Pathania M. Tubercular granuloma mimicking pyogenic liver abscess. J Family Med Prim Care. 2020;9:424-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Wang WJ, Tao Z, Wu HL. Etiology and clinical manifestations of bacterial liver abscess: A study of 102 cases. Medicine (Baltimore). 2018;97:e12326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 20. | Lee NK, Kim S, Lee JW, Jeong YJ, Lee SH, Heo J, Kang DH. CT differentiation of pyogenic liver abscesses caused by Klebsiella pneumoniae vs non-Klebsiella pneumoniae. Br J Radiol. 2011;84:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Mojtahedzadeh M, Otoukesh S, Shahsafi MR, Tahbaz MO, Rahvari SK, Poorabdollah M, Sajadi MM. Case report: portal hypertension secondary to isolated liver tuberculosis. Am J Trop Med Hyg. 2012;87:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Freitas M, Magalhães J, Marinho C, Cotter J. Looking beyond appearances: when liver biopsy is the key for hepatic tuberculosis diagnosis. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Singh KK, Muralidhar M, Kumar A, Chattopadhyaya TK, Kapila K, Singh MK, Sharma SK, Jain NK, Tyagi JS. Comparison of in house polymerase chain reaction with conventional techniques for the detection of Mycobacterium tuberculosis DNA in granulomatous lymphadenopathy. J Clin Pathol. 2000;53:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Agarwala R, Dhooria S, Khaire NS, Mishra S, Verma S, Shah J, Mandavdhare HS, Kumari S, Dutta U, Sharma V. Xpert MTB/RIF for diagnosis of tubercular liver abscess. A case series. Infez Med. 2020;28:420-424. [PubMed] |
| 25. | Mert A, Ozaras R, Tabak F, Ozturk R, Bilir M. Localized hepatic tuberculosis. Eur J Intern Med. 2003;14:511-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368:745-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 559] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 27. | Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007;370:2030-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Niemiec SM, Vinetz JM, Sicklick JK. Porta Hepatis Mass. JAMA Surg. 2016;151:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Luther VP, Bookstaver PB, Ohl CA. Corticosteroids in the treatment of hepatic tuberculosis: case report and review of the literature. Scand J Infect Dis. 2010;42:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |