Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9652
Peer-review started: May 23, 2021
First decision: June 25, 2021
Revised: July 18, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 6, 2021
Processing time: 159 Days and 0.3 Hours
Primary mediastinal leiomyosarcomas are extremely rare. We report a case of leiomyosarcoma around the thoracic and abdominal aorta, mimicking an aortic hematoma, and discuss the diagnostic value of ultrasound.
A 63-year-old female was hospitalized for abdominal pain. Initial computed tomography angiography revealed an enhanced mass around the lower thoracic and upper abdominal aorta. Aortic hematoma was strongly suspected, and stents were placed by interventional surgery. About 1 mo postoperatively, the patient was re-hospitalized because of progressive abdominal pain. Ultrasound showed that the mass had a heterogeneous echo. In contrast-enhanced ultrasound, the hyperechoic regions were filled with contrast medium after the aortic region was, indicating that the blood supply was abundant but had no direct connection with the aorta. There was no obvious contrast medium-filling in the hypoechoic area. These findings were similar to those of malignant tumors with liquefaction and necrosis. Positron emission tomography/computed tomography confirmed that the mass had a high metabolic signal similar to that of a malignant tumor. Leiomyosarcoma was confirmed by postoperative pathology.
Symptoms of mediastinal leiomyosarcoma surrounding the aorta may mimic aortic hematoma. Contrast-enhanced ultrasound can provide valuable and unique diagnostic clues.
Core Tip: This study not only reports a case of leiomyosarcoma around the thoracic and abdominal aorta, mimicking an aortic hematoma, but also proposes a new diagnostic strategy. Diagnosis of leiomyosarcomas of the aorta is always challenging because of its relatively low incidence as well as its similar clinical presentation and computed tomography angiography features to an aortic hematoma. Ultrasound and contrast-enhanced ultrasound can provide valuable and unique diagnostic clues. If the acoustic characteristics are abnormal, it is recommended that tumor detection be improved. We believe this strategy can minimize the risk of missed diagnosis and additional medical costs.
- Citation: Xie XJ, Jiang TA, Zhao QY. Diagnostic value of contrast-enhanced ultrasonography in mediastinal leiomyosarcoma mimicking aortic hematoma: A case report and review of literature . World J Clin Cases 2021; 9(31): 9652-9661
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9652.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9652
Mediastinal sarcomas are rare, accounting for only 1.4% of soft tissue sarcomas. Primary mediastinal leiomyosarcomas account for approximately 9%–11% of mediastinal sarcomas and 0.15% of mediastinal tumors[1-4]. Leiomyosarcomas reportedly originate from mesenchymal tissue[5] and usually develop from the esophagus, inferior vena cava, pulmonary artery, superior vena cava, mediastinal soft tissue, and other organs such as the heart. Leiomyosarcomas that only grow around the aorta are quite rare[1,6-10]. The clinical presentation of leiomyosarcomas is often determined by their specific anatomic location. Although masses around the aorta can easily be detected by computed tomography angiography (CTA), it is difficult to clearly distinguish whether the mass is a dissecting hematoma or a tumor, and if there are no other tumor symptoms, differential diagnostic tests for tumors are rarely used for initial differential diagnosis.
We herein report a case of a mediastinal leiomyosarcoma that only grew around the thoracic abdominal aorta, with recurrent abdominal pain as the only symptom.
A 63-year-old female visited our hospital for recurrent abdominal radicular pain that had lasted for approximately 2 mo. She had no other accompanying signs or sym
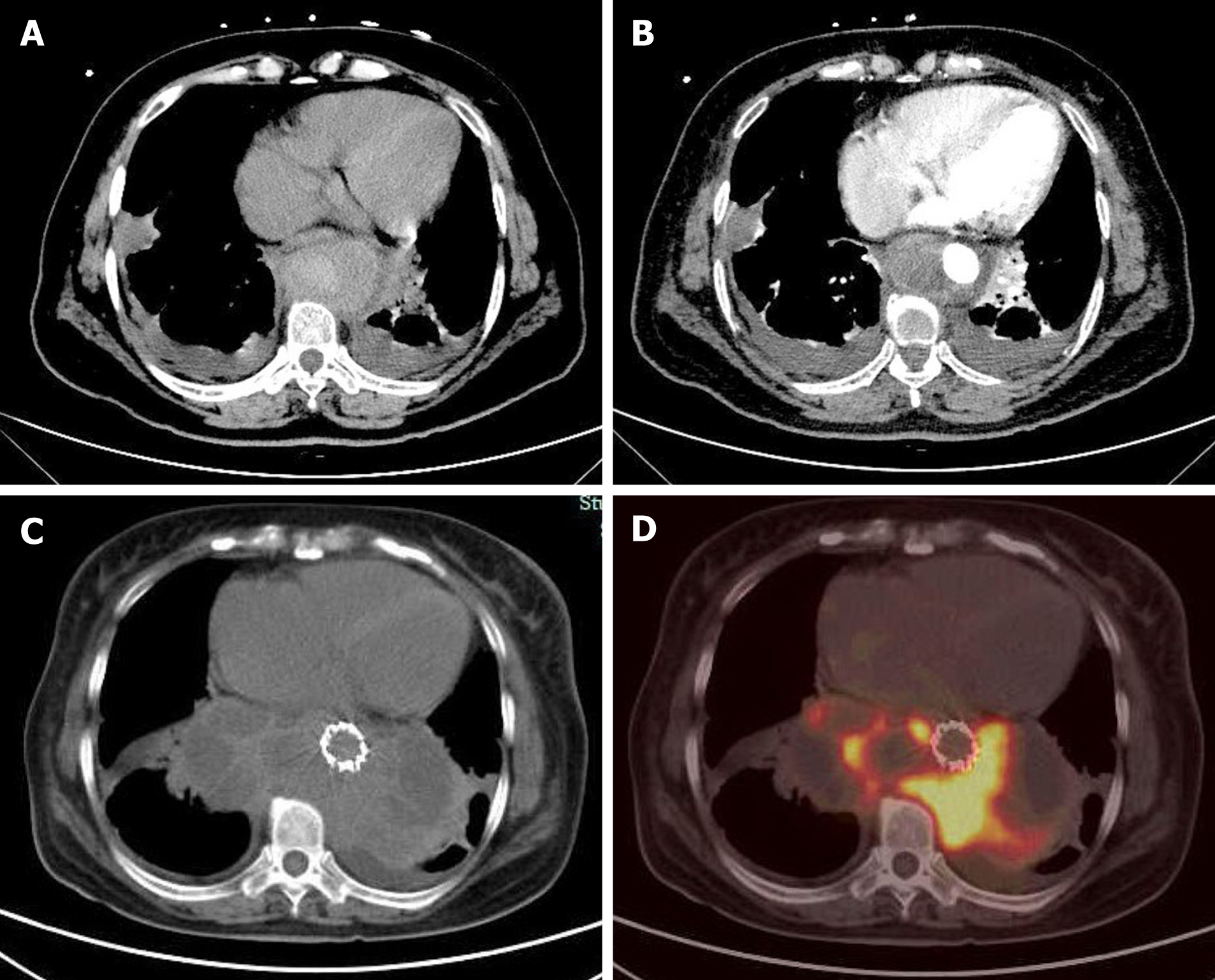
On initial CTA, a thick circular mass with patchy high density enhanced signal was found around the lower thoracic aorta and upper abdominal aorta. The mass showed heterogeneous enhancement on the contrast-enhanced CT scan (Figure 1). Based on the patient’s medical history and imaging test results, we initially thought that the patient had an aortic hematoma, and two stents (CUFF, 24 mm × 80 mm and 28 mm × 80 mm; AnkuraTM, LifeTech Scientific Co., Guangdong, China) were inserted into the aorta through endovascular interventional surgery. However, the patient’s abdominal pain was not alleviated after discharge, leading to rehospitalization approximately 1 mo later.
The patient had a history of hypertension for more than 10 years and was maintained on oral irbesartan and felodipine. She had undergone radiofrequency ablation for atrial fibrillation approximately 2 years prior, and took warfarin postoperatively. There were no other known chronic diseases.
-
There were no positive findings on physical examination, except an irregular heart rate.
Laboratory test results showed carbohydrate antigen 125 Level of 124.4 U/mL (normal range: 0-35 U/mL) and serum ferritin level of 806 ng/mL (normal range: 7-323 ng/mL). Serum levels of alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 15-3 were all in normal range. D-dimer was 522 µg/L (fibrinogen equivalent units). The results of routine blood tests, liver and kidney function tests, and routine urinalysis were all in normal range.
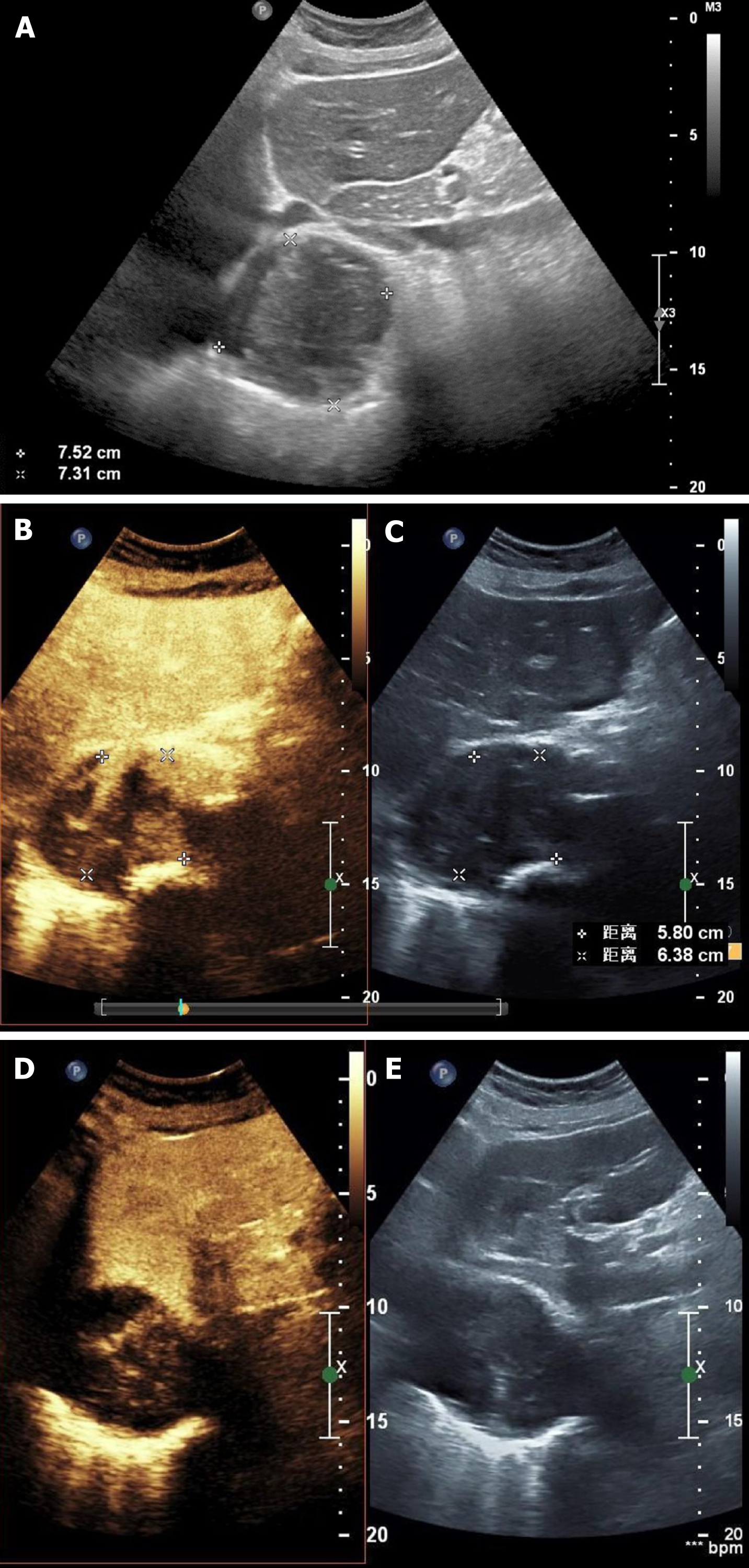
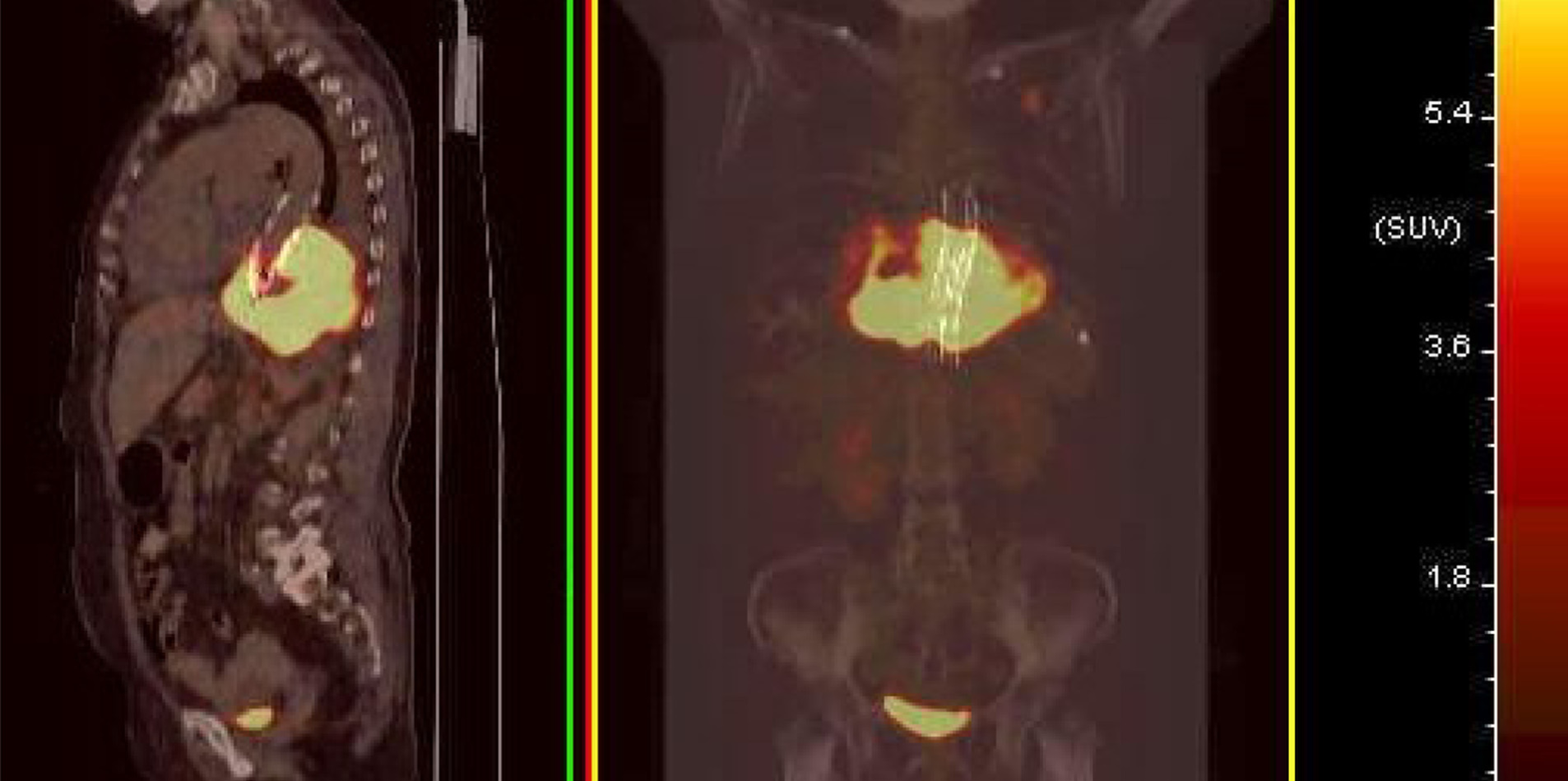
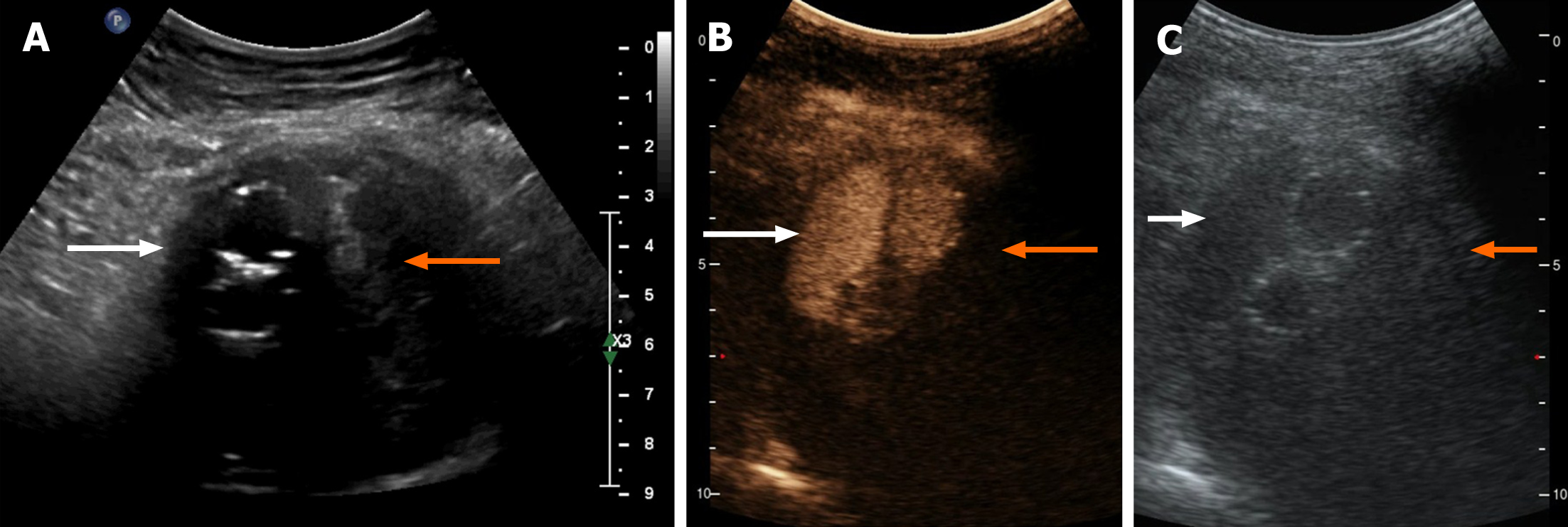
Contrast-enhanced ultrasonography (US) was initially performed at the time of second hospitalization. On two-dimensional US, a heterogeneous echogenic mass was seen around the stent and was larger than it had been 1 mo prior, as measured by CTA. According to the principles of ultrasound, solids reflect ultrasound more strongly than liquids, thus presenting a higher signal. The principle of medical ultrasound imaging is also based on this theory. This implies that hyperechoic regions are mainly composed of solid tissue, whereas hypoechoic regions have more liquid components. On contrast-enhanced US (CEUS), the ultrasound contrast agent (SonoVue®; Bracco Imaging, Milan, Italy) was injected in a bolus through the peripheral vein. Delayed contrast filling was observed in the part with the lesions, and the contrast medium was dispersed rather than forming cords or clumps. No contrast medium was found in the hypoechoic area, which differs from typical endoleaks. The contrast-enhanced images closely resembled a malignant tumor (Figure 2). Positron emission tomography (PET)/CT showed a mass surrounding the descending aorta with a heterogeneous but clear boundary. The high-density part of the mass had increased fluorodeoxyglucose (FDG) metabolism, whereas the low-density part had decreased FDG metabolism. Malignant tumors were initially considered. A nodule with high FDG metabolism was observed in the left upper lobe, and was considered as metastatic foci. No abnormal increase in FDG metabolism was observed in the rest of the body, including the brain (Figure 1 and 3).
Primary mediastinal leiomyosarcoma.
Rapid freezing pathology showed pleomorphic soft tissue tumors rich in sinusoids.
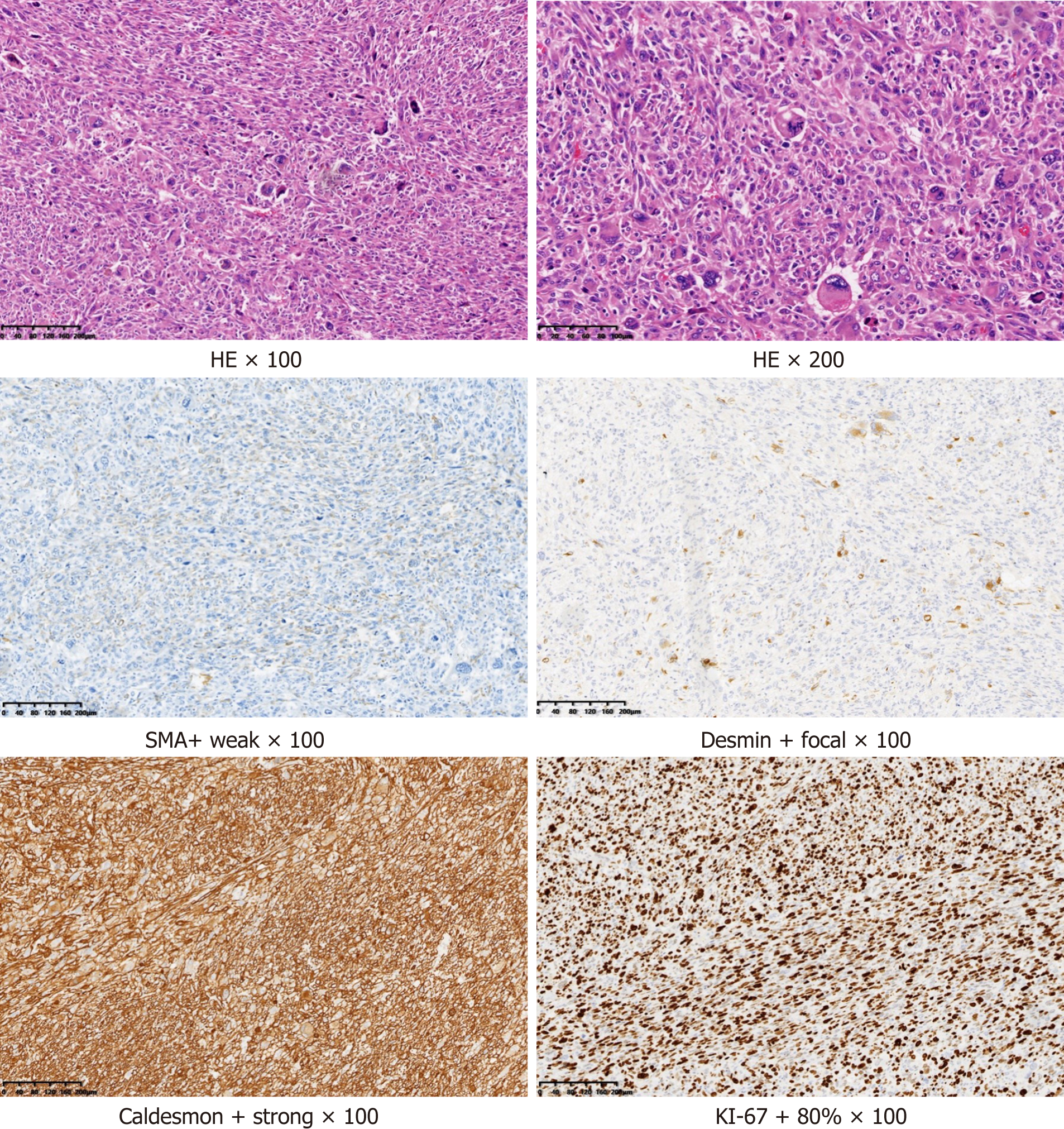
Routine pathology showed that the size of the resected tumor tissue was about 12 cm × 7 cm × 4.5 cm, with hemorrhage and necrosis. Under microscopy (hematoxylin and eosin staining), atypical, short fusiform or epithelioid cells, and multinucleated, pleomorphic giant tumor cells were seen. Mitosis was also present, which indicated rapid cell growth. Immunohistochemistry revealed that the tumor was positive for smooth muscle actin, desmin, and caldesmon, which suggested that the tumor originated from the smooth muscle. The tumor was negative for cytokeratin, epithelial membrane antigen, synaptophysin, S-100, chromogranin A, and cluster of differentiation 56, which indicated that it was not epithelial, neurogenic, neuroendocrine, or neuroectodermal in origin. Overall, these findings suggested that the tumor was a highly malignant soft tissue sarcoma, specifically a pleomorphic leiomyosarcoma (Figure 4). We determined that the primary leiomyosarcoma was located in the mediastinum because it did not adhere to the aorta and the mediastinal volume was larger than the retroperitoneum volume.
During surgery, an incision was made from the sixth intercostal space to the outer edge of the left rectus abdominis. The costal arch and diaphragm were severed after, into the thoracic cavity and retroperitoneum. The tumor grew in the mediastinum and retroperitoneum, surrounded the thoracic aorta, and invaded the left lung. The tumor and lung metastasis were all completely removed. No treatment was performed for the aorta, as it was not invaded by the tumor. No signs of aortic dilatation or endoleak were observed during the operation.
The patient did not receive chemotherapy or radiotherapy, and was discharged 10 d after the operation. There were no complications during the perioperative period. When discharged, the patient had no discomfort and could walk with assistance. The first postoperative follow-up was scheduled 1 mo later, and the follow-up treatment plan was supposed to be determined according to the test results. However, the patient did not return to the hospital for follow-up, and since hospital discharge, we have not been able to contact the patient.
Leiomyosarcoma has been reported in all mediastinal anatomic regions, including the anterior, middle and posterior mediastinum, but the most common location is the posterior mediastinum[1,10,11]. The histologic origin of primary mediastinal leiomyosarcomas is unclear. Some studies have suggested that sarcomas originate from the smooth muscle cells of small vessels in the soft mediastinal tissue, smooth muscle cells that shed from the esophageal wall during development, or heterotrophic smooth muscle cells displaced during embryonal development[12-14]. Research on retroperitoneal leiomyosarcomas has shown that leiomyosarcomas may originate from the smooth muscle remaining in the retroperitoneum, veins, or embryonic Wolffian remnants[15].
Retroperitoneal leiomyosarcomas can be divided into three categories according to the major growth patterns when diagnosed. Completely extravascular retroperitoneal leiomyosarcomas are the most common (62%), followed by extravascular and intravascular (33%) retroperitoneal leiomyosarcomas. Completely intravascular retroperitoneal leiomyosarcomas are the least common (5%)[15,16]. The lungs and liver are common distant metastatic organs[1,5,17]. It has been reported that foreign bodies such as bullets, laparotomy sponges, and bone wax may be associated with sarcoma causes, but the exact mechanisms are unclear[18,19]. Initial primary mediastinal leiomyosarcoma symptoms lack specificity and depend on the tumor location[1,2,10,20,21]. Posterior mediastinal tumors often do not cause obvious clinical symptoms and discomfort in the early stage[9].
The diagnosis of such malignancies is often delayed[21-24], because of their extremely low incidence rate, vague symptoms, similarities between periarterial leiomyosarcoma imaging and aortic hematoma imaging, endoleak after endovascular aortic aneurysm repair (EVAR), or graft infection[22,25]. CT and magnetic resonance imaging (MRI) can demonstrate anatomical relationships between masses and surrounding tissues but are not sufficient for judging histiocyte types[26,27]. Leiomyosarcomas usually present on CT as lobulated noncalcified masses, with or without internal low-density areas that appear similar to necrosis or cystic nodules. The mass does not contain any fat density, distinguishing it from liposarcomas[8]. On MRI, leiomyosarcomas often show a homogeneous intermediate signal intensity on T1-weighted imaging and obvious enhancement. These characteristics help differentiate leiomyosarcomas from thrombi, because thrombi usually have a high signal intensity on both T1 and T2 sequences, and have no enhancement after gadolinium administration[2,28]. PET/CT can provide very reliable diagnostic information[14,22,29,30]. Moreover, ultrasound- or CT-guided aortic punctures can be used for tissue biopsies. However, for reasons such as avoiding unnecessary radiation risks, economy, and safety, PET/CT or biopsy is not performed on each aortic mass as a primary test. The diagnosis is not difficult but is very challenging using safe and economical methods[22].
Additionally, CEUS can be used for the differential diagnosis of malignant tumors and has lower radiation and contrast agent risks than other diagnostic methods[29,31]. On our CEUS, the echo of the mass around the artery was heterogeneous and was comprised of liquid anechoic and solid hyperechoic areas. There was no enhancement signal of the contrast medium in the liquid anechoic area, but a high signal of the contrast medium in the solid hyperechoic area was present, opposite to that for endoleaks. In this study, the contrast medium in the solid hyperechoic region was dispersed, and the rising tides and fades were slower than those for the aorta. It was thought that the hyperechoic regions in this study were solid tissues rich in blood supply and capillaries. Accordingly, the mass was more likely a malignant tumor with liquefaction and necrosis than endoleaks.
Endoleaks are the main EVAR complications and are defined as blood in arteries flowing inside the aneurysm sac and outside the stent, leading to expansion and rupture of the aneurysm. Under ultrasound, endoleaks are composed of liquid anechoic and solid hyperechoic regions. In typical endoleak case, the anechoic regions contained flowing arterial blood, which showed enhancement upon the administration of contrast agent. In contrast, the solid tissue was either coagulated or an organized thrombus with less blood supply; thus, there was no obvious contrast agent signal in the enhancement phase (Figure 5). For Type 1, 3, and 4 endoleaks, contrast en
However, it is difficult to clearly detect all mediastinal masses using ultrasound. The mediastinal area that can be detected by ultrasound is affected by the heart, lung, sternum, ribs, and spine. There may be a blind spot in the posterior mediastinum and thoracic segment of descending aorta. The European Federation of Societies for Ultrasound in Medicine and Biology guidelines and recommendations on the clinical practice of CEUS suggest CEUS as a prior choice for detecting and characterizing endoleaks after abdominal aortic aneurysm repair and endoleak follow-up (recommendation level of A; 1A)[33]. Sonographers should pay more attention to periarterial sarcomas and to whether acoustic characteristics tend to indicate tumors during follow-up, regardless of the initial diagnosis.
The prognosis of patients with leiomyosarcoma depends on complete surgical resection of the tumor[9]. The practical treatment plan is mainly based on sarcoma invasion sites. Currently, there are no widely recognized chemotherapy and targeted treatment options. In this study, initial CTA results were not typical, and the presence of aortic calcification, hypertension, and warfarin history made the diagnosis more confusing. Aortic hematoma was considered to be the most likely diagnosis. However, the inability to directly visualize the lesion and obtain a biopsy led to a 1-mo delay in diagnosis. EVAR with stenting is currently the primary treatment standard for aortic hematoma[34]. So, the initial purpose of aortic stenting in this patient was not to treat the leiomyosarcoma. Considering the relatively higher aortic syndrome incidence and treatment urgency, stenting surgery has plausible benefits despite data that suggest difficulty in evaluating its protective effects on patients with leiomyosarcoma.
In conclusion, the incidence of mediastinal leiomyosarcomas is relatively low and clinical presentations are often associated with specific anatomic locations. When the tumor only grows around the aorta, it may have similar clinical symptoms and CTA features as an aortic hematoma, making the diagnosis challenging. Although aortic hematoma or dissection has a high incidence rate and needs immediate treatment, the possibility of the existence of malignant tumors around or in the aorta should be considered. Ultrasound and CEUS can provide valuable and unique diagnostic clues. If acoustic characteristics are abnormal, it is recommended that tumor detection be improved. We believe that this strategy can minimize the risk of missed diagnosis and medical costs.
In conclusion, the incidence of mediastinal leiomyosarcomas is relatively low, and clinical presentations are often associated with specific anatomic locations. When the tumor only grows around the aorta, it may have similar clinical symptoms and CTA features to an aortic hematoma, making the diagnosis challenging. Although aortic hematoma or dissection has a high incidence rate and more urgent treatment needs, the possibility of the existence of malignant tumors around or in the aorta should be considered. Ultrasound and CEUS can provide valuable and unique diagnostic clues. If acoustic characteristics are abnormal, it is recommended that tumor detection be improved. We believe that this strategy can minimize the risk of missed diagnosis and medical costs.
We are grateful to Dr. Jin Wang for providing the audio recording and editing the audio core tip that accompanies this paper.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Karavaş E, Marickar F, MARTINO A, Matsubara S, Ostojic A, Samara AA, Tahtabasi M S-Editor: Liu M L-Editor: A P-Editor: Zhang YL
| 1. | Kimura K, Ogawa H, Jimbo N, Hokka D, Tanaka Y, Maniwa Y. A case of leiomyosarcoma originating from a bronchogenic cyst: A case report. Mol Clin Oncol. 2020;12:244-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Xue X, Liang W, Zhang W. Anterior mediastinal leiomyosarcoma mimicking thymoma: A case report. Medicine (Baltimore). 2018;97:e11132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Burt M, Ihde JK, Hajdu SI, Smith JW, Bains MS, Downey R, Martini N, Rusch VW, Ginsberg RJ. Primary sarcomas of the mediastinum: results of therapy. J Thorac Cardiovasc Surg. 1998;115:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Hirano H, Kizaki T, Sashikata T, Maeda T, Yoshii Y. Leiomyosarcoma arising from soft tissue tumor of the mediastinum. Med Electron Microsc. 2003;36:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27:957-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Kawashima H, Ariizumi T, Ogose A, Umezu H, Okamoto T, Oike N, Endo N. Aortic mural leiomyosarcoma with spinal involvement. J Thorac Cardiovasc Surg. 2020;159:e249-e254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Masaki N, Ogasawara T, Matsuki K. Differential diagnosis and management of a mural mass in the aortic arch. J Thorac Cardiovasc Surg. 2017;153:e81-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Athanasopoulos LV, Bicknell CD, Anderson JR, Baig K, Goldin R, Hamady M, Foale R, Cheshire NJ. Leiomyosarcoma of the thoracic aorta in a patient with hypertrophic cardiomyopathy. Ann Vasc Surg. 2015;29:841.e1-841.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Moran CA, Suster S, Perino G, Kaneko M, Koss MN. Malignant smooth muscle tumors presenting as mediastinal soft tissue masses. A clinicopathologic study of 10 cases. Cancer. 1994;74:2251-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Iijima Y, Akiyama H, Nakajima Y, Kinoshita H, Hirata T. A Case of Primary Mediastinal Leiomyosarcoma in Which Long-Term Survival Was Achieved. Ann Thorac Cardiovasc Surg. 2020;26:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | den Bakker MA, Marx A, Mukai K, Ströbel P. Mesenchymal tumours of the mediastinum--part II. Virchows Arch. 2015;467:501-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Eroğlu A, Kürkçüoğlu C, Karaoğlanoğlu N, Gürsan N. Primary leiomyosarcoma of the anterior mediastinum. Eur J Cardiothorac Surg. 2002;21:943-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Labarca E, Zapico A, Ríos B, Martinez F, Santamarina M. Superior vena cava syndrome due to a leiomyosarcoma of the anterior mediastinum: A case report and literature overview. Int J Surg Case Rep. 2014;5:984-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Iwata T, Miura T, Inoue K, Hanada S, Inoue H, Miyamoto Y. Primary leiomyosarcoma of the anterior mediastinum encasing the aortic arch, left common carotid and left subclavian arteries. Ann Thorac Cardiovasc Surg. 2012;18:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Cantwell CP, Stack J. Abdominal aortic invasion by leiomyosarcoma. Abdom Imaging. 2006;31:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hartman DS, Hayes WS, Choyke PL, Tibbetts GP. From the archives of the AFIP. Leiomyosarcoma of the retroperitoneum and inferior vena cava: radiologic-pathologic correlation. Radiographics. 1992;12:1203-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 104] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Farshid G, Pradhan M, Goldblum J, Weiss SW. Leiomyosarcoma of somatic soft tissues: a tumor of vascular origin with multivariate analysis of outcome in 42 cases. Am J Surg Pathol. 2002;26:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Stewart B, Manglik N, Zhao B, Buryanek J, Khalil K, Aronson JF, Buja LM. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Alexander JJ, Moawad J, Cai D. Primary intimal sarcoma of the aorta associated with a dacron graft and resulting in arterial rupture. Vasc Endovascular Surg. 2006;40:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Wu X, Huang Y, Li Y, Wang H, Jiang L. A Case of Mediastinal Leiomyosarcoma Demonstrated on FDG PET/CT Imaging. Clin Nucl Med. 2019;44:e158-e160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Székely E, Kulka J, Miklós I, Kaliszky P. Leiomyosarcomas of great vessels. Pathol Oncol Res. 2000;6:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kamran M, Fowler KJ, Mellnick VM, Sicard GA, Narra VR. Multimodality Imaging Approach towards Primary Aortic Sarcomas Arising after Endovascular Abdominal Aortic Aneurysm Repair: Case Series Report. Cardiovasc Intervent Radiol. 2016;39:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Golli M, Said M, Gamra H, Kriaa S, Saad S, Ganouni A. Primary leiomyosarcoma of the thoracic aorta mimicking aortic dissection. Ann Saudi Med. 2000;20:265-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Yoshida M, Ando S, Naito Y, Yano H. Mediastinal leiomyosarcoma concurrent with intra-aortic thrombosis. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Sultan S, Mustafa M, Bennani F, Atteia E, Acharya Y, Hynes N. Challenges in diagnosing aortic leiomyosarcoma post endovascular repair of abdominal aortic aneurysm. J Vasc Surg Cases Innov Tech. 2020;6:666-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Vaziri M. Primary mediastinal leiomyosarcoma. Gen Thorac Cardiovasc Surg. 2012;60:522-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Cakir O, Topal U, Bayram AS, Tolunay S. Sarcomas: rare primary malignant tumors of the thorax. Diagn Interv Radiol. 2005;11:23-27. [PubMed] |
| 28. | Bendel EC, Maleszewski JJ, Araoz PA. Imaging sarcomas of the great vessels and heart. Semin Ultrasound CT MR. 2011;32:377-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Mastoraki A, Leotsakos G, Mastoraki S, Papanikolaou IS, Danias N, Smyrniotis V, Arkadopoulos N. Challenging diagnostic and therapeutic modalities for leiomyosarcoma of inferior vena cava. Int J Surg. 2015;13:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ogawa M, Hara M, Ozawa Y, Moriyama S, Yano M, Shimizu S, Shibamoto Y. Benign metastasizing leiomyoma of the lung with malignant transformation mimicking mediastinal tumor. Clin Imaging. 2011;35:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Jiang H, Wang YX, Li B, Jiang YY, Miao CL, Liao DX, Zhao RH, Luo CH. Surgical management of leiomyosarcoma of the inferior vena cava. Vascular. 2015;23:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, Fanelli F, Di Marzo L, Wlderk A, Cirelli C, Catalano C, Di Leo N, Di Segni M, Malpassini F, D'Ambrosio F. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015;84:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, Claudon M, Clevert DA, Correas JM, D'Onofrio M, Drudi FM, Eyding J, Giovannini M, Hocke M, Ignee A, Jung EM, Klauser AS, Lassau N, Leen E, Mathis G, Saftoiu A, Seidel G, Sidhu PS, ter Haar G, Timmerman D, Weskott HP. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 680] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 34. | Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, Timaran CH, Upchurch GR Jr, Veith FJ; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50:S2-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 470] [Article Influence: 29.4] [Reference Citation Analysis (0)] |