Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9440
Peer-review started: March 15, 2021
First decision: April 4, 2021
Revised: April 15, 2021
Accepted: September 29, 2021
Article in press: September 29, 2021
Published online: November 6, 2021
Processing time: 228 Days and 8.7 Hours
The identification of risk factors for recurrence in patients with minor ischemic stroke (MIS) is a critical medical need.
To develop a nomogram for individualized prediction of in-hospital recurrence in MIS patients.
Based on retrospective collection, a single-center study was conducted at the First Affiliated Hospital of Anhui Medical University from January 2014 to December 2019. Univariate and multivariate logistic regression analyses were used to determine the risk factors associated with MIS recurrence. The least absolute shrinkage and selection operator regression was performed for preliminary identification of potential risk factors. Uric acid, systolic blood pressure, serum total bilirubin (STBL), and ferritin were integrated for nomogram construction. The predictive accuracy and calibration of the nomogram model were assessed by the area under the receiver operating characteristic curve (AUC-ROC) and Hosmer-Lemeshow test, respectively.
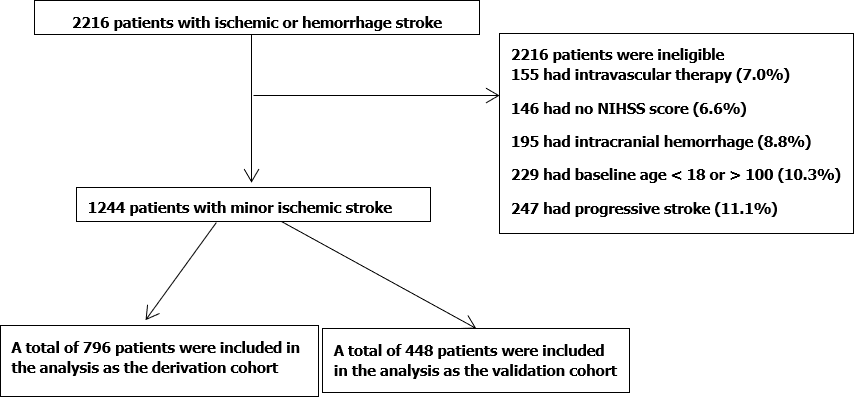
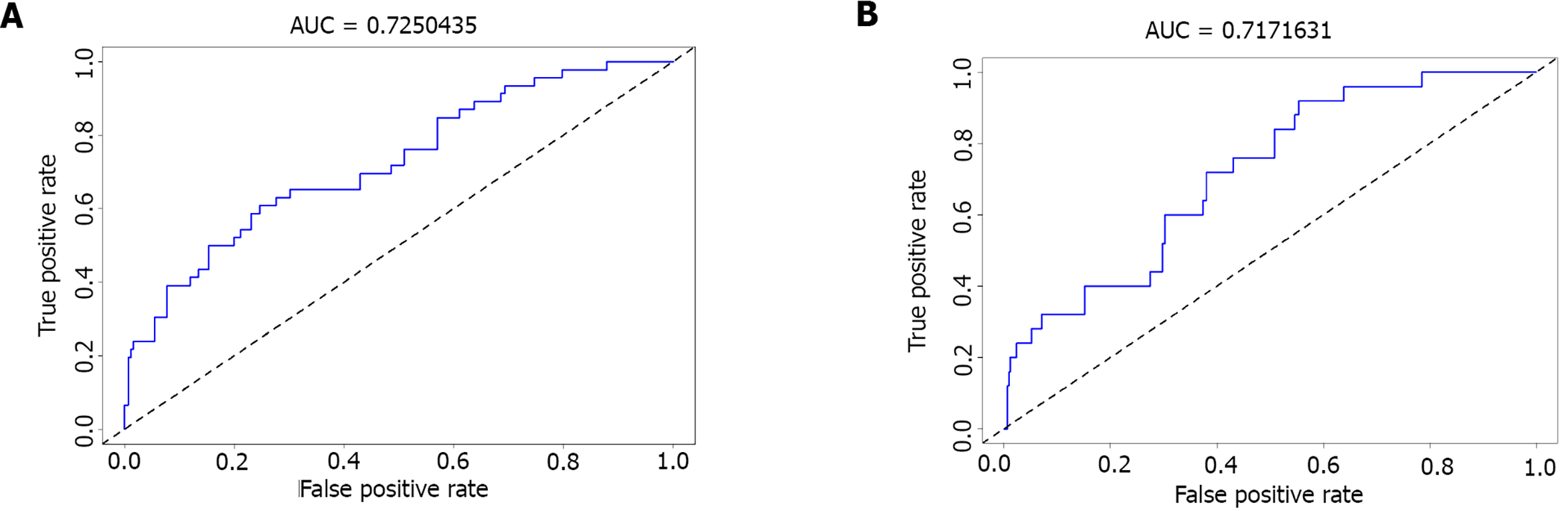
A total of 2216 MIS patients were screened. Among them, 155 were excluded for intravascular therapy, 146 for unknown National Institutes of Health Stroke Scale score, 195 for intracranial hemorrhage, and 247 for progressive stroke. Finally, 1244 patients were subjected to further analysis and divided into a training set (n = 796) and a validation set (n = 448). Multivariate logistic regression analysis revealed that uric acid [odds ratio (OR): 0.997, 95% confidence interval (CI): 0.993-0.999], ferritin (OR: 1.004, 95%CI: 1.002-1.006), and STBL (OR: 0.973, 95%CI: 0.956-0.990) were independently associated with in-hospital recurrence in MIS patients. Our model showed good discrimination; the AUC-ROC value was 0.725 (95%CI: 0.646-0.804) in the training set and 0.717 (95%CI: 0.580-0.785) in the validation set. Moreover, the calibration between nomogram prediction and the actual observation showed good consistency. Hosmer-Lemeshow test results confirmed that the nomogram was well-calibrated (P = 0.850).
Our present findings suggest that the nomogram may provide individualized prediction of recurrence in MIS patients.
Core tip: The identification of risk factors for recurrence in patients with minor ischemic stroke (MIS) is a critical medical need. Based on retrospective collection, a single-center study was conducted at the First Affiliated Hospital of Anhui Medical University from January 2014 to December 2019. Univariate and multivariate logistic regression analyses were used to determine the risk factors associated with MIS recurrence. The developed nomogram may provide individualized prediction of recurrence of MIS inpatients.
- Citation: Yu XF, Yin WW, Huang CJ, Yuan X, Xia Y, Zhang W, Zhou X, Sun ZW. Risk factors for relapse and nomogram for relapse probability prediction in patients with minor ischemic stroke. World J Clin Cases 2021; 9(31): 9440-9451
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9440.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9440
Stroke is the third leading cause of disability and the second leading cause of death in the world[1]. Minor ischemic stroke (MIS) refers to ischemic stroke with minor symptoms and mild neurological impairment; one out of every three patients with acute ischemic cerebrovascular disease is with MIS[2]. While the MIS symptoms are mild, its prognosis is not optimistic. Studies have shown that the recurrence rates of cerebral infarction 7 d and 1 mo after onset are as high as 12% and 15%[3,4]. With the extension of life expectancy, the number of patients with disabilities caused by MIS is expected to rise in the future. Therefore, predicting the possibility for MIS recurrence is critically important.
Previous studies have established that the D-dimer level predicts a poor outcome in MIS patients[5]. Furthermore, moderate-to-vigorous physical activity and visceral fat level were reported to be risk factors for recurrent ischemic stroke[6]. In addition, Wang et al[7] found that metabolic syndrome is a strong risk factor for MIS occurrence and subsequent vascular events. In another study, Vermeer et al[8] suggested that impaired glucose tolerance increased the risk for the development of MIS in nondiabetic patients. Moreover, age, heart disease, and infarction diameter could be used as assessment indicators for prognosis in MIS patients[9]. Although the ABCD2 score has been shown to be a useful tool for predicting short-term and long-term risk of stroke after MIS[10], no easy-to-use tool has been developed to visualize the predicted probability in the prognosis of MIS recurrence.
A nomogram is a statistical graphic visualization tool used to calculate the continuous probability of a specific outcome of a single patient. It can provide an estimated numerical prognosis. Moreover, it can combine different data, forming a continuous scoring system for the prediction of the risk of individuals. For example, Cheng et al[11] developed a nomogram to predict early isolated deep vein thrombosis in acute ischemic stroke patients. In addition, Cappellari et al[12] used a nomogram to predict unfavorable outcomes in patients receiving oral anticoagulants for atrial fibrillation after stroke. Therefore, nomogram has been used as a risk stratification tool in routine clinical practice, including the treatment and prognosis of cancer and cardiovascular diseases[13,14]. However, a nomogram predicting the probability of recurrence of stroke after MIS has not yet been developed.
Therefore, the present study aimed to develop a nomogram for the prediction of the possibility of stroke reoccurrence in MIS patients.
This retrospective study was conducted at the Stroke Center of the Department of Neurology of the First Affiliated Hospital of Anhui Medical University. MIS was diagnosed based on the Guidelines for the Diagnosis and Treatment of High-risk Non-disabling Ischemic Cerebral Artery Events compiled by the Chinese Stroke Association in 2016[15]. The following inclusion criteria were applied: (1) Age ≥ 18 years but ≤ 100 years; (2) Being diagnosed as having MIS; and (3) MIS patients not receiving endovascular therapy such as thrombolysis and stenting and not having signs of intracranial hemorrhage, progressive stroke, and unknown National Institutes of Health Stroke Scale (NIHSS). The study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. As it was a retrospective study, written informed consent was waived.
Data including age, sex, information on hypertension, diabetes, previous heart disease, fasting plasma glucose (FPG), uric acid (UA), blood urea nitrogen (BUN), homocysteine (Hcy), C-reactive protein (CRP), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDLC), high-density lipoprotein cholesterol (HDLC), stable plaques, vulnerable plaque, apolipoprotein A (APOA), apolipoprotein B (APOB), creatinine (Cr), serum total bilirubin (STBL), ferritin, glycosylated hemoglobin (GHb), smoking, alcohol drinking, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were collected and extracted from the medical chart. All patients were examined using magnetic resonance imaging (MRI) to determine whether there was recurrence or aggravation. Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. Diabetes was defined as FPG ≥ 7.0 mmol/ L or random glucose ≥ 11.1 mmol/ L.
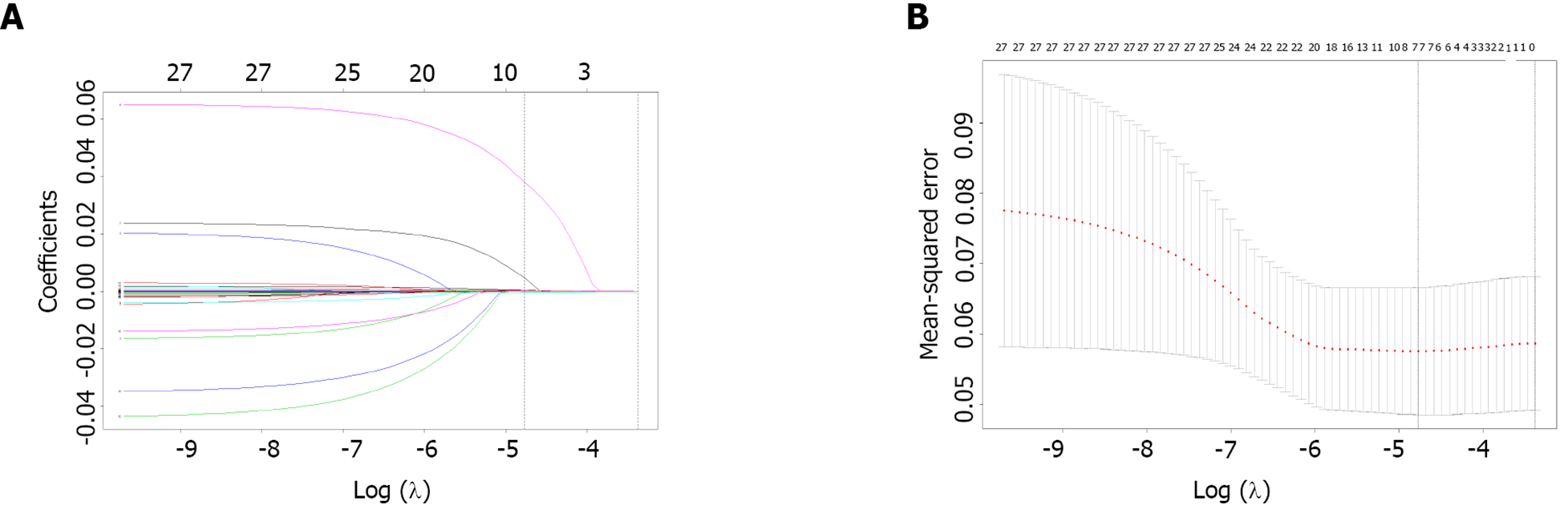
Statistical analyses were performed using R 3.6.2 (https://www.r-project.org/) and STATA 15.0 (Stata Corp., College Station, TX, United States). Descriptive data are presented as medians with interquartile ranges, and categorical variables are expressed as numbers or percentages. Inter-group comparisons of continuous data were made using Mann-Whitney U-test. The Chi-square test was applied for categorical data comparisons. Univariate and multivariate logistic regression analyses were used to determine the risk factors associated with MIS recurrence. In the multivariate logistic regression analysis, variables with P < 0.20 in the univariate regression analysis were included. P < 0.08 was used for nomogram construction in the multivariable logistic regression. The least absolute shrinkage and selection operator (LASSO) regression analysis was implemented for preliminary identification of potential risk factors.
For nomogram construction and validation, the patients were allocated either to a training or a validation cohort. A nomogram was then constructed using the regression coefficient obtained from the multivariable logistic regression model and the “rms” package in R software (version 3.6.1).
The performance of the monogram was evaluated by discrimination (the ability of the proposed model to distinguish patients with different outcomes) and calibration. The accuracy of the nomogram model for predicting the probability of unfavorable outcome was assessed by calculation of the area under the receiver operating characteristic curve (AUC-ROC). The calibration of the risk prediction model was evaluated by a plot comparing the observed probability of an unfavorable outcome against the predicted one and by using the Hosmer-Lemeshow test. A P value less than 0.05 was considered statistically significant.
From January 2014 to December 2019, a total of 2216 MIS patients were screened. Among them, 155 were excluded for intravascular therapy, 146 for unknown NIHSS score, 424 for intracranial hemorrhage, and 247 for progressive stroke. As a result, finally, 1244 patients remained for further analysis. The derivation cohort contained 796 MIS patients, whereas 448 were included in the validation cohort (Figure 1). Their demographic and laboratory data are listed in Table 1. Among the included clinical features, demographic and laboratory data were reduced to seven potential predictors based on the information of patients in the training cohort (Figure 2). These features were nonzero coefficients that were used in the logistic regression model.
| Derivation cohort (n = 796) | Validation cohort (n = 448) | ||||||
| Favorable outcome | Unfavorable outcome | P | Favorable outcome | Unfavorable outcome | P | ||
| n = 750 | n = 46 | n = 423 | n = 25 | ||||
| Age, median (IQR) | 63 (52, 71) | 64.5 (59, 72) | 0.26 | 63 (52, 70) | 69 (62, 75) | 0.03 | |
| Sex | 0.87 | 0.01 | |||||
| Female | 217 (28.9%) | 14 (30.4%) | 132 (31.2%) | 2 (8.0%) | |||
| Male | 533 (71.1%) | 32 (69.6%) | 291 (68.8%) | 23 (92.0%) | |||
| Hypertension | 469 (62.5%) | 26 (56.5%) | 0.44 | 261 (61.7%) | 17 (68.0%) | 0.67 | |
| Diabetes | 229 (30.5%) | 18 (39.1%) | 0.25 | 99 (23.4%) | 3 (12.0%) | 0.23 | |
| FPG, median (IQR) | 5.6 (5, 7.33) | 5.915 (4.66, 7.22) | 0.67 | 5.41 (4.89, 6.65) | 4.85 (4.57, 5.45) | 0.02 | |
| Cardiopathy | 59 (7.9%) | 7 (15.2%) | 0.09 | 40 (9.5%) | 7 (28.0%) | < 0.01 | |
| Smoking | 188 (25.1%) | 15 (32.6%) | 0.29 | 100 (23.6%) | 11 (44.0%) | 0.03 | |
| UA, median (IQR) | 298 (243, 359) | 280 (179, 332) | 0.04 | 306 (238, 364) | 298 (179, 354) | 0.61 | |
| UN, median (IQR) | 5.3 (4.48, 6.54) | 4.82 (3.81, 6.36) | 0.11 | 5.5 (4.38, 6.98) | 5.8 (3.81, 6.3) | 0.34 | |
| Hcy, median (IQR) | 13.96 (11.07, 19.03) | 13.56 (10.01, 18.4) | 0.33 | 14.04 (10.53, 17.6) | 16.43 (10.01, 26.82) | 0.18 | |
| CRP, median (IQR) | 1.415 (0.51, 6.13) | 1.325 (0.6, 3.9) | 0.58 | 1.62 (0.56, 4.7) | 1.56 (0.89, 3.57) | 0.94 | |
| TC, median (IQR) | 4.435 (3.84, 5.2) | 4.89 (3.96, 5.36) | 0.08 | 4.4 (3.89, 5.04) | 5.02 (3.49, 5.75) | 0.16 | |
| TG, median (IQR) | 1.52 (1.08, 2.12) | 1.72 (1.32, 2.45) | 0.09 | 1.47 (1.06, 2.05) | 1.38 (0.89, 1.86) | 0.46 | |
| LDLC, median (IQR) | 2.615 (2.04, 3.25) | 3.15 (2.34, 3.52) | 0.03 | 2.57 (2.14, 3.19) | 3.26 (2.3, 3.71) | 0.04 | |
| Stable plaques | 288 (38.4%) | 18 (39.1%) | 1.00 | 147 (34.8%) | 6 (24.0%) | 0.39 | |
| Vulnerable plaque | 400 (53.3%) | 21 (45.7%) | 0.36 | 217 (51.3%) | 10 (40.0%) | 0.31 | |
| Alcohol drinking | 256 (34.1%) | 18 (39.1%) | 0.52 | 131 (31.0%) | 11 (44.0%) | 0.19 | |
| HDLC, median (IQR) | 1.1 (0.94, 1.36) | 1.145 (0.9, 1.28) | 0.56 | 1.12 (0.95, 1.37) | 1.14 (1.05, 1.46) | 0.47 | |
| APOA, median (IQR) | 1.35 (1.21, 1.55) | 1.415 (1.19, 1.58) | 0.56 | 1.35 (1.22, 1.54) | 1.46 (1.19, 1.53) | 0.47 | |
| APOB, median (IQR) | 0.925 (0.73, 1.09) | 1.1 (0.84, 1.22) | < 0.01 | 0.91 (0.73, 1.08) | 1 (0.75, 1.16) | 0.46 | |
| LP(a), median (IQR) | 208.5 (112, 340) | 173.5 (124, 371) | 0.78 | 187 (106, 329) | 151 (129, 371) | 0.99 | |
| Cr, median (IQR) | 55.65 (14.72, 72.1) | 65.1 (48.6, 80) | < 0.01 | 55.9 (14.7, 69.8) | 70.2 (48.8, 78.3) | 0.01 | |
| STBL, median (IQR) | 17.985 (11.7, 58) | 15.11 (11.5, 19.03) | 0.02 | 17.23 (11.7, 60.2) | 14.86 (11.5, 17.3) | 0.19 | |
| Ferritin, median (IQR) | 220.85 (136.59, 324.3) | 255.57 (218.1, 426.1) | < 0.01 | 207.18 (131.2, 318.78) | 252.2 (191.83, 350.6) | 0.09 | |
| GHb, median (IQR) | 6.66 (5.82, 7.94) | 7.18 (6.3, 9.09) | 0.14 | 5.82 (4.68, 7.4) | 5.91 (4.53, 6.72) | 0.33 | |
| SBP, median (IQR) | 142 (130, 158) | 147 (139, 163) | 0.08 | 140 (129, 155) | 140 (120, 154) | 0.49 | |
| DBP, median (IQR) | 82 (75, 92) | 84.5 (80, 90) | 0.48 | 82 (75, 91) | 89 (81, 90) | 0.28 | |
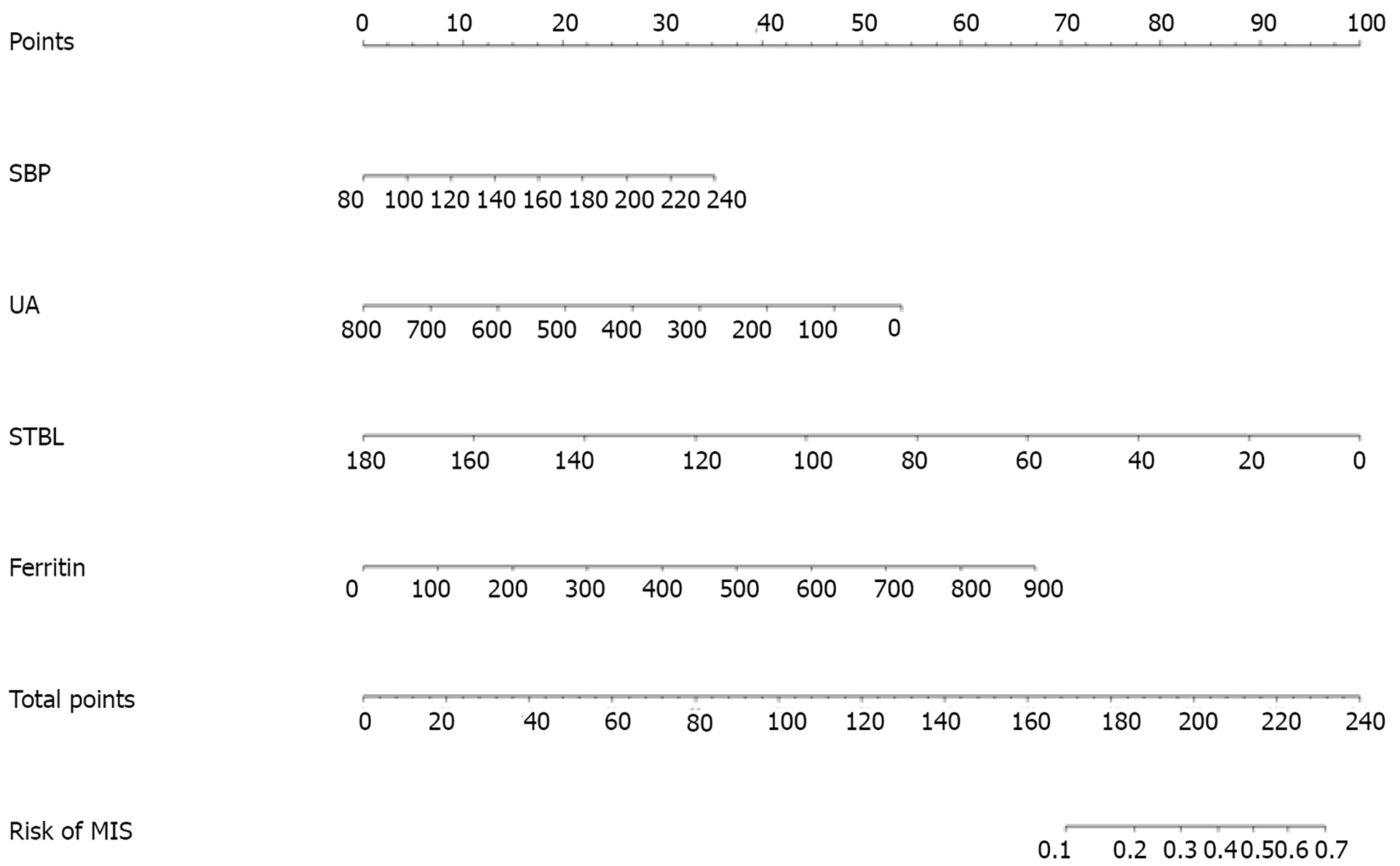
The predictive variables screened by LASSO regression are highly consistent with those by the stepwise regression method. As can be seen in Table 2, UA [odds ratio (OR): 0.997, 95%CI: 0.993-0.999], ferritin (OR: 1.004, 95%CI: 1.002-1.006), STBL (OR: 0.973, 95%CI: 0.956-0.990) were independently associated with in-hospital recurrence in MIS patients. In addition, SBP (OR: 1.012, 95%CI: 0.999-1.025) was moderately associated with the recurrence of MIS. The result of logistic prediction model was: Log [p(x)/1-p(x) = -4.927 - (0.003 × UA) - (0.027 × STBL) + (0.004 × ferritin) + (0.012 × SBP)], where p(x) is the probability of recurrence in MIS patients during hospitalization.
| Univariate analysis | Multivariate analysis | |||||
| OR | 95%CI | P | OR | 95%CI | P | |
| Age | 1.027 | 0.994-1.060 | 0.109 | 1.014 | 0.987-1.043 | 0.315 |
| Sex | 0.853 | 0.364-1.998 | 0.714 | – | ||
| Hypertension | 0.633 | 0.311-1.290 | 0.208 | – | ||
| Diabetes | 1.550 | 0.668-3.597 | 0.308 | – | ||
| FPG | 0.896 | 0.741-1.084 | 0.260 | – | ||
| Cardiopathy | 2.087 | 0.803-5.422 | 0.131 | 2.021 | 0.816-5.003 | 0.128 |
| Smoking | 1.375 | 0.588-3.217 | 0.462 | – | ||
| Uric acid | 0.997 | 0.994-1.001 | 0.179 | 0.997 | 0.993-0.999 | 0.038 |
| UN | 0.877 | 0.715-1.076 | 0.207 | – | ||
| Homocysteine | 0.994 | 0.958-1.031 | 0.751 | – | ||
| CRP | 0.995 | 0.972-1.018 | 0.642 | – | ||
| TC | 0.997 | 0.968-1.027 | 0.833 | – | ||
| TG | 1.000 | 0.896-1.116 | 0.996 | – | ||
| LDLC | 1.037 | 0.878-1.225 | 0.665 | – | ||
| Vulnerable plaque | 0.620 | 0.244-1.576 | 0.315 | – | ||
| Stable plaque | 0.634 | 0.250-1.606 | 0.337 | – | ||
| Alcohol drinking | 0.983 | 0.443-2.181 | 0.967 | – | ||
| HDLC | 0.745 | 0.245-2.265 | 0.604 | – | ||
| APOA | 1.061 | 0.646-1.742 | 0.815 | – | ||
| APOB | 0.981 | 0.750-1.283 | 0.891 | – | ||
| LP a | 1.000 | 0.999-1.001 | 0.929 | – | ||
| Cr | 1.001 | 0.992-1.010 | 0.851 | – | ||
| STBL | 0.975 | 0.956-0.995 | 0.013 | 0.973 | 0.956-0.990 | 0.002 |
| Ferritin | 1.003 | 1.001-1.005 | 0.001 | 1.004 | 1.002-1.006 | < 0.001 |
| GHb | 1.067 | 0.843-1.352 | 0.588 | – | ||
| SBP | 1.012 | 0.995-1.031 | 0.169 | 1.012 | 0.999-1.025 | 0.071 |
| DBP | 1.007 | 0.978-1.037 | 0.633 | – | ||
Based on the multivariate regression analysis, a nomogram incorporating STBL, ferritin, cardiopathy, and SBP was generated, which is presented in Figure 3.
Next, we used ROC curves to evaluate the discrimination ability of the nomogram cohort. As visible in Figures 4A and 4B, the AUC-ROC of the nomogram of the training cohort was 0.737 (95%CI: 0.676-0.798). In the validation cohort, the AUC-ROC value was 0.706 (95%CI: 0.532-0.881).
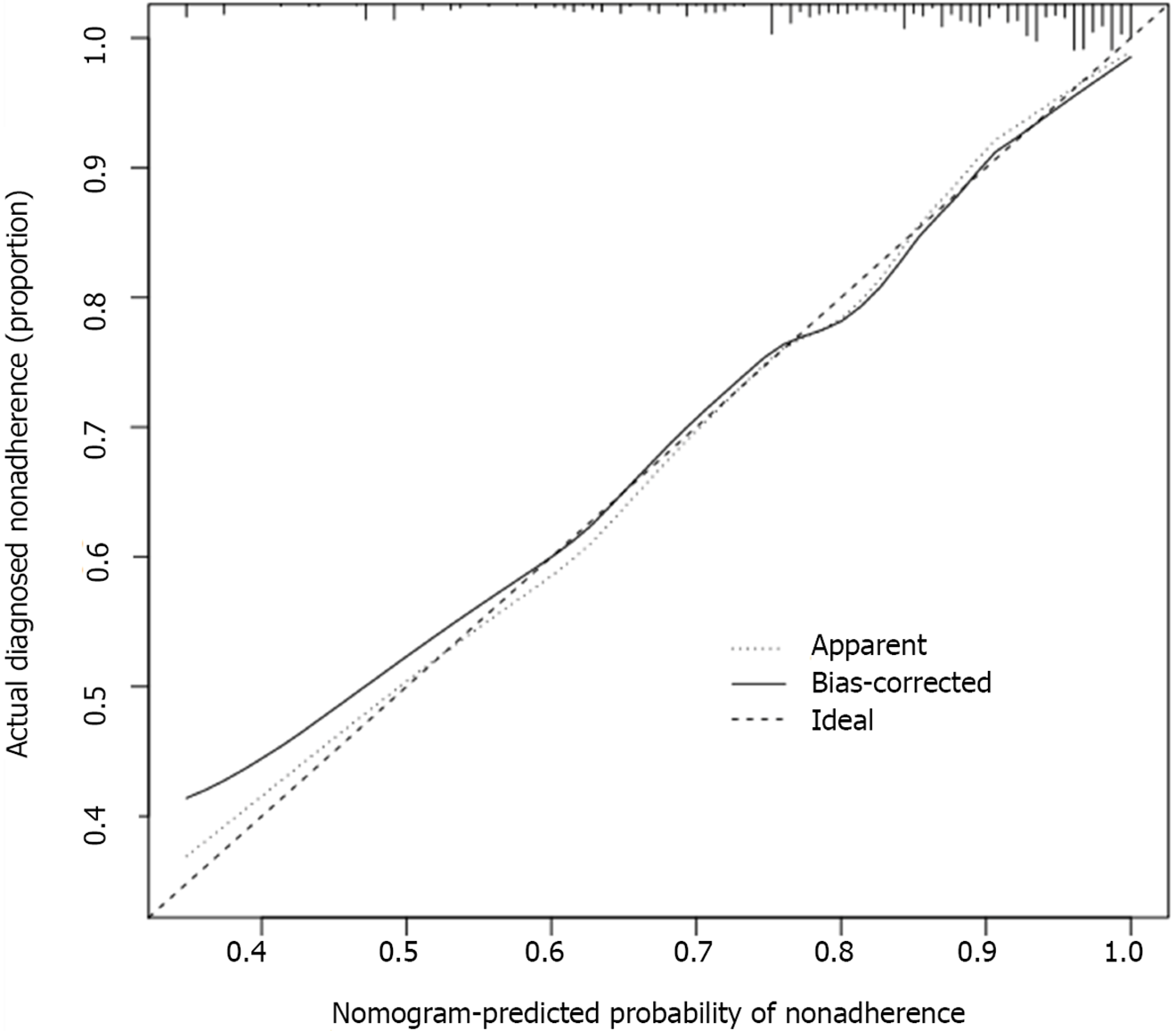
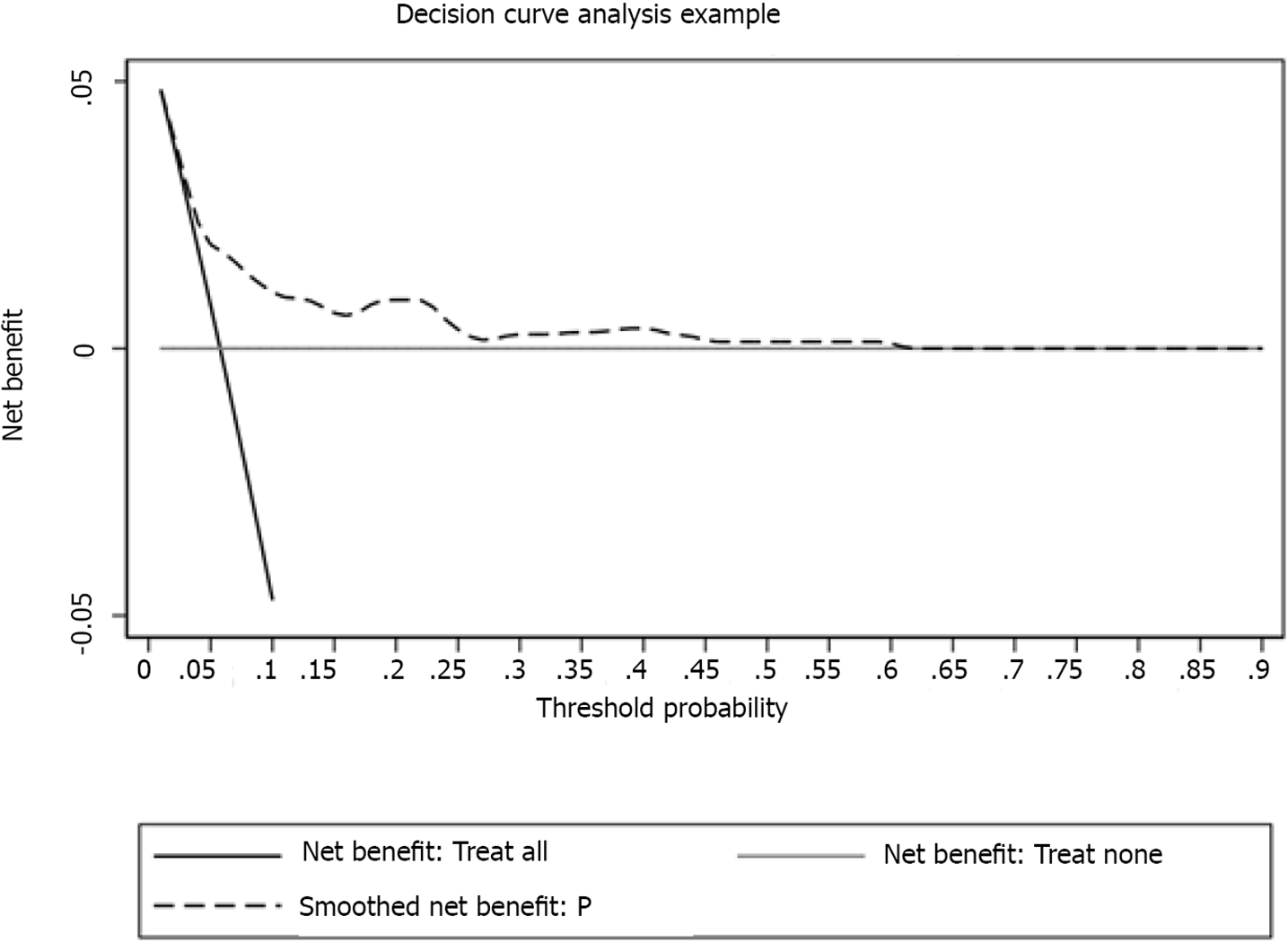
The calibration curve of the nomogram model showed a sufficient consistency between the predicted values calculated by the nomogram and the actual results (Figure 5). Hosmer-Lemeshow goodness-of-fit test revealed that the nomogram was well-calibrated (P = 0.850). The error rate in the confusion matrix of the model in the training cohort was 0.057, and in the validation cohort, it was 0.056 (Table 3). Moreover, decision curve analysis (DCA) was utilized to assess the clinical validity of the nomogram (Figure 6), which showed good calibration.
| Actual outcome | Actual outcome | ||||
| No relapse during hospitalization | Relapse during hospitalization | No relapse during hospitalization | Relapse during hospitalization | ||
| Predicted outcome | No relapse during hospitalization | 750 | 45 | 423 | 25 |
| Relapse during hospitalization | 0 | 1 | 0 | 0 | |
In the present study, we found that UA, STBL, SBP, and ferritin were independently associated with MIS recurrence. We developed and validated a nomogram containing four variable combinations, which could be straightforwardly used to predict the probability of relapse during the hospitalization of MIS patients.
A previous investigation revealed that the level of serum UA is an independent protective factor for cognitive impairment in MIS patients, and a lower level of UA is prone to deteriorate the cognitive function in patients[16]. An animal study showed that apoptosis and brain tissue injury produce generated oxygen species in a rat middle cerebral artery occlusion model, but after the addition of an appropriately high concentration of UA, the degree of brain tissue injury and the production of reactive oxygen species were reduced[17]. Zhang et al[18] established that UA exerted neuroprotective effects in acute ischemic stroke, and a relevant concentration of UA was beneficial to the prognosis of adolescent stroke. Meta-analysis findings support the notion that serum UA level has a protective influence on the prognosis of neurological function after acute ischemic stroke, and a high UA level at onset was a biomarker with better prognosis potential in patients with acute ischemic stroke[19]. Our study showed that there was a negative correlation between a high concentration of UA and recurrence of MIS, which is consistent with these previous findings.
In the present study, we found that ferritin was closely related to the progression of MIS. Previous reports indicated that the increase of ferritin concentration in plasma and cerebrospinal fluid within 24 h after the onset of ischemic stroke was related to the early deterioration of neurological function, and the increase of iron reserve may lead to stroke progression by enhancing the cytotoxic mechanism of cerebral ischemia[20]. Additionally, Davalos et al[21] also showed that the concentration of serum ferritin was associated with the progression of cerebral infarction. In this study, we found a positive correlation between the ferritin level and the recurrence of MIS, which is in agreement with the findings of the aforementioned reports.
The role of total bilirubin as an independent risk factor for MIS was also established[22]. Bilirubin is an antioxidant that can oxidize lipids and lipoproteins and is involved in atherosclerosis prevention. Furthermore, the level of bilirubin was established to be negatively correlated with the level of atherosclerosis, which was closely related to the occurrence of cerebral infarction[23]. Another examination showed that higher levels of total bilirubin were associated with a lower risk of asymptomatic cerebral infarction[24]. These findings suggest that bilirubin exerts protective effects in stroke patients. In the present study, we found that STBL was also a protective factor against MIS recurrence.
Previous research showed that SBP was associated with stroke recurrence[25]. Turana et al[26] also found that SBP was positively correlated with stroke incidence, and adherence to hypertension treatment is to be the main goal in the prevention of stroke occurrence in several countries in Asia. In addition, in a cohort study, Zhuo et al[27] identified SBP as a risk factor for 2-year post-ischemic stroke recurrence prediction. These aforementioned studies suggested that SBP was a risk factor for both stroke onset and recurrence. In the present investigation, although SBP was not significantly correlated with MIS occurrence (P = 0.08) in the multiple regression model, we also included SBP in the prediction model and nomogram construction.
The parameters used for the nomogram construction were derived from clinical practice and could be collected by non-invasive procedures during follow-up. Moreover, the nomogram is easy to use since it does not require imaging results, making it more feasible in neurological disorder prognosis evaluation and treatment. For instance, bland diet, fruit consumption, sleep status, and cigarette cessation were used to generate a nomogram to evaluate the probability of recurrence of large-vessel ischemic stroke[28]. Additionally, age, baseline NIHSS score, collateral circulation, fast blood glucose, and recanalization were combined to predict malignant cerebral edema[29]. In the present study, we validated a nomogram model through the use of a validation cohort and DCA, which showed good fitness. Hence, this easy-to-use nomogram is potentially clinically applicable.
Nevertheless, certain limitations of this study should be acknowledged. First, the data utilized were retrospectively extracted from a single-center registry, which might have introduced information bias, limiting its statistical power. Second, the number of MIS recurrences in both cohorts was small. In addition, the follow-up duration was relatively short. Moreover, an actual set is may be different from the studied cohorts, and thus external verification is necessary.
We have constructed and validated a nomogram for predicting the recurrence of MIS, which is a rapid and clinically easily applicable tool for the evaluation of the outcome in MIS patients. However, prospective multicenter clinical studies are needed to confirm our present findings.
The identification of risk factors for recurrence in patients with minor ischemic stroke (MIS) is a critical medical need.
To develop a nomogram for individualized prediction of in-hospital recurrence in MIS patients.
To develop a nomogram for individualized prediction of in-hospital recurrence in MIS patients.
The predictive accuracy of a nomogram model to predict the probability of unfavorable outcome was assessed by calculation of the area under the receiver operating characteristic curve (AUC-ROC). Calibration of the risk prediction model was assessed by a plot comparing the observed probability of unfavorable outcome against the predicted, and by using the Hosmer–Lemeshow test.
A total of 2216 MIS patients were screened. Among them, 155 were excluded for intravascular therapy, 146 for unknown National Institutes of Health Stroke Scale (NIHSS) score, 424 for intracranial hemorrhage, and 247 for progressive stroke. Finally, 1244 patients were subjected for further analysis and divided into a training set (n = 796) and a validation set (n = 448). Multivariate logistic regression analysis revealed that uric acid [odds ratio (OR): 0.997, 95% confidence interval (CI): 0.993-0.999], ferritin (OR: 1.004, 95%CI: 1.002-1.006), and serum total bilirubin (OR: 0.973, 95%CI: 0.956-0.990) were independently associated with in-hospital recurrence in MIS patients. Our model showed good discrimination; the AUC-ROC value was 0.725 (95%CI: 0.646-0.804) in the training set and 0.717 (95%CI: 0.580-0.785) in the validation set. Moreover, the calibration between nomogram prediction and the actual observation showed good consistency. Hosmer-Lemeshow test results confirmed that the nomogram was well-calibrated (P = 0.850).
This study has developed and verified that the nomogram can provide individualized, intuitive, and accurate prediction for the recurrence of mild ischemic stroke inpatients in China.
Our present findings suggest that the nomogram may provide individualized prediction of recurrence in MIS patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3027] [Cited by in RCA: 3578] [Article Influence: 325.3] [Reference Citation Analysis (0)] |
| 2. | Ju Y, Zhao XQ, Wang CX, Wang YL, Liu GF, Wang YJ. Neurological deterioration in the acute phase of minor ischemic stroke is an independent predictor of poor outcomes at 1 year: results from the China National Stroke Registry (CNSR). Chin Med J (Engl). 2013;126:3411-3416. [PubMed] |
| 3. | Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 554] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 4. | Ois A, Gomis M, Rodríguez-Campello A, Cuadrado-Godia E, Jiménez-Conde J, Pont-Sunyer C, Cuccurella G, Roquer J. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke. 2008;39:1717-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Sato T, Sato S, Yamagami H, Komatsu T, Mizoguchi T, Yoshimoto T, Takagi M, Ihara M, Koga M, Iwata H, Matsushima M, Toyoda K, Iguchi Y. D-dimer level and outcome of minor ischemic stroke with large vessel occlusion. J Neurol Sci. 2020;413:116814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ushio M, Kanaoka M, Kinoshita Y, Maeno S, Fujita K. Moderate-to-vigorous physical activity and the risk of stroke recurrence in patients with a history of minor ischemic stroke in Japan: a retrospective analysis. Top Stroke Rehabil. 2018;25:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Wang GS, Tong DM, Chen XD, Yang TH, Zhou YT, Ma XB. Metabolic Syndrome Is a Strong Risk Factor for Minor Ischemic Stroke and Subsequent Vascular Events. PLoS One. 2016;11:e0156243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Vermeer SE, Sandee W, Algra A, Koudstaal PJ, Kappelle LJ, Dippel DW; Dutch TIA Trial Study Group. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke. 2006;37:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | You W, Li Y, Ouyang J, Li H, Yang S, Hu Q, Zhong J. Predictors of Poor Outcome in Patients with Minor Ischemic Stroke by Using Magnetic Resonance Imaging. J Mol Neurosci. 2019;69:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yang J, Fu JH, Chen XY, Chen YK, Leung TW, Mok V, Soo Y, Wong KS. Validation of the ABCD2 score to identify the patients with high risk of late stroke after a transient ischemic attack or minor ischemic stroke. Stroke. 2010;41:1298-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Cheng HR, Huang GQ, Wu ZQ, Wu YM, Lin GQ, Song JY, Liu YT, Luan XQ, Yuan ZZ, Zhu WZ, He JC, Wang Z. Individualized predictions of early isolated distal deep vein thrombosis in patients with acute ischemic stroke: a retrospective study. BMC Geriatr. 2021;21:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Cappellari M, Seiffge DJ, Koga M, Paciaroni M, Forlivesi S, Turcato G, Bovi P, Yoshimura S, Tanaka K, Shiozawa M, Yoshimoto T, Miwa K, Takagi M, Inoue M, Yamagami H, Caso V, Tsivgoulis G, Venti M, Acciarresi M, Alberti A, Toni D, Polymeris A, Bonetti B, Agnelli G, Toyoda K, Engelter ST, De Marchis GM; SAMURAI-NVAF, RAF-NOAC, NOACISP LONG-TERM, and Verona Study Groups. A nomogram to predict unfavourable outcome in patients receiving oral anticoagulants for atrial fibrillation after stroke. Eur Stroke J. 2020;5:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Chang B, He W, Ouyang H, Peng J, Shen L, Wang A, Wu P. A Prognostic Nomogram Incorporating Depth of Tumor Invasion to Predict Long-term Overall Survival for Tongue Squamous Cell Carcinoma With R0 Resection. J Cancer. 2018;9:2107-2115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Callegaro D, Miceli R, Bonvalot S, Ferguson P, Strauss DC, Levy A, Griffin A, Hayes AJ, Stacchiotti S, Pechoux CL, Smith MJ, Fiore M, Dei Tos AP, Smith HG, Mariani L, Wunder JS, Pollock RE, Casali PG, Gronchi A. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 338] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Zhao X, Liu X. Guidelines for the diagnosis and treatment of high-risk non-disabling ischemic cerebrovascular events. Chinese Journal of Stroke. 2016;11:481-491. |
| 16. | Li R, Huang C, Chen J, Guo Y, Tan S. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: a review of literature. Neurol Sci. 2015;36:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Zhang B, Yang N, Lin SP, Zhang F. Suitable Concentrations of Uric Acid Can Reduce Cell Death in Models of OGD and Cerebral Ischemia-Reperfusion Injury. Cell Mol Neurobiol. 2017;37:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Zhang B, Gao C, Yang N, Zhang W, Song X, Yin J, Pu S, Yi Y, Gao Q. Is elevated SUA associated with a worse outcome in young Chinese patients with acute cerebral ischemic stroke? BMC Neurol. 2010;10:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Wang Z, Lin Y, Liu Y, Chen Y, Wang B, Li C, Yan S, Wang Y, Zhao W. Serum Uric Acid Levels and Outcomes After Acute Ischemic Stroke. Mol Neurobiol. 2016;53:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Petzold A, Michel P, Stock M, Schluep M. Glial and axonal body fluid biomarkers are related to infarct volume, severity, and outcome. J Stroke Cerebrovasc Dis. 2008;17:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Dávalos A, Castillo J, Marrugat J, Fernandez-Real JM, Armengou A, Cacabelos P, Rama R. Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology. 2000;54:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Tao X, Wu J, Wang A, Xu C, Wang Z, Zhao X. Lower Serum Indirect Bilirubin Levels are Inversely Related to Carotid Intima-Media Thickness Progression. Curr Neurovasc Res. 2019;16:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712-16719. [PubMed] |
| 24. | Li RY, Cao ZG, Zhang JR, Li Y, Wang RT. Decreased serum bilirubin is associated with silent cerebral infarction. Arterioscler Thromb Vasc Biol. 2014;34:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Dai L, Cheng A, Hao X, Xu J, Zuo Y, Wang A, Meng X, Li H, Wang Y, Zhao X. Different contribution of SBP and DBP variability to vascular events in patients with stroke. Stroke Vasc Neurol. 2020;5:110-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Turana Y, Tengkawan J, Chia YC, Nathaniel M, Wang JG, Sukonthasarn A, Chen CH, Minh HV, Buranakitjaroen P, Shin J, Siddique S, Nailes JM, Park S, Teo BW, Sison J, Ann Soenarta A, Hoshide S, Tay JC, Prasad Sogunuru G, Zhang Y, Verma N, Wang TD, Kario K; HOPE Asia Network. Hypertension and stroke in Asia: A comprehensive review from HOPE Asia. J Clin Hypertens (Greenwich). 2021;23:513-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Zhuo Y, Wu J, Qu Y, Yu H, Huang X, Zee B, Lee J, Yang Z. Clinical risk factors associated with recurrence of ischemic stroke within two years: A cohort study. Medicine (Baltimore). 2020;99:e20830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Huang ZX, Yuan S, Li D, Hao H, Liu Z, Lin J. A Nomogram to Predict Lifestyle Factors for Recurrence of Large-Vessel Ischemic Stroke. Risk Manag Healthc Policy. 2021;14:365-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Du M, Huang X, Li S, Xu L, Yan B, Zhang Y, Wang H, Liu X. A Nomogram Model to Predict Malignant Cerebral Edema in Ischemic Stroke Patients Treated with Endovascular Thrombectomy: An Observational Study. Neuropsychiatr Dis Treat. 2020;16:2913-2920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |