Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9577
Peer-review started: March 22, 2021
First decision: May 28, 2021
Revised: May 30, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: November 6, 2021
Processing time: 212 Days and 7.4 Hours
Hepatic encephalopathy (HE) is a frequent and debilitating complication of chronic liver disease. Recurrent HE is strongly linked with spontaneous portosystemic shunts (SPSSs). Intrahepatic arterioportal fistulas (IAPFs) occur rarely but pose a major clinical challenge and may lead to or worsen portal hypertension. Herein, we present a rare case of recurrent HE secondary to a SPSS combined with an IAPF.
A 63-year-old female with primary biliary cirrhosis presented with recurrent disturbance of consciousness for 4 mo. SPSS communicating the superior mesenteric vein with the inferior vena cava and IAPF linking the intrahepatic artery with the portal vein were found on contrast-enhanced abdominal computed tomography. The patient did not respond well to medical treatment. Therefore, simultaneous embolization of SPSS and IAPF was scheduled. After embolization, the symptoms of HE showed obvious resolution.
The presence of liver vascular disorders should not be neglected in patients with chronic liver disease, and interventional therapy is a reasonable choice in such patients.
Core Tip: Hepatic encephalopathy (HE) is characterized by neurological dysfunction due to cirrhosis or portal-systemic shunting. The relationship between recurrent HE and spontaneous portosystemic shunts (SPSSs) has been demonstrated. Intrahepatic arterioportal fistulas (IAPFs), as an uncommon cause of portal hypertension, are rarely reported in HE. Herein, we present a case of recurrent HE secondary to a SPSS and IAPF. Endovascular embolization of the SPSS and IAPF was performed. Our case highlights the hemodynamic changes caused by SPSSs and IAPFs. Simultaneous embolization of an SPSS and IAPF should be considered as the optimal therapy for such patients.
- Citation: Liu GF, Wang XZ, Luo XF. Simultaneous embolization of a spontaneous porto-systemic shunt and intrahepatic arterioportal fistula: A case report. World J Clin Cases 2021; 9(31): 9577-9583
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9577.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9577
Hepatic encephalopathy (HE) is a neurological dysfunction caused by liver disease or portal-systemic shunting. HE severity ranges from subtle cognitive slowing to a completely comatose state[1]. Spontaneous portosystemic shunts (SPSSs) are abnormal communications between the portal system and systemic circulation. The morbidity of SPSS increases to 40% in patients with cirrhosis[2], probably as a consequence of worsening portal hypertension. The relationship between HE and SPSS has been clearly demonstrated, SPSS deviates neurotoxins from the intestine via the portal vein to the systemic circulation bypassing the liver and leads to episodes of HE[3].
Intrahepatic arterioportal fistulas (IAPFs) are vascular malformations between the intrahepatic artery and portal venous system[4,5]. Most patients with IAPFs are asymptomatic. With the widespread use of radiologic techniques, the reported prevalence of IAPF has increased[6]. The presence of an IAPF may increase portal pressure and aggravate the complications of portal hypertension, which leads to gastrointestinal bleeding, refractory ascites, diarrhea, and HE[5].
Vascular embolization is a safe and effective treatment for hepatic vascular disorders, and it has been confirmed to significantly alleviate the symptoms caused by SPSSs and IAPF, respectively[7,8].
In this report, we present the case of a 63-year-old female with recurrent HE secondary to SPSS combined with IAPF who underwent embolization for both types of vascular malformation.
A 63-year-old female with primary biliary cirrhosis was admitted to our hospital due to recurrent disturbance of consciousness for 4 mo, accompanied by fatigue, upper abdominal discomfort, and edema of both lower extremities.
The patient began to experience fatigue, upper abdominal discomfort, and edema of both lower extremities 1 year ago and was diagnosed with primary biliary cirrhosis combined with gastroesophageal varices and splenomegaly in a local hospital.
Four months ago, the patient had one episode of coma 1 wk following splenectomy and portoazygos disconnection surgery in a local hospital. The symptoms recurred following a high-protein diet or constipation, manifested by coma, hyperreflexia, and hypermyotonia. No obvious abnormalities were found on head computed tomography (CT) and cardiac ultrasound, but a large portosystemic shunt was found on contrast-enhanced abdominal CT. The patient was awake but began to be disoriented with slurred speech after treatment with lactulose, rifaximin, and ornithine aspartate in a local hospital. Similar symptoms recurred in the next few months despite persistent use of the above-mentioned drugs. To resolve the above symptoms completely, she was transferred to our hospital.
History of previous HE, epilepsy, stroke, or brain trauma was denied.
The patient had no relevant family history.
The patient appeared to be delirious and presented with count disturbance and gross disorientation. Asterixis, hyperreflexia, and hypermyotonia were detected. She also had marked edema of both lower limbs. No signs of jaundice, anemia, bloating and abdominalgia were found.
Clinical laboratory examination revealed increased levels of aspartate aminotransferase (100 IU/L, 2.9 × upper limit of normal), alanine aminotransferase (58 IU/L, 1.5 × upper limit of normal), alkaline phosphatase (304 IU/L, 2.3 × upper limit of normal), serum ammonia (213.5 μmol/L, 6.5 × upper limit of normal), and a decreased level of serum albumin (31.9 g/L, 0.8 × lower limit of normal). Hemoglobin, total bilirubin, prothrombin time and international normalized ratio results were normal. The patient scored 8 points on the Child-Pugh score and the Model for End-stage Liver Disease score was 11 points.
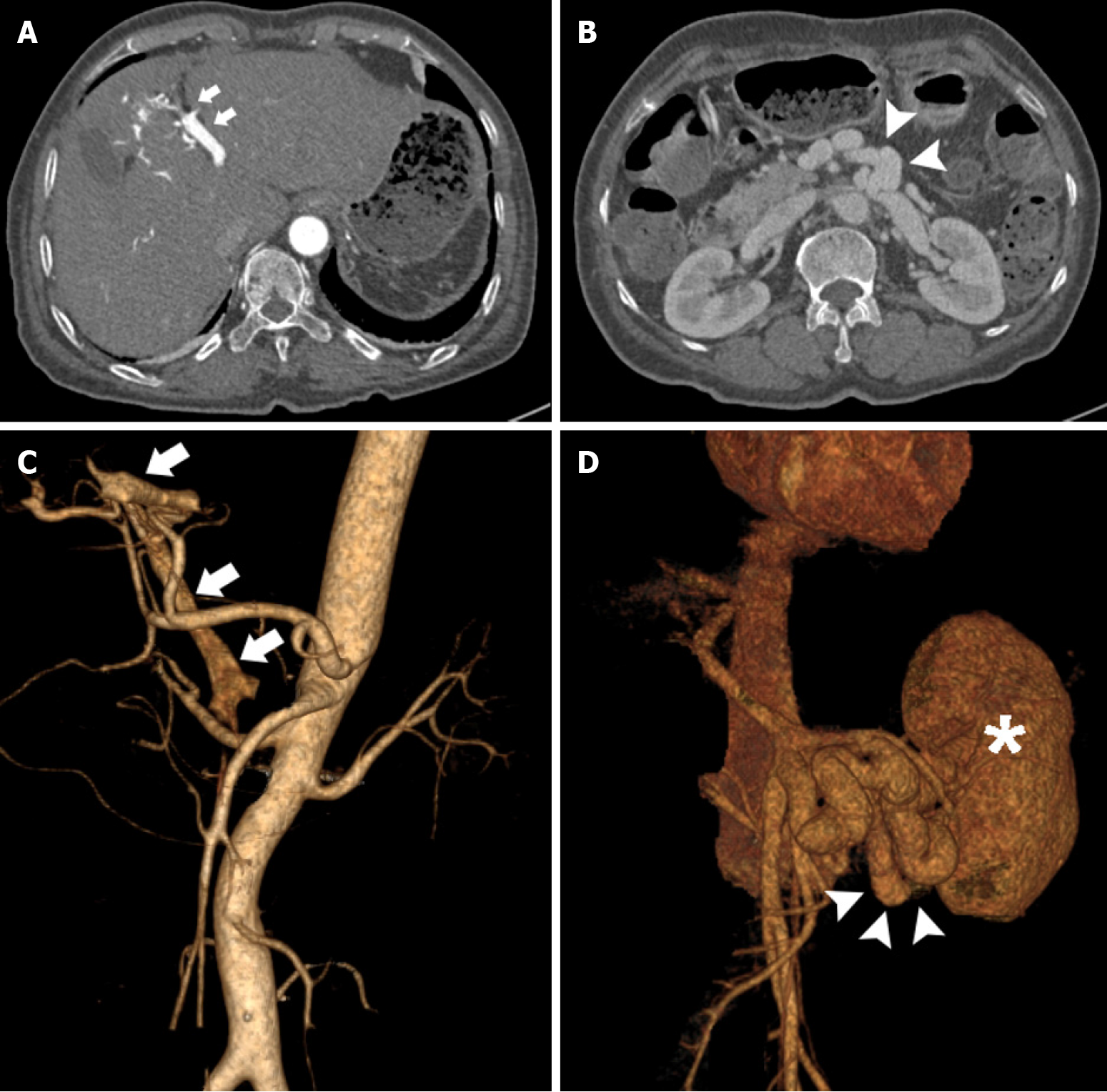
Contrast-enhanced abdominal CT revealed early visualization of the left branch of the portal vein in the arterial phase which was grossly dilated. Malformed collateral vessels were noted between the superior mesenteric vein (SMV) and inferior vena cava (IVC) (Figure 1). Gastroscopy showed the presence of moderate gastroesophageal varices.
The final diagnosis was HE caused by SPSS and IAPF.
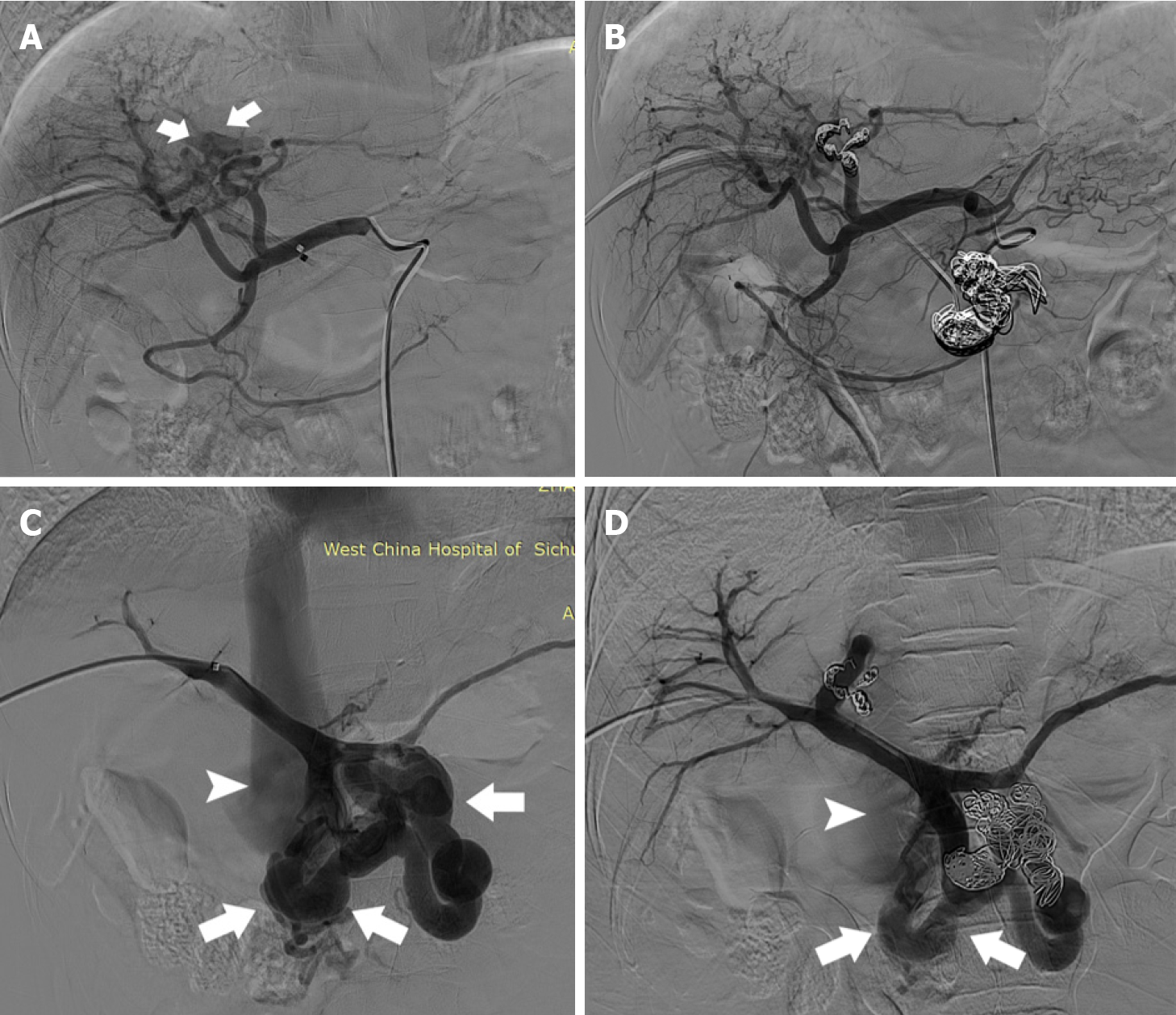
Considering the severity of HE (Grade 4)[1], the coexistence of a large SPSS and IAPF, and poor response to medical treatment, endovascular embolization of the SPSS and IAPF was scheduled. Under local anesthesia, the common hepatic artery was catheterized using a 5F catheter via the femoral artery approach. Selective angiography of the common hepatic artery revealed that the left hepatic artery branches communicated with the left branch of the portal vein, leading to fistulous communications (Figure 2A). The hepatic arterial branch feeding the IAPF was successfully embolized using metal coils via a microcatheter (Figure 2B). In addition, an ultrasound-guided, percutaneous, transhepatic puncture of the right anterior portal vein was performed. Venography confirmed grossly dilated malformed vessels between the SMV and IVC (Figure 2C). Shunt embolization using metal coils was performed (Figure 2D).
The symptoms of unconsciousness, gross disorientation, and slurred speech disappeared gradually, and her reflexes and muscular tone returned to normal post-intervention.
Serum ammonia decreased to 69.1 μmol/L three days after embolization. The Child-Pugh score improved from B (8 points) to A (6 points) after embolization. However, the patient presented with left lower abdominal pain two days later. Physical examination revealed mild tenderness. Abdominal CT revealed fresh thrombus in the portal vein trunk, and the patient was administered low-molecular-weight heparin as an anticoagulant therapy.
She was discharged six days after embolization. Ligation was scheduled in 2-4 wk intervals to prevent hemorrhage induced by recurrent esophageal varices.
According to the telephone follow-up results, the patient showed no signs of HE for 9 mo after the procedure. The latest examinations in the local hospital showed that the level of serum ammonia was 30.7 μmol/L.
This case describes the successful interventional treatment of recurrent HE due to SPSS and IAPF. The presence of SPSS diverts the portal blood flow directly into the systematic circulation, increasing the bioavailability of intestinal ammonia and leading to episodes of HE[9]. Moreover, IAPF may have aggravated the existing portal-systemic shunt by increasing the portal vein pressure in our patient.
SPSS frequently develops as liver function deteriorates and in the presence of portal hypertension in patients with cirrhosis[10]. Recent findings indicate that patients who suffer from cirrhosis in combination with SPSS developed more frequent episodes of portal hypertension-related complications (HE, bleeding, or ascites) and had lower transplantation-free survival than patients without SPSS[11]. Doppler ultrasound imaging and CT/A were singly the most frequently used methods to detect SPSSs[12]. Patients with recurrent HE are often refractory to standard medical therapies[13]. Recent reports have demonstrated that angiographic embolization of a large SPSS is an effective treatment and can improve the symptoms of portosystemic encephalopathy, decrease the hospitalization rate, and potentially alleviate the need for liver transplantation[14]. Moreover, An et al[15] found that embolization of a large SPSS could improve survival and liver function in patients and prevent recurrent episodes of HE.
As an uncommon potential cause of portal hypertension, an IAPF forms a connection between a high-pressure hepatic artery and a branch of the low-pressure portal vein to communicate[5]. IAPFs are generally found in patients with hepatocellular carcinoma, hepatic vascular malformations, or benign neoplasms. The more common acquired cause is iatrogenic, representing more than 50% of all published cases[16]. The incidence of arterioportal fistulas resulting from liver biopsy varies from 5.4% to 38%[17]. The treatment depends on the size, location, the number of IAPFs, and patients’ clinical manifestations. Small arterioportal fistulae can regress spontaneously and thus only regular follow-up is suggested[18]. The treatment involves either surgical or endovascular occlusion of the fistula. Endovascular embolization is a less invasive, safe and effective treatment modality for IAPFs, and surgery will only be indicated in rare instances after the failure of radiological intervention[5].
Given that two types of vascular abnormalities (an SPSS and IAPF) were detected in our patient, simultaneous embolization of the SPSS and IAPF was considered to be an ideal treatment option. The interventional procedure was performed successfully. In general, the symptoms of HE disappeared gradually, and the level of serum ammonia decreased from 213.5 μmol/L to 69.1 μmol/L 3 d post-intervention in our patient, and the reduction in the Child-Pugh score might be associated with the improvement of HE after embolization of SPSS and IAPF.
Our case highlights the hemodynamic changes caused by SPSSs and IAPFs. A large SPSS reduces liver perfusion and contributes to compromising liver function as well as the occurrence of HE[19]. The presence of an IAPF increases the portal venous flow[18]. We speculate that increased portal vein pressure caused by the IAPF may increase the shunt flow and contribute to the recurrent episodes of HE in the present case. Hence, the two vascular malformations were embolized together.
In conclusion, the coexistence of an SPSS and IAPF is rare among patients with cirrhosis. In the present case, the presence of SPSS and IAPF may contribute to diverting flow away from the liver and the occurrence of recurrent HE. Endovascular embolization is a safe and effective treatment for both SPSSs and IAPFs. Moreover, the presence of liver vascular disorders should not be neglected in patients with chronic liver disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salimi M S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Liu JH
| 1. | Córdoba J, Mínguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Zardi EM, Uwechie V, Caccavo D, Pellegrino NM, Cacciapaglia F, Di Matteo F, Dobrina A, Laghi V, Afeltra A. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Riggio O, Efrati C, Catalano C, Pediconi F, Mecarelli O, Accornero N, Nicolao F, Angeloni S, Masini A, Ridola L, Attili AF, Merli M. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology. 2005;42:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Krishan S, McPherson S, Pine J, Hayden J. Current management of mesenteric extrahepatic arterioportal fistulas: report of a case treated with a gastroduodenal artery stent graft and literature review. Vasc Endovascular Surg. 2010;44:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Vauthey JN, Tomczak RJ, Helmberger T, Gertsch P, Forsmark C, Caridi J, Reed A, Langham MR Jr, Lauwers GY, Goffette P, Lerut J. The arterioportal fistula syndrome: clinicopathologic features, diagnosis, and therapy. Gastroenterology. 1997;113:1390-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M, Jalan R, Brookes J, Thalassinos E, Burroughs AK, Cordoba J, Nevens F; EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Bapuraj JR, Kalra N, Rao KL, Suri S, Khandelwal N. Transcatheter coil embolization of a traumatic intrahepatic arterioportal fistula. Indian J Pediatr. 2001;68:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Nardelli S, Riggio O, Gioia S, Puzzono M, Pelle G, Ridola L. Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects. World J Gastroenterol. 2020;26:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (3)] |
| 10. | Praktiknjo M, Simón-Talero M, Römer J, Roccarina D, Martínez J, Lampichler K, Baiges A, Low G, Llop E, Maurer MH, Zipprich A, Triolo M, Maleux G, Fialla AD, Dam C, Vidal-González J, Majumdar A, Picón C, Toth D, Darnell A, Abraldes JG, López M, Jansen C, Chang J, Schierwagen R, Uschner F, Kukuk G, Meyer C, Thomas D, Wolter K, Strassburg CP, Laleman W, La Mura V, Ripoll C, Berzigotti A, Calleja JL, Tandon P, Hernandez-Gea V, Reiberger T, Albillos A, Tsochatzis EA, Krag A, Genescà J, Trebicka J; Baveno VI-SPSS group of the Baveno Cooperation. Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis. J Hepatol. 2020;72:1140-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | Simón-Talero M, Roccarina D, Martínez J, Lampichler K, Baiges A, Low G, Llop E, Praktiknjo M, Maurer MH, Zipprich A, Triolo M, Vangrinsven G, Garcia-Martinez R, Dam A, Majumdar A, Picón C, Toth D, Darnell A, Abraldes JG, Lopez M, Kukuk G, Krag A, Bañares R, Laleman W, La Mura V, Ripoll C, Berzigotti A, Trebicka J, Calleja JL, Tandon P, Hernandez-Gea V, Reiberger T, Albillos A, Tsochatzis EA, Augustin S, Genescà J; Baveno VI-SPSS group from the Baveno Cooperation. Association Between Portosystemic Shunts and Increased Complications and Mortality in Patients With Cirrhosis. Gastroenterology. 2018;154:1694-1705.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 12. | Matthews TJ, Trochsler MI, Bridgewater FH, Maddern GJ. Systematic review of congenital and acquired portal-systemic shunts in otherwise normal livers. Br J Surg. 2014;101:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Choudhary NS, Baijal SS, Saigal S, Agarwal A, Saraf N, Khandelwal R, Jain V, Khandelwal AH, Kapoor A, Jain D, Misra SR, Puri R, Sud R, Soin AS. Results of Portosystemic Shunt Embolization in Selected Patients with Cirrhosis and Recurrent Hepatic Encephalopathy. J Clin Exp Hepatol. 2017;7:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Lynn AM, Singh S, Congly SE, Khemani D, Johnson DH, Wiesner RH, Kamath PS, Andrews JC, Leise MD. Embolization of portosystemic shunts for treatment of medically refractory hepatic encephalopathy. Liver Transpl. 2016;22:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | An J, Kim KW, Han S, Lee J, Lim YS. Improvement in survival associated with embolisation of spontaneous portosystemic shunt in patients with recurrent hepatic encephalopathy. Aliment Pharmacol Ther. 2014;39:1418-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Kim TK, Choi BI, Han JK, Chung JW, Park JH, Han MC. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998;208:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Okuda K, Musha H, Nakajima Y, Takayasu K, Suzuki Y, Morita M, Yamasaki T. Frequency of intrahepatic arteriovenous fistula as a sequela to percutaneous needle puncture of the liver. Gastroenterology. 1978;74:1204-1207. [PubMed] |
| 18. | Strodel WE, Eckhauser FE, Lemmer JH, Whitehouse WM Jr, Williams DM. Presentation and perioperative management of arterioportal fistulas. Arch Surg. 1987;122:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kumamoto M, Toyonaga A, Inoue H, Miyakoda K, Morita Y, Emori K, Sakamoto Y, Oho K, Sata M. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |