Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9535
Peer-review started: January 21, 2021
First decision: February 11, 2021
Revised: February 24, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 6, 2021
Processing time: 281 Days and 5.6 Hours
Adenoid cystic carcinoma (ACC) occurs mainly in the head and neck. Tracheal ACC (TACC) is uncommon. Primary resection is recommended as the main treatment of choice, and radiotherapy is considered for residual disease in the postoperative setting. Definitive radiotherapy is an alternative approach to cure unresectable TACC. As the status of radiosensitivity in TACC is uncertain, the evidence for radiotherapy in unresectable TACC is not well established, especially in terms of the optimal dosage and its response evaluation. Herein, we report a case of locally advanced TACC.
A 49-year-old woman was diagnosed with TACC, which included a range of lesions arising in the upper trachea extending caudally 2 cm to 7 cm of the glottis. She was treated with definitive radiotherapy, given the low likelihood of complete resection of the disease. Due to the indolent growth and the propensity for infiltration along the airways, the scheduled radiation dose of 76 Gy in 38 fractions with 6-MV X-ray delivered by intensity-modulated radiotherapy was conducted to the primary tumor volume. After irradiation of 40 Gy, the patient’s dyspnea on exertion was dramatically relieved and bronchoscopy revealed that the previous large polypoid intra-luminal mass was significantly eliminated, with near-complete response. The patient completed two phases of scheduled radiotherapy, and acute reactions to treatment included subjective chest tightness and grade 2 esophagitis, managed medically. After 5 years of treatment, the patient is alive without recurrent disease, and there were no serious late radiation esophagus and lung damage, with only slight dysphagia without perforation and fistula.
Taken together, TACC is uncommon and the treatment of unresectable TACC is challenging. This case indicated that patients with unresectable TACC who rapidly respond to radiation may benefit from primary radical radiotherapy. Radiotherapy may be considered an effective alternative treatment modality.
Core Tip: Tracheal adenoid cystic carcinoma (TACC) is uncommon. Primary resection is recommended as the main treatment of choice, and radiotherapy is considered for residual disease in the postoperative setting. Definitive radiotherapy is an alternative approach to cure unresectable TACC. As the status of radiosensitivity in TACC is uncertain, the evidence for radiotherapy in unresectable TACC is not well established, especially in terms of the optimal dosage and its response evaluation. Herein, we report a case of locally advanced TACC who rapidly responded to radiotherapy.
- Citation: Wu Q, Xu F. Rapid response to radiotherapy in unresectable tracheal adenoid cystic carcinoma: A case report. World J Clin Cases 2021; 9(31): 9535-9541
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9535.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9535
Adenoid cystic carcinoma (ACC) is a rare tumor, which is most commonly found in salivary glands of the head and neck[1]. Tracheal ACC (TACC), originating from the submucosal glands of the trachea, is much less common, accounting for approximately 10%-20% of malignant tracheal tumors[2]. The standard treatment of TACC includes primary resection and postoperative radiotherapy for residual disease. Most patients, however, present with locally advanced disease, due to the slow and indolent growth of the tumor and the propensity for invasion into adjacent structures[3]. Primary radiotherapy is the main approach for unresectable TACC. As ACC is prone to indolent growth and the radiosensitivity status is uncertain, most tumors respond poorly to radiotherapy and local progression often occurs shortly after completion of treatment. Herein, we present a case of inoperable TACC, who rapidly responded to radiotherapy. This study was approved by the ethical review committee of West China Hospital of Sichuan University.
A 49-year-old woman presented with increasing dyspnea on exertion for 3 wk, occasionally accompanied by chest pain and cough.
No special history of past illness.
Physical examination was notable for stridor at rest.
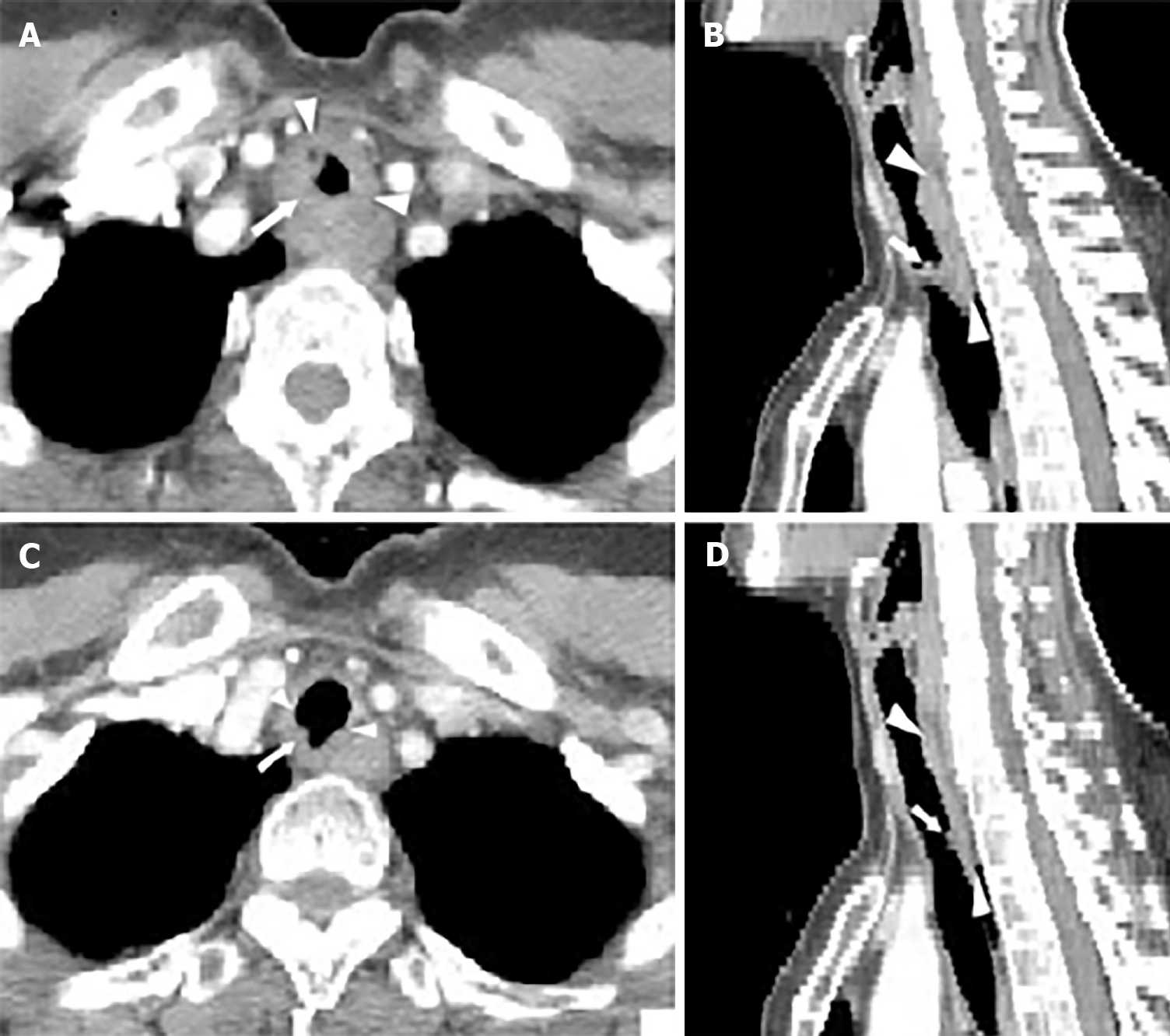
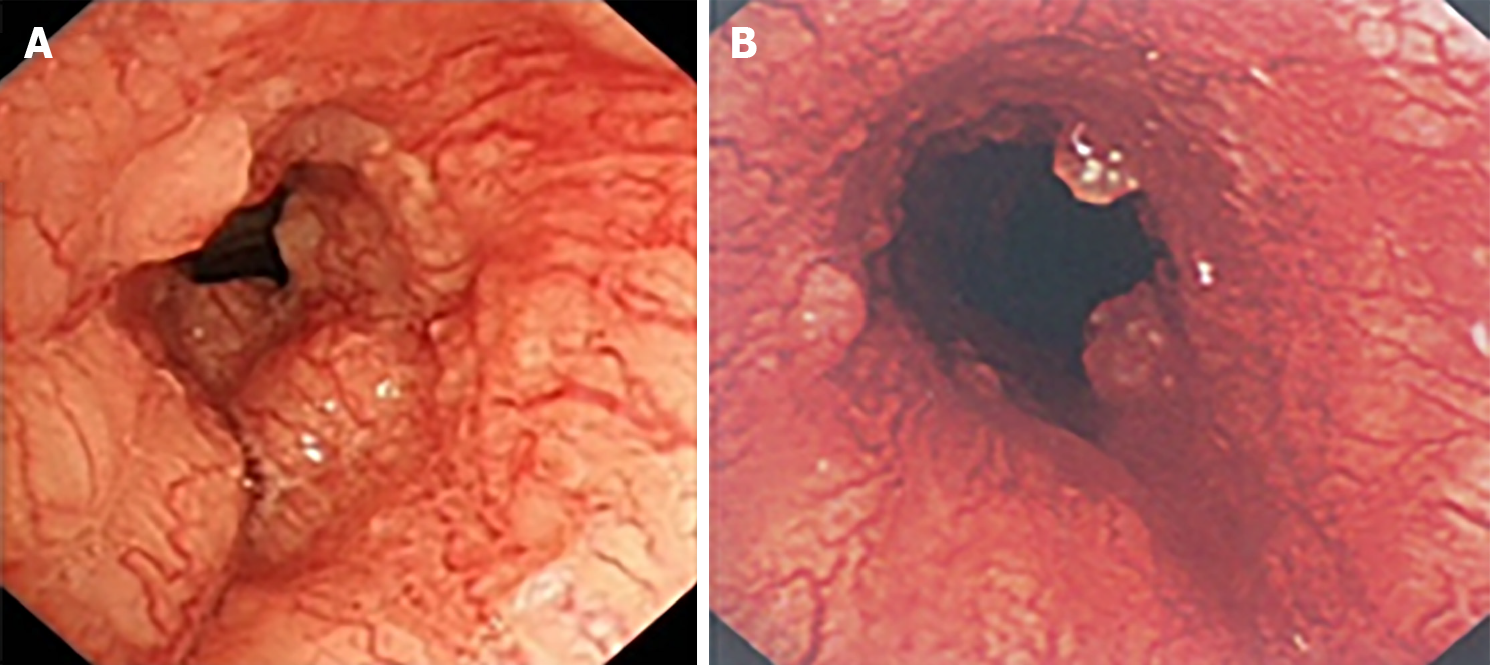
After she was admitted to the local hospital in March 2015, a chest computed tomography (CT) scan showed a soft tissue mass surrounding the inner surface of the upper trachea, leading to irregular lumen stenosis of the trachea (Figure 1A and B). Thus, she was referred to our hospital, in order to initially undergo resection, which is considered the most effective treatment for tracheal tumor. The patient experienced stridor at rest. Bronchoscopy revealed that a large polypoid intra-luminal mass, with its surface presenting rich vascular networks and nodular protrusions, was present in the upper trachea extending caudally 2 cm to 7 cm of the glottis (Figure 2A).
Bronchoscopic biopsy confirmed an ACC, with immunohistochemistry of AE1/AE3 (+), CEA (-), GFAP (-), S-100 (+).
Radioisotope bone scans, brain magnetic resonance imaging, and abdominal CT did not reveal enlarged lymph nodes or metastatic disease. After a discussion of this case in our daily multidisciplinary tumor board, she was treated with definitive radiotherapy given the low likelihood of complete resection of disease.
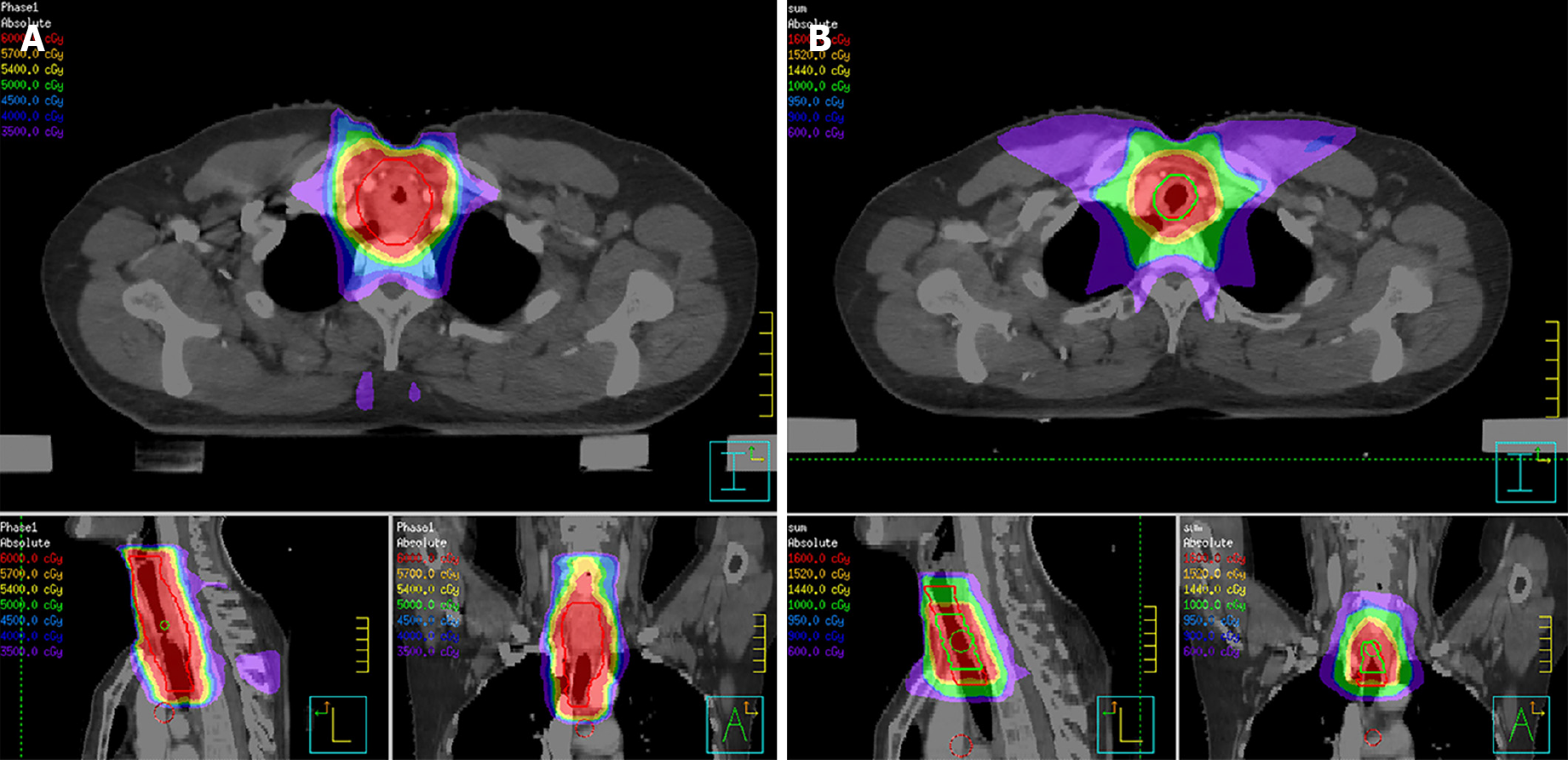
According to the suggestion from the QUANTEC (quantitative analysis of normal tissue effects in the clinic) report[4], the total irradiated dose in our case did not exceed 80 Gy in order to reduce the risk of central airway stenosis. In view of large volume lesions, the therapeutic regimen, which was planned to deliver 76 Gy to the gross tumor volume (GTV) with an additional margin to account for microscopic extension of disease, respiratory motion, and daily variance in patient positioning, was divided into two phases. In the first treatment phase, she received 60 Gy in 30 fractions, using 6-MV X-ray delivered by an intensity-modulated radiotherapy (IMRT) technique. Astonishingly, the patient’s dyspnea on exertion was dramatically relieved after 40 Gy of irradiation. Bronchoscopy was conducted which revealed that the previous large polypoid intra-luminal mass was significantly eliminated and the narrow trachea was freed (Figure 2B). However, considering the probable low inherent radiosensitivity of ACC, a prophylactic dose of 60 Gy to the low-risk clinical target volume (CTVlow), referring to the region for low-risk lymphatic metastasis, was delivered as planned. In the second CT scan of radiotherapy simulation, there was evident regression of the tumor with increased luminal opening of the trachea (Figure 1C and D). The patient received an additional 10 Gy for the high-risk clinical target volume (CTVhigh), referring to the region for high-risk lymphatic metastasis, and an another 6 Gy for the GTV at 2 Gy/d in the second phase of radiotherapy with 6-MV X-ray delivered by IMRT (Figure 3). Thus, the GTV received the prescribed dosage of 76 Gy per 2 Gy in total. The radiotherapy plan beam arrangement consisted of anterior, left, and right anterior oblique, posterior, and left and right posterior oblique fields. The weights of the three posterior fields were reduced compared to the others, in order to minimize the irradiated dose and volume to the esophagus and spinal cord. Due to longitudinal invasion along the trachea, margins of 4-5 cm in the craniocaudal axis were included as suggested by Kaminski[5]. Considering the high-risk of local-regional recurrence, the CTVhigh encompassed the entire GTV with a 2-cm margin of adjacent superior and inferior structures, and with a 0.5-cm margin of adjacent anterior, posterior, and bilateral structures. Given the tumor’s propensity for invasion into local nerves, the CTVlow covered the entire CTVhigh with a 3-cm margin of adjacent superior and inferior structures, and with a 0.5-cm margin of adjacent anterior, posterior and bilateral structures (Figure 3).
The patient successfully completed the scheduled two phases of radiotherapy, and acute reactions to treatment included subjective chest tightness and grade 2 esophagitis, managed medically. After 5 years of treatment, the patient is alive without recurrent disease, and no serious late radiation esophagus and lung damage, only slight dysphagia without perforation and fistula.
ACC, formerly known as “cylindroma” and “adenocystic carcinoma,” occurs mainly in the head and neck. However, its presence in the pulmonary system is uncommon. Most tumors occur in the trachea and mainstem bronchi, but peripheral tumors from glands of small bronchi have occasionally been reported[6-8]. Women are more often affected than men, and the peak age of incidence occurs between the 4th and 5th decades of life, which is earlier than lung cancer[9,10]. Smoking does not affect the incidence. ACC is well known for its prolonged clinical course, its propensity for peritracheal soft tissue and perineural invasion, and delayed onset of distant metastases[3]. The most common site of distant metastasis is the lung, followed by bone, liver, and brain[11].
Owing to its slow and indolent growth, the clinical course of TACC is prolonged and asymptomatic. When the tumor invades local nerves and structures, patients will present varying symptoms, which are related to airway obstruction. Cough, dyspnea, and hemoptysis are the most common complaints. However, those symptoms often lead to a misdiagnosis of asthma or bronchitis. Chest CT and bronchoscopy are the main diagnostic methods for the diagnosis of TACC. On CT, this tumor usually has a marked tendency toward submucosal extension that manifests as an intraluminal mass with longitudinal extension[12], as seen in our case.
Once diagnosed, the tumor should be assessed for surgical resection. Several relatively large studies showed that surgery can lead to a significantly better survival than non-surgical management. Two large studies[13,14] reported that the overall 5-year and 10-year survival rates of resected TACC, regardless of complete or incomplete resection, were 52%-88.7% and 29%-43.2%, compared with 33% and 10% in the non-resected group, respectively. Compared with incomplete resection, complete resection with negative airway margins resulted in higher survival with 5-year and 10-year survival rates of 75%-90% and 45%-75%, respectively. It was noted that the incomplete resection of TACC was quite high, with 59%-84.4% R1 resection rates, probably because it tends to infiltrate along the airways and invade peritracheal soft tissue and perineural tissue. However, the 15-year long-term follow-up results showed that surgery still benefits patients with incomplete resected TACC, compared with the unresectable group (15-year overall survival: 14.5% vs 0%)[13]. For TACC patients with incomplete resection (R1), postoperative radiotherapy significantly reduces local-regional failure and improves overall survival. This evidence suggests that a pathologically negative margin might not always be required, and adjuvant radiotherapy is needed in the postoperative setting in such cases. The benefit of adjuvant radiotherapy in R0 resection remains uncertain.
If patients have technically or medically inoperable tumors, radiotherapy should be considered as an alternative local treatment. Nevertheless, the optimal dosage of radical radiotherapy for TACC is not well known, due to the uncertain status of radiosensitivity in TACC. ACC is deemed to be a relatively radioresistant tumor, due to its indolent growth and low inherent radiosensitivity. Lee et al[2] assessed the efficacy of irradiation in patients with unresectable TACC, after immediately completing primary radiotherapy. The results showed that among 13 inoperable patients treated with primary radiation of 60 Gy, the objective response rate was only 1/13 (one had a partial response), while four had stable disease, and eight had progressive disease. A Chinese study[9] revealed that two patients with locally advanced disease and invasion of the pulmonary artery received primary radio
TACC is uncommon and the treatment of unresectable TACC is challenging. This case indicates that patients with unresectable TACC who rapidly respond to radiation may persistently benefit from primary radical radiotherapy. Radiotherapy may be considered an effective alternative treatment modality. Future research in the form of more case reports, case series, or small-scale clinical trials could add evidence on this phenomenon.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arias F, Dubinsky P S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wu RR
| 1. | Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Lee JH, Jung EJ, Jeon K, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ, Shim YM, Kim J, Han J, Um SW. Treatment outcomes of patients with adenoid cystic carcinoma of the airway. Lung Cancer. 2011;72:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med. 2011;135:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK, Jackson A. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70-S76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 793] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 5. | Kaminski JM, Langer CJ, Movsas B. The role of radiation therapy and chemotherapy in the management of airway tumors other than small-cell carcinoma and non-small-cell carcinoma. Chest Surg Clin N Am. 2003;13:149-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gallagher CG, Stark R, Teskey J, Kryger M. Atypical manifestations of pulmonary adenoid cystic carcinoma. Br J Dis Chest. 1986;80:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Vallonthaiel AG, Jain D, Singh V, Kaur K, Madan K, Kumar V, Iyer VK, Sharma MC. c-Myb Overexpression in Cytology Smears of Tracheobronchial and Pulmonary Adenoid Cystic Carcinomas. Acta Cytol. 2017;61:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Shinohara S, Hanagiri T, Takenaka M, Oka S, Chikaishi Y, Shimokawa H, Nakagawa M, Uramoto H, So T, Tanaka F. Primary Adenoid Cystic Carcinoma of the Peripheral Lungs. J UOEH. 2015;37:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Hu MM, Hu Y, He JB, Li BL. Primary adenoid cystic carcinoma of the lung: Clinicopathological features, treatment and results. Oncol Lett. 2015;9:1475-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Chen F, Huang M, Xu Y, Li T, Xie K, Zhang L, Cheng D, Liu L, Che G, Hou M, Wang J, Luo Z, Lu Y. Primary tracheal adenoid cystic carcinoma: adjuvant treatment outcome. Int J Clin Oncol. 2015;20:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Maziak DE, Todd TR, Keshavjee SH, Winton TL, Van Nostrand P, Pearson FG. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg. 1996;112:1522-31; discussion 1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 171] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Kwak SH, Lee KS, Chung MJ, Jeong YJ, Kim GY, Kwon OJ. Adenoid cystic carcinoma of the airways: helical CT and histopathologic correlation. AJR Am J Roentgenol. 2004;183:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Gaissert HA, Grillo HC, Shadmehr MB, Wright CD, Gokhale M, Wain JC, Mathisen DJ. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78:1889-96; discussion 1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Yang H, Yao F, Tantai J, Zhao Y, Tan Q, Zhao H. Resected Tracheal Adenoid Cystic Carcinoma: Improvements in Outcome at a Single Institution. Ann Thorac Surg. 2016;101:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Bhandari V, Ladia DD, Kausar M, Varma A. Definitive radiotherapy for inoperable adenoid cystic carcinoma of the trachea: A rare case report. Lung India. 2017;34:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Kanematsu T, Yohena T, Uehara T, Ushijima C, Asoh H, Yoshino I, Ichinose Y. Treatment outcome of resected and nonresected primary adenoid cystic carcinoma of the lung. Ann Thorac Cardiovasc Surg. 2002;8:74-77. [PubMed] |
| 17. | Bonner Millar LP, Stripp D, Cooper JD, Both S, James P, Rengan R. Definitive radiotherapy for unresected adenoid cystic carcinoma of the trachea. Chest. 2012;141:1323-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Eom JS, Kim B, Kim H, Jeon K, Um SW, Koh WJ, Suh GY, Chung MP, Kwon OJ. Fibrotic airway stenosis following radiotherapy in patients with adenoid cystic carcinoma. Respirology. 2014;19:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bittner N, Koh WJ, Laramore GE, Patel S, Mulligan MS, Douglas JG. Treatment of locally advanced adenoid cystic carcinoma of the trachea with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |