Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9598
Peer-review started: April 18, 2021
First decision: May 24, 2021
Revised: June 6, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: November 6, 2021
Processing time: 194 Days and 7.3 Hours
Spinal extradural arachnoid cysts (SEACs) are a rare cause of spinal cord compression. Typically, these cysts communicate with the intradural subara
A 79-year-old female presented with pain related to bi-segmental SEACs at the T11-L1 segments. She underwent sequential transforaminal percutaneous endoscopic thoracic cystectomy of the SEACs. Following her first procedure, spinal magnetic resonance imaging demonstrated complete excision of the cyst at the T12-L1 segment. However, the cyst at the T11-T12 segment was still present. Thus, a second procedure was performed to remove this lesion. The patient’s right-sided lumbar and abdominal pain improved significantly postoperatively. Her Japanese Orthopaedic Association score increased from 11 to 25, her visual analogue scale score was reduced from 8 to 1. The physical and mental component summary of the 36-item short-form health survey (SF-36) were 15.5 and 34.375 preoperatively, and had increased to 79.75 and 77.275 at the last follow-up visit, respectively.
Bi-segmental non-communicating SEACs are extremely rare. Endoscopic surgery is a safe, effective, and reliable method for treating these cysts. In the event of bi-segmental SEACs, it is important to identify whether both cysts are commu
Core Tip: Spinal extradural arachnoid cysts (SEACs) are a rare cause of spinal cord compression. Typically, these cysts communicate with the intradural subarachnoid space through a small defect in the dural sac. To date, few articles have reported SEACs that are not in direct communication with the subarachnoid space. For symptomatic SEACs, the standard treatment is to remove the cyst in total with a (hemi)laminectomy or laminoplasty. We present a rare case of bi-segmental non-communicating SEACs and describe our experience of using an endoscopic minimal access technique to treat them.
- Citation: Yun ZH, Zhang J, Wu JP, Yu T, Liu QY. Transforaminal endoscopic excision of bi-segmental non-communicating spinal extradural arachnoid cysts: A case report and literature review. World J Clin Cases 2021; 9(31): 9598-9606
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9598.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9598
Spinal extradural arachnoid cysts (SEACs) are a rare cause of spinal cord compression[1,2]. A mass effect is created by a collection of cerebrospinal fluid (CSF) that originates through a small defect in the dural sac[3,4]. Variation in nomenclature for this pathology including “meningoceles”, “arachnoid cysts”, and “CSF diverticula pseudomeningocele” has led to significant confusion in their classification[5]. It has been estimated by Klekamp et al[6] and Tokmak et al[7] that these cysts account for as few as 1% of all primary spinal mass lesions. These lesions can extend as a single cyst across several spinal segments, or they may occur in the form of multiple cysts with each accompanied by a corresponding dural defect[4,6-9]. In 1988, Nabors et al[10] proposed a classification of arachnoid cysts comprising three categories: SEACs without spinal nerve root fiber involvement (Type I); Type I are further divided into two subtypes, extradural arachnoid cysts (Type Ia) and sacral meningoceles (Type Ib); SEACs with spinal nerve root fiber involvement (Type II); and spinal intradural arachnoid cysts (Type III). Despite multiple efforts to better define these groups, there remains a lack of consensus about how they should be classified[11,12]. SEACs in the spinal canal are usually located on the lateral or posterior side of the dural sac[6,8]. A majority of SEAC cases reported in the literature involve one segment, with very few reporting disease across multiple segments[4,6-9,13-18]. In this case report, we present a rare case of bi-segmental SEACs, and introduce an important practice consideration in foraminal endoscopic surgery.
A 79-year-old female patient presented with severe back and right-sided abdominal pain of one year duration.
The patient presented with severe lumbar and right-sided abdominal pain of one year duration, which had become increasingly severe over the preceding week. The patient did not complain of myelopathic symptoms such as heaviness or stiffness. She had no history of trauma.
The patient’s past medical history included hypertension, type 2 diabetes mellitus, and coronary heart disease. She had previously presented to two other hospitals with similar symptoms, but they advised her that she was too high risk for open surgery.
No relevant personal and family history.
Physical examination revealed lower back tenderness and percussion pain, which radiated to the intercostal region. Superficial sensation across the T12 dermatome on the right-side of the abdomen was decreased and strength in the lower extremities muscle groups was grade four. The right knee-tendon reflex and Achilles-tendon reflex could not be elicited bilaterally, but both the planter reflex and ankle clonus were negative. The patient had no abnormality in muscle tone. There is no urinary or faecal abnormalities. At baseline, the Japanese Orthopaedic Association (JOA) and visual analogue scale (VAS) scores were 11 and 8 points, respectively. The physical and mental component summary of the 36-item short-form health survey (SF-36) were 15.5 and 34.375, respectively.
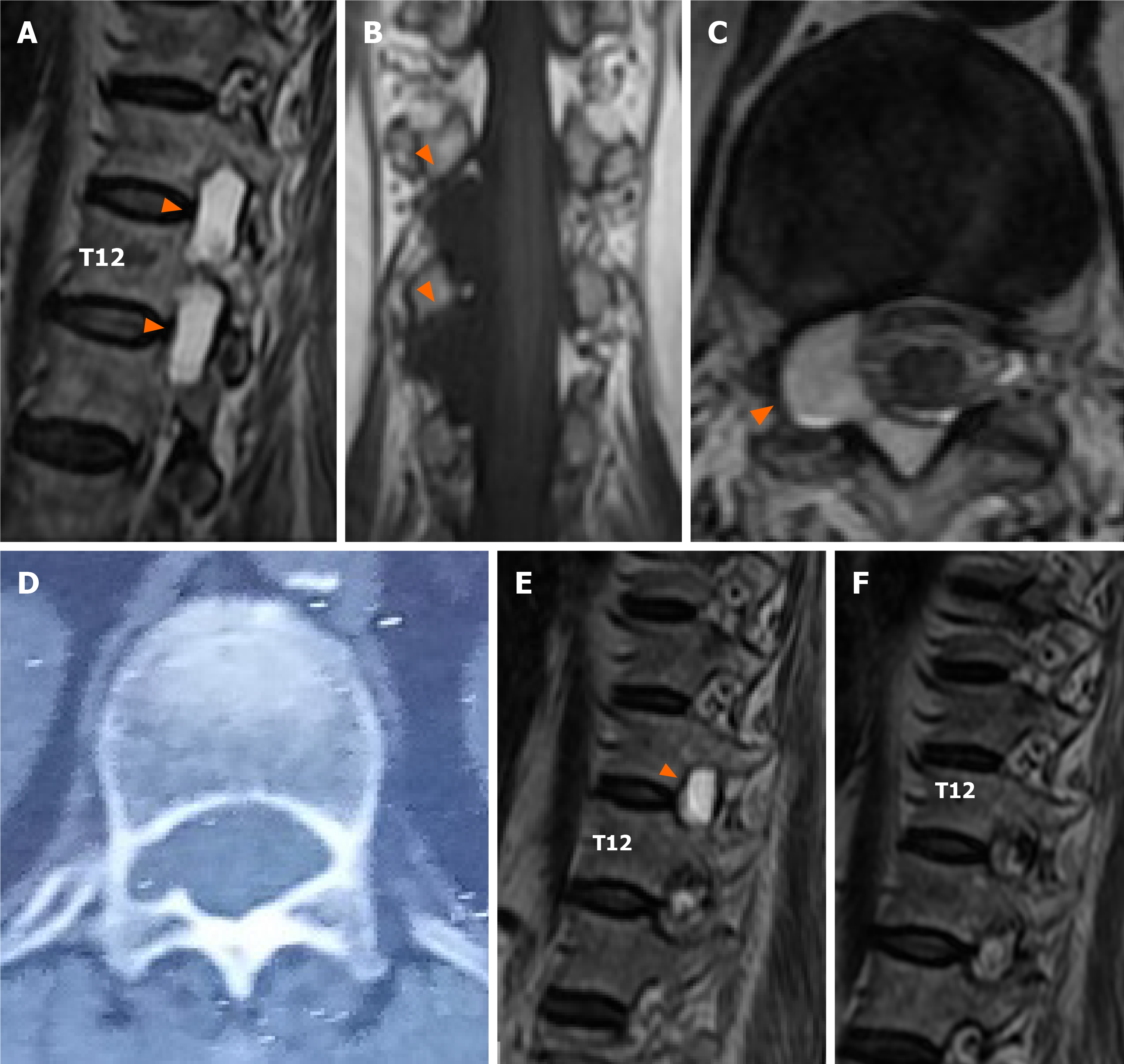
Magnetic resonance imaging (MRI) of the whole spine revealed cystic lesions located adjacent to the nerve roots at the T11-L1 level. The lesion showed a low-intensity signal on T1WI and a high-intensity signal on T2WI. The cystic lesion had caused spinal nerve root compression and foraminal enlargement, without compression of the conus medullaris (Figure 1A-C). MRI with gadolinium (Gd) contrast demonstrated no enhancement of the cysts. No apparent communications between the cyst and the subarachnoid space were detected on MRI. Computed tomography (CT) scan of the thorax, abdomen and pelvis revealed bony erosion, foraminal enlargement, and enlargement of the spinal canal at the level of the cystic lesions (Figure 1D).
Clinical diagnosis was SEACs.
The patient underwent surgical excision of the lesion using a minimally invasive endoscopic technique. We were unable to determine whether the cysts were connected on preoperative MRI. It was our expectation that after removing the cyst at T12-L1, the adjacent cyst at T11-T12 would collapse. If a dural tear were to be found during the operation, we planned to close this with a dural patch and gelfoam without thoracic drainage.
The operation performed was similar to a percutaneous endoscopic lumbar discectomy. Under endoscopic vision, the T12-L1 cyst was observed directly by manipulating the nucleus pulposus using specialised forceps. The nerve root was carefully protected, and the extradural cyst was excised using nucleus pulposus forceps piece by piece. A radiofrequency probe was used to ensure hemostasis in the spinal canal. Under direct vision, we observed that the surface of the dural sac was intact, with no visible defects. The autonomic beat of the nerve root was observed, suggesting intact function. After confirming full decompression of the nerve root and hemostasis, the wound was sutured without any drainage.
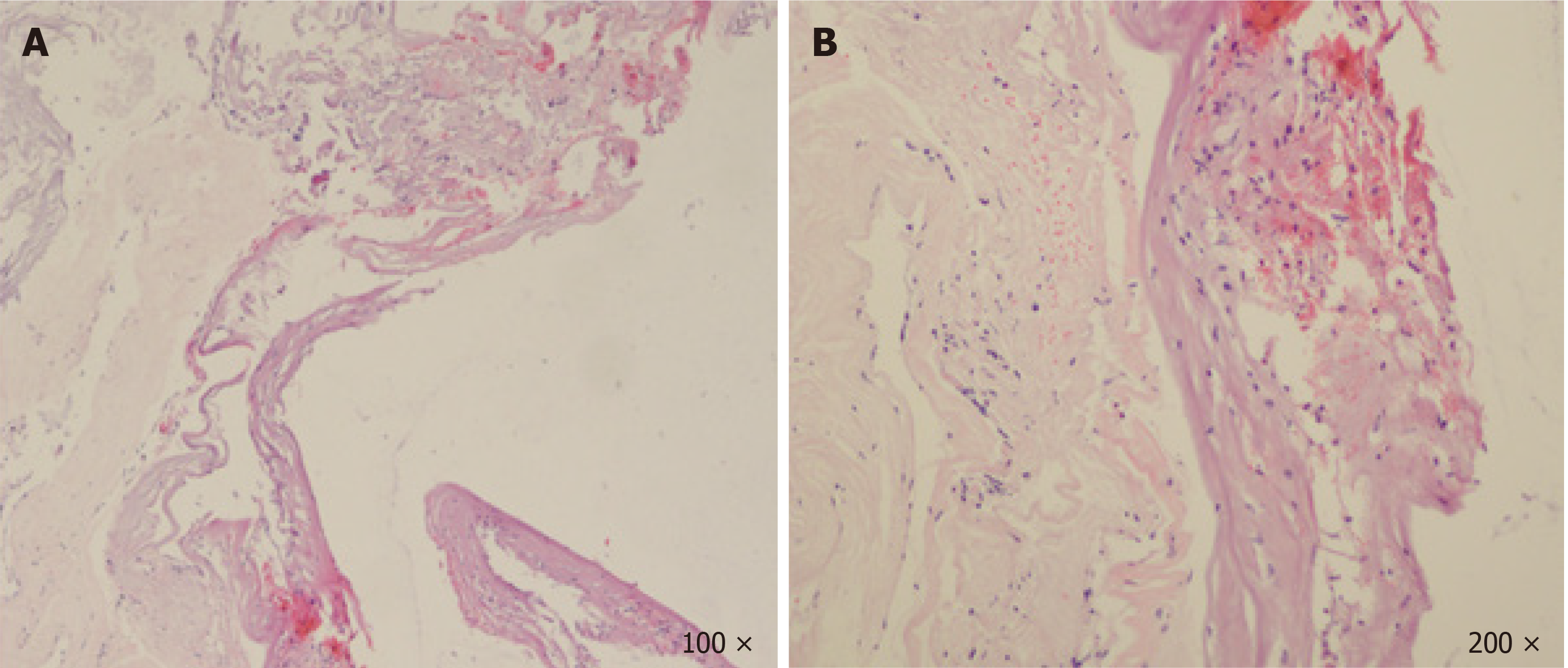
Postoperative MRI demonstrated that the cyst at T12-L1 had been excised in total, but a second cyst at T11-T12 still remained in situ (Figure 1E). A biopsy from the wall of the cyst demonstrated fibrous tissue without evidence of arachnoid features (Figure 2A). The cyst was filled with a blood clot, without any epithelial or stromal components (Figure 2B). No disc materials, nerve tissues, or tumor cells were found in any of the histopathological specimens.
The patient's symptoms recurred on the third postoperative day, and she responded to nerve root block therapy for just one day. Therefore, a decision was made to return for a second transforaminal percutaneous endoscopic cystectomy. The same procedure was carried out using the endoscopic system; however, this time at the level of the T11-T12 disk space instead of T12-L1.
Postoperatively, the patient's right-sided back and abdominal pain had largely resolved. The VAS and JOA scores were improved to 1 and 25 points, respectively. We encouraged the patient to do lower extremity muscle strengthening exercises to prevent muscle atrophy, and allowed the patient to walk from the third day onwards. The physical and mental component summary of SF-36 had increased to 79.75 and 77.275 by the time of the first postoperative follow-up visit. The patient remained asymptomatic during the following two years, and no recurrence was found on MRI (Figure 1F).
PubMed and Web of Science were used to search for articles published before April 2020. The keywords and MeSH terms for retrieval were: “spinal extradural meningeal cysts”, “SEMC”, “spinal extradural arachnoid cysts”, “SEAC*”, “spinal extradural cysts”, ”arachnoid cysts”, ”spinal canal”, “extradural arachnoid cyst”. The language of the search study was restricted to English.
Various cystic lesions can be found in the spinal canal, including intramedullary, intradural, epidural, perineural, synovial, and intervertebral disc cysts[12]. As the reliability of MRI has developed, SEACs can now be easily distinguished from other spinal cysts[1,19]. Characteristically their composition may include fibrous connective tissue and inner single-cell arachnoid lining (although this lining is sometimes not present upon histopathological examination)[4,6,20,21]. Most SEACs reported in the literature affect just one segment[4,6-9,13-18]. In this case report we diagnosed and treated a rare presentation of simultaneous bi-segmental SEACs.
The pathogenesis of SEACs remains unclear. Authors have described links to congenital abnormalities or acquired, degenerative changes secondary to trauma; however, the majority seem to be idiopathic[3,12]. Ogura et al[22] reported that the transcription pathway mediated by HOXD4 and FOXC2 may play an important role in the developing dura mater, and therefore could have a role in the pathoaetiology of SEACs. Trauma and local mechanical stress, infection, or degenerative changes may all cause acquired dural defects[1,4,8,23]. These defects may allow the arachnoid and its closed subarachnoid space to protrude through the dura, where SEACs are formed[3]. In this case, the patient had no history of trauma and was an older adult. As such, we suspected that the cyst was idiopathic.
The mechanism of cyst enlargement also remains unclear. Some mainstream hypotheses include a one-way valve system, hyperosmolar fluid concentration within the cyst, or secretion of fluid from the cyst lining[3,24,25]. Rohrer et al[24] reported that a one-way valve can be caused by the meninges folding at the ostium of the cyst. However, in a series by Morizane et al[20], in 7 of 12 patients the nerve root fiber may have acted as an alternative valve mechanism. This "one-way valve" may prevent or hinder the CSF from flowing back into the intradural space[1,3,14,26-29]. Gradual expansion of the cyst may cause erosion of surrounding bony structures through repetitive micro-stress[1,26]. Many authors have argued against the hyperosmolar fluid concentration theory because the cyst is likely to have the same fluid concentration as CSF[30]. However, Gortvai et al[26] found xanthochromic fluid in SEACs which may increase the osmolarity, in support of this theory. The theory of fluid secretion is considered the least likely because cystic walls largely consist of simple connective tissue and often lack an inner arachnoid lining[9].
The clinical symptoms of SEACs are related to the size and location of the cyst. In addition to possible pain involving the dermatone of the corresponding segment, cysts may also cause symptoms of spinal cord, nerve root and/or cauda equina compression. As the cyst grows, typically the associated symptoms are also exacerbated[31]. Diagnosing SEACs from symptoms alone is difficult and imaging examination is essential. X-ray imaging typically demonstrates a mass effect of the cystic lesion including spinal bone erosion, foraminal enlargement, and spinal canal enlargement[1]. MRI is the most useful modality for imaging SEACs because this technique can determine the location, extent and relationship of the cysts to the spinal dura mater. Thin slice and contrast-enhanced MRI are also helpful in excluding other diagnoses, such as cystic tumors, synovial cysts and inflammatory cysts[1,32]; typically SEACs show no enhancement after Gd administration[19]. In this case, whilst physical examination demonstrated tenderness in the lower back, we believe that this was unlikely to be due to the cyst itself. There was no compression of the conus medullaris on imaging. The strength in the lower extremity muscle groups was grade four. This was likely to be due to the patient’s age (79 years), with no features suggestive of an upper motor neuron lesion or lower motor neuron lesion. The knee-tendon reflex and Achilles-tendon reflex were abnormal, which again may be due to expected variation between patients rather than directly related to the SEAC.
At present, (hemi)laminectomy or laminoplasty with closure of the dural defect is considered the standard method of treating SEACs[2,33]. However, several reports suggest that closure of the dural defect without resection of the cyst may be as effective as a complete cyst resection, whilst maintaining a minimally invasive approach[8]. Lee et al[34] proposed a "twist technique" as another treatment method, but Shanbhag et al[31] responded that it may be dangerous to twist the cyst wall without a thorough examination of the inside of the cyst and this has not yet been widely performed.
Spinal endoscopic surgery is now widely used for the treatment of many lumbar degenerative diseases such as foraminal stenosis and lumbar disc herniation[35,36]. In this case report, because we were unable to assess whether the two cysts were continuous or discontinuous preoperatively, the patient had to undergo a re-do transforaminal percutaneous endoscopic procedure to remove the second cyst. If possible, we recommend that future clinicians presented with bi-segmental disease determine whether the cysts are connected before proceeding to surgery. Compared with traditional open surgery, endoscopic spinal surgery has several advantages, including preserving the paraspinal muscle structure, less blood loss, and faster postoperative recovery[37-39]. The endoscope can reach the lesion through a small puncture wound to effectively remove a cyst. This approach preserves the integrity and stability of the spine as the vertebral plate is not removed. Finally, direct vision under endoscopy allows the surgeon to ensure the cyst has been removed in total. Dural tear has been a disadvantage of endoscopic spinal surgery[40], but new methods proposed by Kim et al[40] reduce this risk significantly. In this case report the patient recovered rapidly with a clear improvement in symptoms postoperatively; we attribute this positive outcome to the use of foraminal endoscopic surgery.
Strangely, no cyst or dural sac communication was found during the operation, and postoperative MRI showed no CSF leakage indicating a dural defect. A review of the literature revealed few other cases of non-communicating SEAC[9,41]. Liu et al[9] hypothesised that non-communicating cysts may originate from SEACs, but enlargement of the cyst eventually disrupts the communication with the subarachnoid space due to Laplace’s law[42]. Proliferation of arachnoid cells may eventually lead to closure of the dural defects leaving a non-communicating cyst[9]. This is more likely in thoracic segment disease as the CSF pressure is close to zero in the upright position, which is beneficial for early closure[9]. Kim et al[41] reported a case of huge non-communicating SEACs with myelopathy. These authors believed that the communication was likely to have closed as the cysts continued to expand. Compared with communicating SEACs, surgeons treating non-communicating SEACs do not need to deal with any communication between the cyst and the dura, such as dural defects, arachnoid pedicles or fistulas[41]. We hypothesise that non-communicating SEACs likely develop directly from communicating SEACs. When SEACs develop and begin eroding bony structures surrounding them, the pressure increases inside the cysts. At this time, the pressure in the arachnoid space becomes than that in the cyst, so the CSF cannot open the "one-way valve" and enter the cyst to fill further. As time progresses, the channel gradually closes, and the defect disappears.
This article has several limitations. Firstly, this was a single case report at risk of bias. Multicentre studies of this treatment method are required to obtain more valid results. Secondly, endoscopic cystectomy surgery may not be suitable for multi-segment SEACs (e.g., involving more than three segments). Thirdly, constructive interference in steady-state MRI and CT myelography with delayed scanning can be used to explore a communication between cysts. However, these examinations were not performed in our case. Finally, in the first operation, only one segment was treated, and the cyst in the second segment was not excised. As a result, the patient was still symptomatic after the index operation and had to return for a second procedure.
In conclusion, bi-segmental non-communicating SEACs are extremely rare. Endoscopic surgery is a safe, effective, and reliable method for treating SEACs. However, in the event of bi-segmental SEACs, it is important to identify whether both cysts are communicating before surgery, and if not, to remove both cysts separately during the index surgery to avoid re-operation.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Romano L, Soliman MAR S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Li JH
| 1. | Oh JK, Lee DY, Kim TY, Yi S, Ha Y, Kim KN, Shin H, Kim DS, Yoon DH. Thoracolumbar extradural arachnoid cysts: a study of 14 consecutive cases. Acta Neurochir (Wien). 2012;154:341-348; discussion 348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Choi SW, Seong HY, Roh SW. Spinal extradural arachnoid cyst. J Korean Neurosurg Soc. 2013;54:355-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Liu JK, Cole CD, Kan P, Schmidt MH. Spinal extradural arachnoid cysts: clinical, radiological, and surgical features. Neurosurg Focus. 2007;22:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Tsuchimochi K, Morioka T, Murakami N, Yamashita F, Kawamura N. Huge multiple spinal extradural meningeal cysts in infancy. Childs Nerv Syst. 2019;35:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Yanni DS, Mammis A, Thaker NG, Goldstein IM. Traumatic fracture of thin pedicles secondary to extradural meningeal cyst. J Surg Tech Case Rep. 2011;3:40-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Klekamp J. A New Classification for Pathologies of Spinal Meninges, Part 1: Dural Cysts, Dissections, and Ectasias. Neurosurgery. 2017;81:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Tokmak M, Ozek E, Iplikcioglu AC. Spinal Extradural Arachnoid Cysts: A Series of 10 Cases. J Neurol Surg A Cent Eur Neurosurg. 2015;76:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Fukumoto H, Samura K, Katsuta T, Miki K, Fukuda K, Inoue T. Extensive Multilocular Spinal Extradural Meningeal Cyst That Developed 16 Years After Traumatic Brachial Plexus Injury: A Case Report. World Neurosurg. 2016;86:510.e5-510.10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Liu JK, Cole CD, Sherr GT, Kestle JR, Walker ML. Noncommunicating spinal extradural arachnoid cyst causing spinal cord compression in a child. J Neurosurg. 2005;103:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Nabors MW, Pait TG, Byrd EB, Karim NO, Davis DO, Kobrine AI, Rizzoli HV. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988;68:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 369] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Thorpe Lowis CG, Zhang M, Amin NF. Fine Configuration of Thoracic Type II Meningeal Cysts: Macro- and Microscopic Cadaveric Study Using Epoxy Sheet Plastination. Spine (Phila Pa 1976). 2016;41:E1195-E1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Sun JJ. Classification, mechanism and surgical treatments for spinal canal cysts. Chin Neurosurg J. 2016;2:106-116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | de Oliveira RS, Amato MC, Santos MV, Simão GN, Machado HR. Extradural arachnoid cysts in children. Childs Nerv Syst. 2007;23:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Marbacher S, Barth A, Arnold M, Seiler RW: Multiple spinal extradural meningeal cysts presenting as acute paraplegia. Case report and review of the literature. J Neurosurg Spine. 2007;6:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Payer M, Brühlhart K. Spinal extradural arachnoid cyst: review of surgical techniques. J Clin Neurosci. 2011;18:559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Samura K, Morioka T, Miyagi Y, Nagata S, Mizoguchi M, Mihara F, Sasaki T: Surgical strategy for multiple huge spinal extradural meningeal cysts. Case report. J Neurosurg. 2007;107:297-302. [PubMed] [DOI] [Full Text] |
| 17. | Suryaningtyas W, Arifin M: Multiple spinal extradural arachnoid cysts occurring in a child. Case report. J Neurosurg. 2007;106:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Takagaki T, Nomura T, Toh E, Watanabe M, Mochida J. Multiple extradural arachnoid cysts at the spinal cord and cauda equina levels in the young. Spinal Cord. 2006;44:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Netra R, Min L, Shao Hui M, Wang JC, Bin Y, Ming Z. Spinal extradural meningeal cysts: an MRI evaluation of a case series and literature review. J Spinal Disord Tech. 2011;24:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Morizane K, Fujibayashi S, Otsuki B, Sakamoto T, Tsutsumi R, Odate S, Kusuba A, Matsuda S. Clinical and radiological features of spinal extradural arachnoid cysts: Valve-like mechanism involving the nerve root fiber as a possible cause of cyst expansion. J Orthop Sci. 2018;23:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Tanaka T, Boddepalli RS, Miller DC, Cao Z, Sindhwani V, Coates JR, Govindarajan R, Litofsky NS. Electrodiagnostic and Advanced Neuroimaging Characterization for Successful Treatment of Spinal Extradural Arachnoid Cyst. World Neurosurg. 2018;109:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Ogura Y, Miyake N, Kou I, Iida A, Nakajima M, Takeda K, Fujibayashi S, Shiina M, Okada E, Toyama Y, Iwanami A, Ishii K, Ogata K, Asahara H, Matsumoto N, Nakamura M, Matsumoto M, Ikegawa S. Identification of HOXD4 Mutations in Spinal Extradural Arachnoid Cyst. PLoS One. 2015;10:e0142126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Woo JB, Son DW, Kang KT, Lee JS, Song GS, Sung SK, Lee SW. Spinal Extradural Arachnoid Cyst. Korean J Neurotrauma. 2016;12:185-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Rohrer DC, Burchiel KJ, Gruber DP. Intraspinal extradural meningeal cyst demonstrating ball-valve mechanism of formation. Case report. J Neurosurg. 1993;78:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Roski RA, Rekate HL, Kurczynski TW, Kaufman B. Extradural meningeal cyst. Case report and review of the literature. Childs Brain. 1984;11:270-279. |
| 26. | Gortvai P. Extradural cysts of the spinal canal. J Neurol Neurosurg Psychiatry. 1963;26:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Sun JJ, Wang ZY, Teo M, Li ZD, Wu HB, Yen RY, Zheng M, Chang Q, Yisha Liu I. Comparative outcomes of the two types of sacral extradural spinal meningeal cysts using different operation methods: a prospective clinical study. PLoS One. 2013;8:e83964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Bowman JJ, Edwards CC 2nd. Extradural arachnoid cyst with bony erosion: a rare case report. J Spine Surg. 2020;6:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kadono Y, Yuguchi T, Ohnishi Y, Iwatsuki K, Yoshimine T. A symptomatic spinal extradural arachnoid cyst with lumbar disc herniation. Case Rep Orthop. 2015;2015:250710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Cloward RB. Congenital spinal extradural cysts: case report with review of literature. Ann Surg. 1968;168:851-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Shanbhag NC, Duyff RF, Groen RJM. Symptomatic Thoracic Nerve Root Herniation into an Extradural Arachnoid Cyst: Case Report and Review of the Literature. World Neurosurg. 2017;106:1056.e5-1056.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Menezes AH, Hitchon PW, Dlouhy BJ. Symptomatic spinal extradural arachnoid cyst with cord compression in a family: case report. J Neurosurg Spine. 2017;341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine (Phila Pa 1976). 2004;29:E426-E430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Lee SH, Shim HK, Eun SS. Twist technique for removal of spinal extradural arachnoid cyst: technical note. Eur Spine J. 2014;23:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Kim M, Kim HS, Oh SW, Adsul NM, Singh R, Kashlan ON, Noh JH, Jang IT, Oh SH. Evolution of Spinal Endoscopic Surgery. Neurospine. 2019;16:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 36. | Heo DH, Lee DC, Park CK. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurg Focus. 2019;46:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 37. | Choi KC, Shim HK, Hwang JS, Shin SH, Lee DC, Jung HH, Park HA, Park CK. Comparison of Surgical Invasiveness Between Microdiscectomy and 3 Different Endoscopic Discectomy Techniques for Lumbar Disc Herniation. World Neurosurg. 2018;116:e750-e758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | Chen HT, Tsai CH, Chao SC, Kao TH, Chen YJ, Hsu HC, Shen CC, Tsou HK. Endoscopic discectomy of L5-S1 disc herniation via an interlaminar approach: Prospective controlled study under local and general anesthesia. Surg Neurol Int. 2011;2:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Bresnahan LE, Smith JS, Ogden AT, Quinn S, Cybulski GR, Simonian N, Natarajan RN, Fessler RD, Fessler RG. Assessment of Paraspinal Muscle Cross-sectional Area After Lumbar Decompression: Minimally Invasive Versus Open Approaches. Clin Spine Surg. 2017;30:E162-E168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Kim HS, Pradhan RL, Adsul N, Jang JS, Jang IT, Oh SH. Transforaminal Endoscopic Excision of Intradural Lumbar Disk Herniation and Dural Repair. World Neurosurg. 2018;119:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Kim IS, Hong JT, Son BC, Lee SW. Noncommunicating spinal extradural meningeal cyst in thoracolumbar spine. J Korean Neurosurg Soc. 2010;48:534-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | McCrum C, Williams B. Spinal extradural arachnoid pouches. Report of two cases. J Neurosurg. 1982;57:849-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 2.6] [Reference Citation Analysis (0)] |