Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9645
Peer-review started: May 30, 2021
First decision: June 24, 2021
Revised: July 7, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: November 6, 2021
Processing time: 152 Days and 0.8 Hours
Tuberculous myelitis is a rare manifestation of tuberculosis (TB) that is usually caused by hematogenous spread of Mycobacterium tuberculosis (MTB). Neuro
A 56-year-old man presented with numbness and pain of both lower limbs for 2 wk and dysuria for 1 wk. Syphilis serology and cerebrospinal fluid (CSF) analysis supported the diagnosis of neurosyphilis and the patient was treated with intravenous ceftriaxone at first, but symptoms still progressed. Then, magnetic resonance images revealed multiple lesions along the cervicothoracic junction, and chest computed tomography showed a typical TB lesion. MTB DNA was detected in the CSF sample by metagenomic next-generation sequencing. Eventually the patient was diagnosed with tuberculous myelitis combined with asymptomatic neurosyphilis. Subsequently, quadruple anti-TB drug standardized therapy was empirically used and his neurological symptoms improved gradually.
Patients can have coinfection with tuberculous transverse myelitis and asymptomatic neurosyphilis. Patients with neurosyphilis should be examined for other pathogens.
Core Tip: The present case indicates that possibility of coinfection with tuberculous transverse myelitis and asymptomatic neurosyphilis. We need to identify which infection is the main cause of the disease.
- Citation: Gu LY, Tian J, Yan YP. Concurrent tuberculous transverse myelitis and asymptomatic neurosyphilis: A case report. World J Clin Cases 2021; 9(31): 9645-9651
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9645.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9645
Tuberculosis (TB) is now the leading cause of death and disability among adults worldwide[1]. TB can invariably invade any bodily system, including the central nervous system (CNS). The manifestation of CNS TB includes meningitis, tuberculoma, abscesses, pachymeningitis, calvarial TB, and tuberculous myelitis[2]. TB involvement of the spinal cord is usually due to hematogenous spread[3] and compression via spinal TB[4]. The most common clinical symptoms reported are bladder and bowel symptoms (90%), fever (70%) and paraplegia (60%). On magnetic resonance imaging (MRI), the involvement of the cervical/thoracic segment of the spinal cord was most commonly observed (90%). The most consistent finding was hyperintense signals on T2 and iso- or hypointense signals on T1-weighted images, and in some cases, it even presented as longitudinally extensive transverse myelitis[5,6]. Cerebrospinal fluid (CSF) findings are also of note as most patients have increased white blood cell (WBC) count and protein levels, while their glucose levels are either normal or low[7]. In recent years, the development of metagenomic next-generation sequencing (mNGS) has been valuable as it can provide detailed sequencing of the total DNA content of a microorganism, including Mycobacterium tuberculosis (MTB)[8]. This tool has emerged as a sensitive technology capable of detecting pathological organisms. Several studies have proven that CSF mNGS confers high sensitivity, specificity, and positive predictive values in the diagnosis of CNS TB[9,10].
Syphilis is a sexually transmitted disease caused by Treponema pallidum, which can invade the brain and spinal cord, resulting in neurosyphilis[11]. The diagnosis of symptomatic neurosyphilis requires meeting clinical, serological and CSF criteria, while asymptomatic neurosyphilis relies on serological and CSF criteria alone[12]. The clinical manifestation of neurosyphilis include meningitis, dementia, stroke, tabes dorsalis and syphilitic myelitis[13,14]. In general, syphilitic myelitis is a rare manifestation of syphilis and a rare cause of myelopathic syndromes. According to previous cases, the typical MRI appearance of syphilitic myelitis is abnormal enhancement in the superficial parts of the spinal cord parenchyma (candle guttering appearance) and reversed signal intensities on T2-weighted images and gadolinium-enhanced T1-weighted images (flip-flop sign)[15-17]. It is now widely accepted that neurosyphilis can coexist with many diseases, including human immunodeficiency virus (HIV) infection, cryptococcal meningitis, tuberculous meningitis, neuromyelitis optica (NMO), and N-methyl-D-aspartate-receptor encephalitis[18-21].
Here, we report a case in which neurosyphilis coexisted with tuberculous myelitis. The specific mechanism might be that T. pallidum destroys the blood–brain barrier and the patient is more likely to have other CNS infections[18]. Additionally, the potential association between neurosyphilis and tuberculous myelitis will be discussed in detail.
A 56-year-old man presented with numbness and pain in both lower limbs for 2 wk and dysuria for 1 wk.
Two weeks previously, the patient developed numbness and pain in both lower limbs with no obvious origin and dysuria for 1 wk. Gradually, the symptoms of both lower limbs extended upward to the root of the thigh and the hips. There was no fecal incontinence at that time. The patient was referred to a local hospital, where he tested positive for serum syphilis antibody, but enhanced lumbosacral magnetic resonance imaging (MRI) as well as brain MRI showed no obvious abnormalities. The patient was first considered to have neurosyphilis and received 2 d of anti-syphilis therapy (ceftriaxone 2 g b.i.d and dexamethasone 5 mg b.i.d, combined with long-acting penicillin 2.4 MU, intramuscular injection). However, the patient’s symptoms did not improve; thus, he came to our hospital to seek further diagnosis and treatment.
The patient previously had TB, chest computed tomography (CT) found similar lesions 7 years ago, but the patient had no symptoms and no standardized treatment was added.
The patient had no relevant personal history and denied a family history of TB or myelitis.
The muscle strength of the patient’s bilateral lower limbs was graded as level 3 using the muscle strength grading scale (maximum score 5). The examination also revealed impaired pinprick sensation (bilateral) below the T10 dermatomes and hyporeflexia in both legs. The patient had a neurogenic bladder, and his temperature ranged between 37.5 and 38.8C since the disease onset. Other physical examination showed no abnormality.
The patient’s results indicated positive serum syphilis and tuberculous serology [Toluidine red unheated serum test (TRUST) 1:8 and Treponema pallidum particle agglutination assay (TPPA)+, Mycobacterium tuberculosis specific T lymphocyte (T-SPOT)+, respectively]. The rapid HIV test and other blood tests revealed no obvious abnormalities. Lumbar puncture was performed, and the pressure was 185 mmH2O (June 4, 2020). CSF analysis revealed high WBC count (310 × 106/L) with lymphocytic predominance (90%), high protein level (159.2 mg/dL; normal range, 8–43 mg/dL), low glucose (0.99 mmol/L, normal range 2.2–3.9 mmol/L), and low chloride levels (118.3 mmol/L; normal range, 120–130 mmol/L). CSF TRUST and TPPA were also reactive with a titer of 1:1 for the TRUST. Subsequently, tuberculous-infected T cells indicated a positive T-SPOT result. For making a definitive diagnosis, mNGS was conducted and MTB DNA was detected in the CSF sample. Routine urine/fecal test and urinary ultrasound were normal.
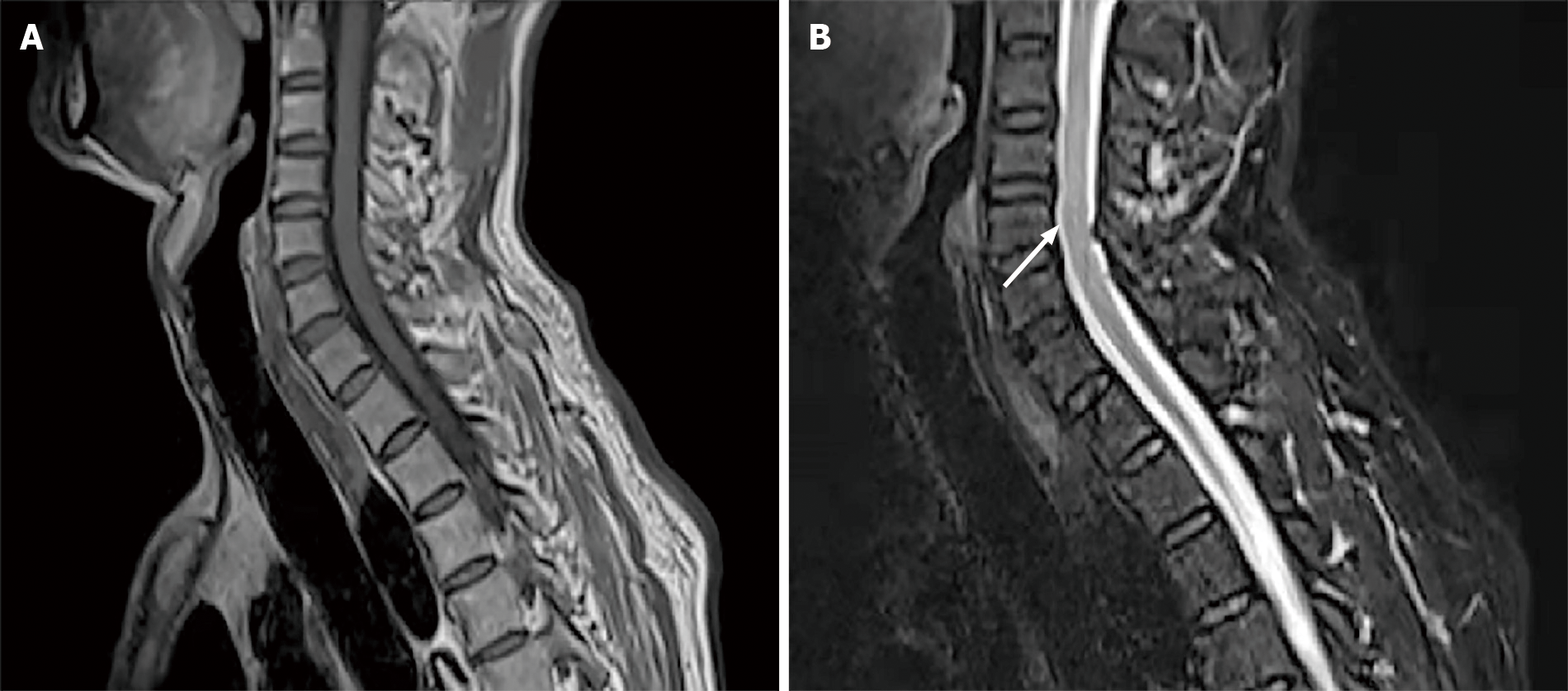
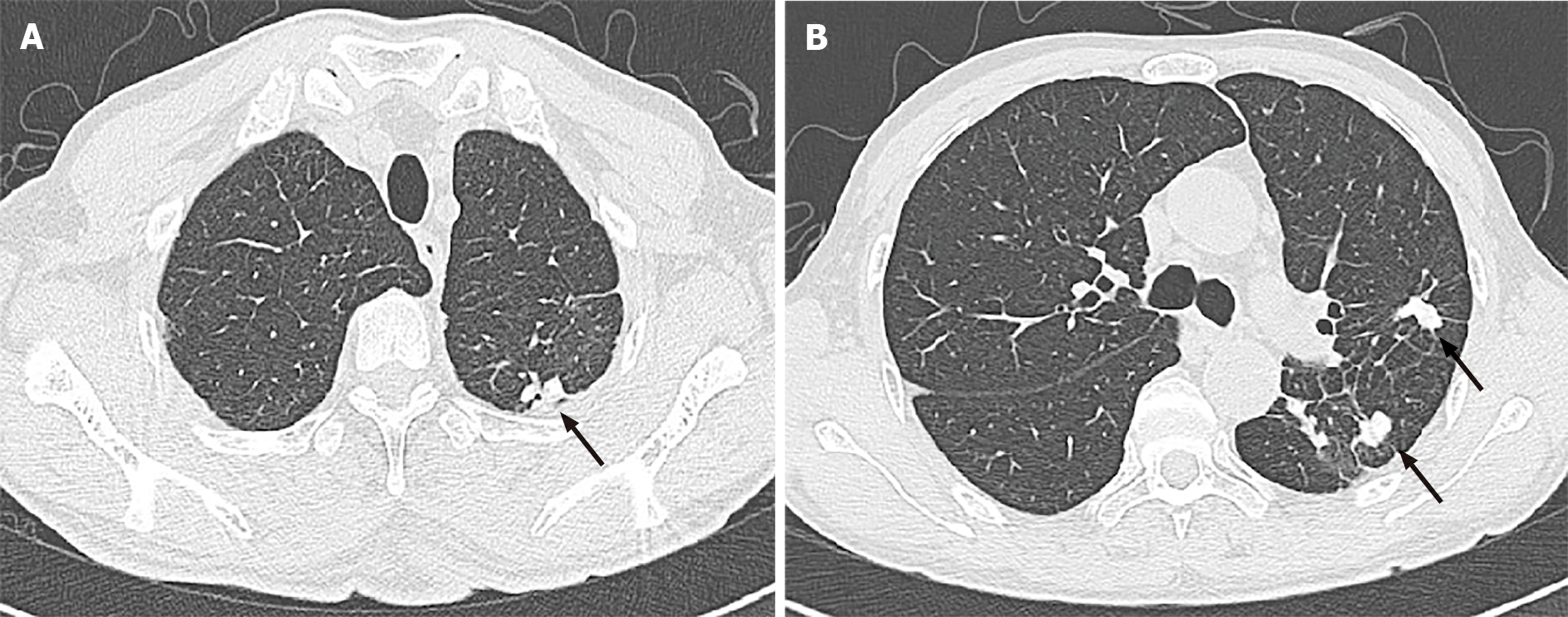
MRI of the thoracic cord showed multiple lesions along the cervicothoracic junction on T2-weighted imaging (Figure 1A, B). Chest CT also showed patchy cord shadows in the apical posterior segment of the left upper lobe and the dorsal segment of the lower lobe (Figure 2A, B). Re-examination of brain MRI did not reveal any abnormalities.
The patient was diagnosed with tuberculous transverse myelitis combined with asymptomatic neurosyphilis and previous pulmonary TB.
Anti-syphilis therapy (ceftriaxone 3 g q.d.) was added at first because the patient was initially diagnosed with neurosyphilis, but the patient complained about progression of symptoms as fecal incontinence appeared. Numbness and pain in both lower limbs was still present, and dysuria and fever did not improve as well. After 1 wk, the results of MRI, mNGS and CSF successively came out. Eventually, tuberculous myelitis was considered and quadruple anti-TB drug therapy was initiated empirically at the first 2 mo (isoniazid 600 mg, pyrazinamide 20 mg/kg, rifampin 600 mg, and ethambutol 15 mg/kg daily), then duplex anti-TB treatment (isoniazid 300 mg and pyrazinamide 500 mg t.i.d.) was used from last September as the symptoms gradually improved.
Some of the clinical symptoms gradually improved, including temperature returning to normal, significant relief of pain in the lower limbs and recovery of muscle strength (grade 4) after 4 mo of anti-TB treatment. However, dysuria, fecal incontinence and numbness of lower limbs were not significantly improved. The CSF results at that time (October 30, 2020) were better than before: CSF pressure, 160 mmH2O; protein, 72.1 mg/dL; glucose, 2.41 mmol/L; leukocyte count, 6 cells/mm3; TPPA+ and TRUST. During 1-year follow-up, the patient still had dysuria, fecal incontinence and numbness below the waist. The latest results of CSF analysis (March 26, 2021) indicated: protein, 59.8 mg/dL; glucose, 2.04 mmol/L; leukocyte count, 4.0/L, suggesting the disease still fluctuated and was not completely cured.
Here, we describe a rare case of concurrent transverse tuberculous myelitis and asymptomatic neurosyphilis. To the best of our knowledge, this is the first case report about the coexistence of these two diseases. Based on the positive serum (TRUST and TPPA) and CSF results, the patient was definitively diagnosed with neurosyphilis at first, which was caused by infection with T. pallidum. Neurosyphilis can also stimulate immune response and delayed-type hypersensitivity participates in the immunopathology of syphilis[22]. In an animal model, opsonization, activated macrophages and pathogen-specific IgG were involved in the immune process of infection with T. pallidum[12]. So, we hypothesized that the state of neurosyphilis was not the same as the immunocompromised state of HIV patients. Additionally, T. pallidum can cause chronic inflammation and invade the CNS via the blood, leading to destruction of the blood–CSF barrier, disintegration of myelin, and loss of nerve fibers[18]. The most common forms of neurosyphilis involving the spinal cord are tabes dorsalis and syphilitic myelitis[14]. However, according to the CSF analysis and MRI, neither tabes dorsalis nor syphilitic myelitis could be diagnosed. The CSF analysis showed increased protein levels, low glucose levels, low chloride levels, and significant lymphocytosis, which supports the diagnosis of tuberculous myelitis. This was confirmed by the detection of MTB DNA in the CSF. The multiple lesions along the cervicothoracic junction on T2-weighted imaging are characteristic manifestations of tuberculous myelitis[6]. Both detection of MTB DNA in the CSF and the observed clinical improvement after treatment with anti-TB drugs confirmed the diagnosis. So, we believe that the patient presented with asymptomatic neurosyphilis.
It has been reported that CNS TB comprises 1% of all TB infections, with 95% of these in the form of TB meningitis and half of them involving the spine[23,24]. Tuberculous myelitis is usually due to hematogenous spread or compression via spinal TB[3]. In our case, the patient’s chest CT showed typical pulmonary TB lesions and he did not receive treatment before. Thus, we hypothesized that the patient was in a possible hypersensitivity state after infection with syphilis, leading to generation of chronic inflammation and destruction of the blood–CSF barrier. Thus, activated MTB is more likely to invade the spinal cord or meninges in patients with neurosyphilis. It is widely accepted that TB can coexist with other immune or infectious diseases such as HIV infection, neurosyphilis, cryptococcal meningitis, and NMO[19-21]. Gonzales Zamora et al[21] have reported a case of neurosyphilis with concomitant cryptococcal and tuberculous meningitis in a patient with AIDS. Zayet et al[19] also found a case of concurrent Devic’s neuromyelitis optica associated with active pulmonary TB. However, there are no reports of tuberculous myelitis in patients with neurosyphilis.
Tuberculous myelitis is predominantly a disease of the thoracic spinal cord. Most spinal cord lesions appear as hyperintense signals on T2 and iso- or hypointense signals on T1-weighted images; the cervical/thoracic segment of the spinal cord was the most commonly observed (90%)[6]. In most patients, tuberculous myelitis affects more than one spinal segment (> 80%)[25]. The typical neuroimaging characteristics of syphilitic myelitis include “candle guttering appearance” and “flip-flop sign”[14-16]. The spinal cord MRI alterations in our patient showed multiple lesions along the cervicothoracic junction on T2-weighted imaging, which is more consistent with tuberculous myelitis lesions. CSF analysis is also the preferred method for diagnosis. As TB is a bacterial infection, increased proteins (> 100 mg/dL), moderate decrease in glucose concentration (< 40 mg/dL), moderate increase in lactate concentration, and increased adenosine deaminase (> 6.0) can be observed in most cases. CSF mNGS has high sensitivity (84.44%), specificity (100%), and positive predictive value (46.15%) in the diagnosis of CNS TB[9]. The CSF results of the current patient are consistent with the above characteristics, and MTB DNA was detected in the CSF sample by mNGS, which supports the diagnosis of tuberculous myelitis.
TB and syphilis are common in the clinical setting; however, the involvement of CNS is rare. T. pallidum can invade the CNS via the blood and cause chronic inflammation, leading to the destruction of the blood–brain barrier[18]. The breakdown of the blood–brain barrier makes it easier for other pathogens, such as MTB and Cryptococcus, to enter the CNS. Therefore, we speculate that the specific cause might be the direct destruction of the blood–brain barrier by T. pallidum, which make the patient susceptible to tuberculous myelitis.
This article describes a rare case of concurrent tuberculous transverse myelitis and asymptomatic neurosyphilis, indicating the possibility of coinfection with the two diseases. Neurosyphilis can sometimes present as asymptomatic. So, in future studies, patients with neurosyphilis should be examined for other pathogens, like MTB.
We are grateful to the patient for his contributions to the study.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious Diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andrianto A, Wani I S-Editor: Wang JL L-Editor: Kerr C P-Editor: Guo X
| 1. | Nathavitharana RR, Friedland JS. A tale of two global emergencies: tuberculosis control efforts can learn from the Ebola outbreak. Eur Respir J. 2015;46:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Bernaerts A, Vanhoenacker FM, Parizel PM, Van Goethem JW, Van Altena R, Laridon A, De Roeck J, Coeman V, De Schepper AM. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol. 2003;13:1876-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Almeida A. Tuberculosis of the spine and spinal cord. Eur J Radiol. 2005;55:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 4. | Rasouli MR, Mirkoohi M, Vaccaro AR, Yarandi KK, Rahimi-Movaghar V. Spinal tuberculosis: diagnosis and management. Asian Spine J. 2012;6:294-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Md Noh MSF, Bahari N, Abdul Rashid AM. Tuberculous Myelopathy Associated with Longitudinally Extensive Lesion: A Clinicoradiological Review of Reported Cases. J Clin Neurol. 2020;16:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Wasay M, Arif H, Khealani B, Ahsan H. Neuroimaging of tuberculous myelitis: analysis of ten cases and review of literature. J Neuroimaging. 2006;16:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Machado Ldos R, Livramento JA, Vianna LS. Cerebrospinal fluid analysis in infectious diseases of the nervous system: when to ask, what to ask, what to expect. Arq Neuropsiquiatr. 2013;71:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, Yao Y, Su Y, Huang Y, Wang M, Li B, Li H, Zhou C, Li C, Ye M, Xu X, Li Y, Hu B. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin Infect Dis. 2018;67:S231-S240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 594] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 9. | Yan L, Sun W, Lu Z, Fan L. Metagenomic Next-Generation Sequencing (mNGS) in cerebrospinal fluid for rapid diagnosis of Tuberculosis meningitis in HIV-negative population. Int J Infect Dis. 2020;96:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Wang S, Chen Y, Wang D, Wu Y, Zhao D, Zhang J, Xie H, Gong Y, Sun R, Nie X, Jiang H, Li W, Liu G, Li X, Huang K, Huang Y, Li Y, Guan H, Pan S, Hu Y. The Feasibility of Metagenomic Next-Generation Sequencing to Identify Pathogens Causing Tuberculous Meningitis in Cerebrospinal Fluid. Front Microbiol. 2019;10:1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Wormser GP, Pavia CS. Neurosyphilis. N Engl J Med. 2019;381:2376-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Gonzalez H, Koralnik IJ, Marra CM. Neurosyphilis. Semin Neurol. 2019;39:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Hook EW 3rd. Syphilis. Lancet. 2017;389:1550-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Tsui EY, Ng SH, Chow L, Lai KF, Fong D, Chan JH. Syphilitic myelitis with diffuse spinal cord abnormality on MR imaging. Eur Radiol. 2002;12:2973-2976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | He D, Jiang B. Syphilitic myelitis: magnetic resonance imaging features. Neurol India. 2014;62:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kikuchi S, Shinpo K, Niino M, Tashiro K. Subacute syphilitic meningomyelitis with characteristic spinal MRI findings. J Neurol. 2003;250:106-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Yang LG, Tucker JD, Yang B, Shen SY, Sun XF, Chen YF, Chen XS. Primary syphilis cases in Guangdong Province 1995-2008: opportunities for linking syphilis control and regional development. BMC Public Health. 2010;10:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Chen HQ, Zhang Y, Wang SB, Song YN, Bai MS, Liu KD, Zhu MQ. Concurrent aquaporin-4-positive NMOSD and neurosyphilis: A case report. Mult Scler Relat Disord. 2019;34:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Zayet S, Zaghdoudi A, Harrabi H, Goubantini A, Tiouiri Benaissa H. Devic's neuromyelitis optica associated with active pulmonary tuberculosis, Tunisia. New Microbes New Infect. 2021;39:100828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Marks M, Jarvis JN, Howlett W, Mabey DCW. Neurosyphilis in Africa: A systematic review. PLoS Negl Trop Dis. 2017;11:e0005880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Gonzales Zamora JA, Espinoza LA, Nwanyanwu RN. Neurosyphilis with Concomitant Cryptococcal and Tuberculous Meningitis in a Patient with AIDS: Report of a Unique Case. Case Rep Infect Dis. 2017;2017:4103858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Carlson JA, Dabiri G, Cribier B, Sell S. The immunopathobiology of syphilis: the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am J Dermatopathol. 2011;33:433-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Alkan G, Emiroğlu M, Kartal A, Peru H, Koplay M. Occult Disseminated Tuberculosis with Holocord Longitudinally Extensive Transverse Myelitis: A Rare Phenomenon in a Child. J Pediatr Neurosci. 2017;12:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Sahu SK, Giri S, Gupta N. Longitudinal extensive transverse myelitis due to tuberculosis: a report of four cases. J Postgrad Med. 2014;60:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Garg RK, Malhotra HS, Gupta R. Spinal cord involvement in tuberculous meningitis. Spinal Cord. 2015;53:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |