Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9542
Peer-review started: January 27, 2021
First decision: July 16, 2021
Revised: July 22, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 6, 2021
Processing time: 274 Days and 23.4 Hours
Pediatric-type follicular lymphoma (PTFL) is a unique pathological type in the 4th edition of hematopoiesis and lymphoid tissue tumor classification revised by World Health Organization. It is unique in clinical practice and seldom seen in adult. PTFL mainly occurs in the head and neck lymph nodes. Most of the cases are short of fever, night sweat, weight loss, and other B symptoms which substitute for lymphadenopathy as the main symptom. PTFL can be disposed of surgical resection and it can achieve long-term tumor-free survival, and it has an excellent outcome.
Two cases of PTFL were reported and their clinicopathological features, differential diagnosis, therapy and prognosis were discussed. PTFL showed gray-brown tough texture in general performance. The histological manifestations of PTFL were similar to that of adult-follicular lymphoma (FL). Under low power microscope, the structure of lymph nodes was destroyed in different degree, the follicles were closely arranged, expanded and irregular, and the mantle zone became thin or disappeared. In addition, the “starry sky phenomenon” could be seen. At high magnification, the follicles were mainly composed of single medium-sized central cells, and some of them mainly consisted of centroblastic cells to characterize scattered chromatin and inconspicuous nucleoli. Immunohistochemical showed the tumor cells expressed CD20, PAX5, CD79a and CD10, BCL6, FOXP-1, which were limited in germinal center; Ki-67 was highly expressed in germinal center. CD21 and CD23 showed nodular and expanded follicular dendritic cells. Immunoglobulin gene rearrangement was positive for IGH and IGK. The two patients underwent surgical resection with no complications. After discharge, the two patients with a close review for 18 mo and 5 mo respectively and showed no evidence of recurrence.
PTFL in adult is generally supposed to be extremely rare. PTFL displayed characteristic morphological, immunophenotypic, and molecular biological changes which are a kind of neoplasm with satisfactory prognosis after surgical excision.
Core Tip: Pediatric-type follicular lymphoma (PTFL) is a unique type of follicular lymphoma, which occurs in childhood and adolescents frequently. PTFL is unique in clinical practice and seldom seen in adult. It has unique clinical and pathological characteristics, which is different from the pathological type of FL in adult. Through the discussion of the cases, we can deepen our understanding of the disease.
- Citation: Liu Y, Xing H, Liu YP. Clinical observation of pediatric-type follicular lymphomas in adult: Two case reports . World J Clin Cases 2021; 9(31): 9542-9548
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9542
Pediatric-type follicular lymphoma (PTFL) is a unique type of follicular lymphoma (FL), which occurs in childhood and adolescents frequently. PTFL is unique in clinical practice and seldom seen in adult. It accounts for 1%-2% of all non-Hodgkin's lymphoma (NHL) cases in childhood approximately[1]. PTFL was classified as a subtype of FL, that is FL occurs in childhood over the past few years. In 2017, the novel classification of hematopoiesis and lymphoid tissue tumor of World Health Organi
Case 1: A 43-year-old male patient presented with posterior occipital scalp mass.
Case 2: A 37-year-old male patient presented with left cervical lymphadenectasis.
Case 1: The male had no apparent inducement to appear posterior occipital scalp mass half a year ago, with the size of 1 cm × 1 cm × 1 cm. The male had no apparent discomfort, local skin fever, local skin variation, and exceptional treatment.
Case 2: The male had no apparent cause of left cervical lymph node enlargement half a year ago and with the size of 3 cm × 2 cm × 2 cm approximately. The male had no apparent indisposition, local skin fever and local skin variation.
Case 1: The patient had an insignificant medical history.
Case 2: The patient had an insignificant medical history.
These two patients had insignificant personal and family history.
No obvious abnormality.
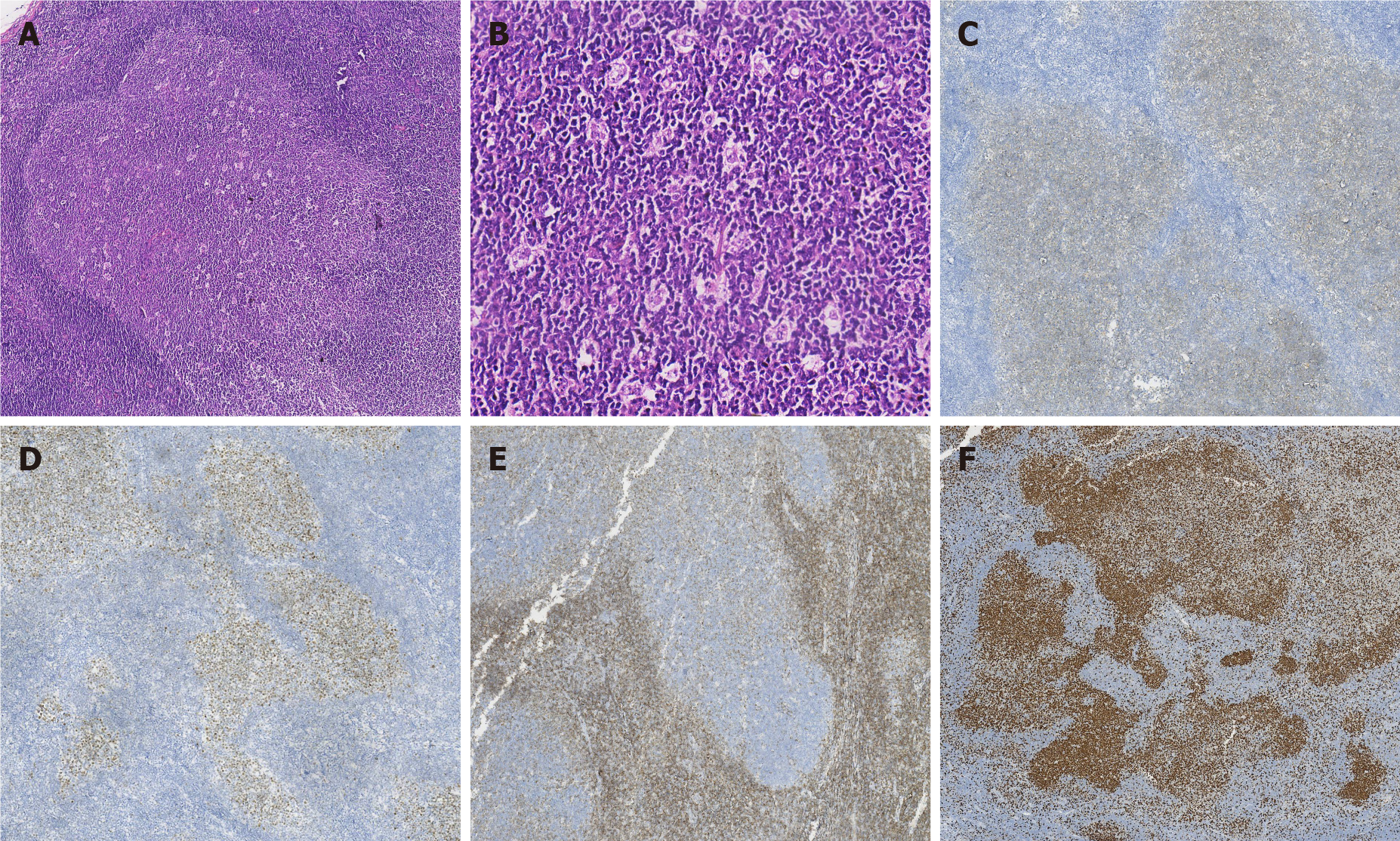
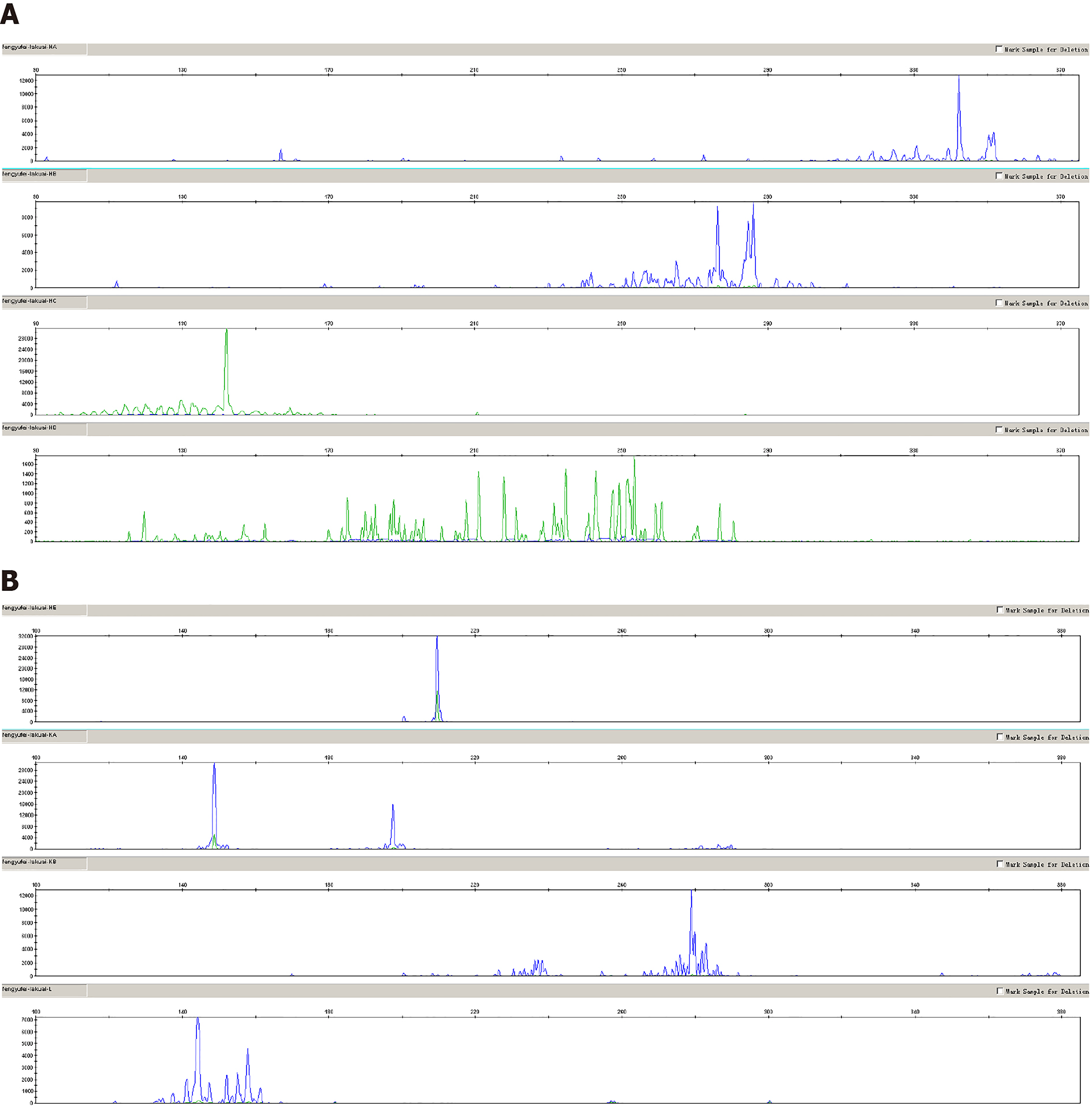
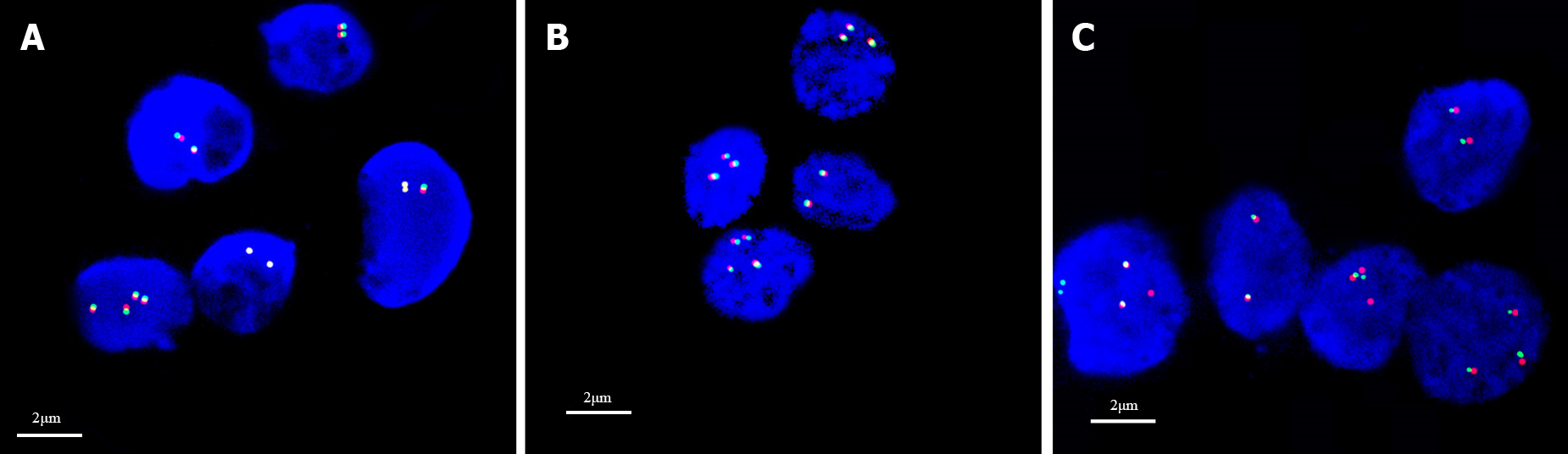
Pathological findings: (1) Visual inspection: As seen by naked eyes, nodule like substance in size of 2 cm × 1.5 cm × 1.5 cm and with a smooth surface, gray-brown tough texture, no bleeding or necrosis region; (2) Microscope observation: PTFL was similar to that of FL, the lymph node structure was destroyed partially or completely, the follicles were arranged closely, expanded and irregular. The mantle zone was thin or disappeared and the follicles were lack of polarity. The bright and dark area was inconspicuous, and the “starry sky phenomenon” could be seen under the low power microscope (Figure 1A). Under the high power microscope, the follicle mainly consists of single medium-sized central cells, some of which are centroblastic cells to characterize scattered chromatin and inconspicuous nucleoli. The histological grade was by following per under the grade 3 standard of FL (Figure 1B); (3) Immunohistochemical staining demonstrated that tumor cells expressed CD20, PAX5, CD79a (B cell markers) and CD10 (Figure 1C), BCL6 (Figure 1D), FOXP-1 (germinal center markers) which were limited to germinal center; IRF4, BCL2 (Figure 1E) and MUM1 were negative; Ki-67 was highly expressed in germinal center (Figure 1F); CD21 and CD23 revealed nodular and expanded follicular dendritic cells (FDC); and (4) Immunoglobulin gene rearrangement was positive for IGH (Figure 2A) and IGK (Figure 2B). BCL2, BCL6 and MYC genes were negative (Figure 3A-C).
Case 1: Ultrasound inspection displayed a hypoechoic nodule in the rear of the neck. It had no apparent enlarged lymph nodes were found in the bilateral perivascular neck, supraclavicular fossa and thoracoabdominal region.
Case 2: Ultrasonography of thyroid and cervical lymph nodes revealed hypoechoic left supraclavicular fossa. There was no else superficial lymph node enlargement.
Based on the above imaging examinations and pathological findings, two patients were diagnosed with PTFL and confirmed by immunoglobulin (IG) gene rearrangement.
The two patients underwent surgical resection, the strategy of observation was adopted in the follow-up.
Case 1 was followed up for 25 mo and case 2 was followed up for 10 mo without recurrence. Two patients come to the hospital regularly for reexamination.
PTFL was a unique clinicopathological type in the fourth edition of hematopoiesis and lymphoid tissue tumor classification revised by WHO[2]. Although PTFL was found in childhood initially and accounting for 1%-2% of all childhood with NHL approximately, but it was discovered that it can be seen in adolescent afterwards, even less in the adult[1]. The median age showed between 7.5 to 14 years old, and most of which were males (male to female ratio > 10:1)[3,4]. Currently, PTFL is considered a lymph node disease that most often arises in the head and neck lymph nodes. It usually isolated peripheral lymph nodes and less involved in the groin and axillary lymph nodes[1,5]. Most of the cases are short of fever, night sweat, weight loss, and other B symptoms which substitute for lymphadenopathy as the main symptom. On account of the differences in histopathological features, molecular structure and clinical behavior, the revised WHO hematopoiesis and lymphoid tissue tumors of the fourth edition excluded the cases of extranodal FL, that including testis, epididymis, gastrointestinal tract, and kidney, which were reported as PTFL previously[1]. Another tumor that should be excluded is a novel tumor namely large B-cell lymphoma with IRF4 gene rearrangement[1,6] which usually occurs in the pharyngeal lymph ring and/or cervical lymph nodes, but it may also occur in the gastrointestinal tract. The tumor growth pattern can be diffuse or follicular hyperplasia or both. Histologically, PTFL was replaced by an enlarged follicular structure partially or completely. Under the low power microscope, there were irregular lymphoid follicles characterize of different sizes and shapes. The germinal center displayed a “starry sky phenomenon” and without a clear distinction between bright and dark areas. In some cases, marginal zone differentiation could be seen around the tumor follicles. The mantle area was thinned or disappeared, the follicles were short of polarity, and some areas were diffuse hyperplasia of lymphoid tissue. In terms of cell morphology, PTFL had a large number of high-grade cells more often than not (so in the old days, the histological grade of this kind cases were mostly 3a or 3b, but in the fourth edition classification, PTFL was isolated, and the grading standard of adult follicular lymphoma was no longer used)[1]. The cell-rich tumor area had a single cell composition, it mainly consisted of medium-sized blastoid cells which characterize the irregular nucleus and unclear nucleolus. The mitosis was easy to found, and some cases contain more typical immature centroblastic cells[7]. In terms of immu
PTFL should be differentiated from FL, large B-cell lymphoma with IRF4 rear
PTFL can be treated by surgical excision and it can combine with low-dose chemotherapy/radiotherapy, or adopting the strategy of "observation and waiting". PTFL usually be in early clinical stage, the long-term tumor-free survival can be obtained after surgical resection, and PTFL is a kind of neoplasm with a satisfactory prognosis.
PTFL in adult is generally supposed to be extremely rare. PTFL displayed characteristic morphological, immunophenotypic, and molecular biological changes which are a kind of neoplasm with satisfactory prognosis after surgical excision. To avoid misdiagnosis, differential diagnosis plays a crucial role in clinical practice.
The authors thank all the clinicians and nurses involved in the treatment of this case and thank other members of the Department of Pathology, Fourth Hospital of Hebei Medical University.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Popa-Fotea NM S-Editor: Zhang H L-Editor: A P-Editor: Wu RR
| 1. | Attarbaschi A, Beishuizen A, Mann G, Rosolen A, Mori T, Uyttebroeck A, Niggli F, Csoka M, Krenova Z, Mellgren K, Kabickova E, Chiang AK, Reiter A, Williams D, Burkhardt B; European Intergroup for Childhood Non-Hodgkin Lymphoma (EICNHL) and the international Berlin-Frankfurt-Münster (i-BFM) Study Group. Children and adolescents with follicular lymphoma have an excellent prognosis with either limited chemotherapy or with a "Watch and wait" strategy after complete resection. Ann Hematol. 2013;92:1537-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Jaffe ES, Harris NL, Siebert R. Paediatric-typefollicular lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2017: 278-279. |
| 3. | Quintanilla-Martinez L, Sander B, Chan JK, Xerri L, Ott G, Campo E, Swerdlow SH. Indolent lymphomas in the pediatric population: follicular lymphoma, IRF4/MUM1+ lymphoma, nodal marginal zone lymphoma and chronic lymphocytic leukemia. Virchows Arch. 2016;468:141-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 4. | Schmidt J, Gong S, Marafioti T, Mankel B, Gonzalez-Farre B, Balagué O, Mozos A, Cabeçadas J, van der Walt J, Hoehn D, Rosenwald A, Ott G, Dojcinov S, Egan C, Nadeu F, Ramis-Zaldívar JE, Clot G, Bárcena C, Pérez-Alonso V, Endris V, Penzel R, Lome-Maldonado C, Bonzheim I, Fend F, Campo E, Jaffe ES, Salaverria I, Quintanilla-Martinez L. Genome-wide analysis of pediatric-type follicular lymphoma reveals low genetic complexity and recurrent alterations of TNFRSF14 gene. Blood. 2016;128:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Louissaint A Jr, Schafernak KT, Geyer JT, Kovach AE, Ghandi M, Gratzinger D, Roth CG, Paxton CN, Kim S, Namgyal C, Morin R, Morgan EA, Neuberg DS, South ST, Harris MH, Hasserjian RP, Hochberg EP, Garraway LA, Harris NL, Weinstock DM. Pediatric-type nodal follicular lymphoma: a biologically distinct lymphoma with frequent MAPK pathway mutations. Blood. 2016;128:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Pittaluga S, Harris NL, Siebert R, Salaverria I. IRF4 B-cell lymphoma with IRF4 rearrangment. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (Eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2017: 280-281. |
| 7. | Attarbaschi A, Abla O, Arias Padilla L, Beishuizen A, Burke GAA, Brugières L, Bruneau J, Burkhardt B, d'Amore ESG, Klapper W, Kontny U, Pillon M, Taj M, Turner SD, Uyttebroeck A, Woessmann W, Mellgren K. Rare non-Hodgkin lymphoma of childhood and adolescence: A consensus diagnostic and therapeutic approach to pediatric-type follicular lymphoma, marginal zone lymphoma, and nonanaplastic peripheral T-cell lymphoma. Pediatr Blood Cancer. 2020;67:e28416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Agostinelli C, Akarca AU, Ramsay A, Rizvi H, Rodriguez-Justo M, Pomplun S, Proctor I, Sabattini E, Linch D, Daw S, Pittaluga S, Pileri SA, Jaffe ES, Quintanilla-Martinez L, Marafioti T. Novel markers in pediatric-type follicular lymphoma. Virchows Arch. 2019;475:771-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Sun S, Zhang B, Yang H. An unusual case of pediatric-type follicular lymphoma: A case report. Medicine (Baltimore). 2019;98:e17567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ. Diffuse large B-cell lymphoma variants: an update. Pathology. 2020;52:53-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Zhou Y, Shi YK. [Progress in the research of gene mutations in follicular lymphoma]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:172-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Schmidt J, Ramis-Zaldivar JE, Nadeu F, Gonzalez-Farre B, Navarro A, Egan C, Montes-Mojarro IA, Marafioti T, Cabeçadas J, van der Walt J, Dojcinov S, Rosenwald A, Ott G, Bonzheim I, Fend F, Campo E, Jaffe ES, Salaverria I, Quintanilla-Martinez L. Mutations of MAP2K1 are frequent in pediatric-type follicular lymphoma and result in ERK pathway activation. Blood. 2017;130:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Chapman JR, Alvarez JP, White K, Sanchez S, Khanlari M, Algashaamy K, Cassidy D, Peng JH, Fan YS, Alencar A, Alderuccio JP, Lossos IS, Vega F. Unusual Variants of Follicular Lymphoma: Case-based Review. Am J Surg Pathol. 2020;44:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Bai DY, Xie JL, Zheng YY, Zhang YL, Ma DY, Zhou XG. [Paediatric nodal marginal zone lymphoma: a clinicopathological study of seven cases]. Zhonghua Bing Li Xue Za Zhi. 2019;48:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |