Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9417
Peer-review started: April 1, 2021
First decision: August 18, 2021
Revised: August 21, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: November 6, 2021
Processing time: 212 Days and 0.3 Hours
The liver as a primary site of lymphoma is rarely seen, they are usually misdiagnosed as hepatocellular carcinoma, etc. In 2017, a review of primary hepatic lymphoma (PHL) was done in immunocompetent diffuse large B-cell lymphoma (DLBCL) patients. Yet questions that include treatment choosing or susceptibility of immunoincompetent patients remain disputable.
To investigate the clinical characteristics of patients with PHL.
We collected PHL cases on PubMed, and extracted demographic and clinicopathological data to perform a systematic analysis. Survival analysis regarding age, lactate dehydrogenase (LDH), liver function abnormality (LFA), and treatment modalities were conducted. The Kaplan-Meier method and Cox regression were used to identify risk factors.
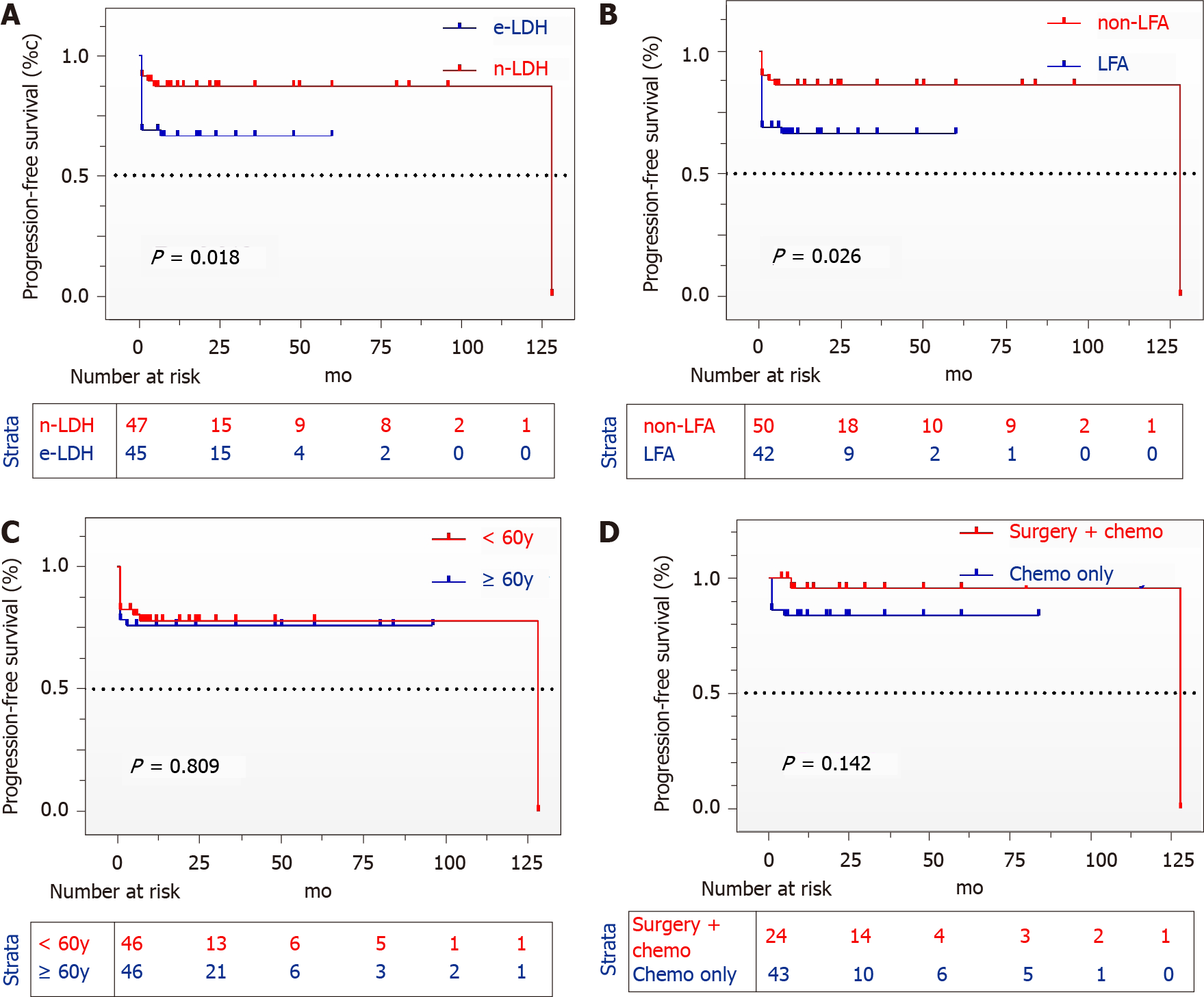
Of 116 PHL patients with DLBCL (62.1%) as the most common subtype. Biopsy methods before surgery produced a 97% positive rate. Progression-free survival (PFS) was significantly shortened in patients with elevated LDH [Hazard ratio (HR): 3.076, 95% confidence interval (CI): 1.207-7.840, P = 0.018] or LFA (HR: 2.909, 95%CI: 1.135-7.452, P = 0.026). Univariate Cox regression analysis suggesting that LDH, liver function, B symptom, hepatosplenomegaly, and lesion were significantly associated with PHL patients survival (P < 0.05). Heavy disease burden was observed in deceased patients. A few PHL patients (3.4%) have slightly higher tumor markers.
PHL patients with elevated LDH and LFA tend to have shorter PFS. Biopsy before treatment in undecided patients with no tumor markers exceeds upper limits has the most essential clinical significance, especially in immunoincompetent patients.
Core Tip: Primary hepatic lymphoma is rarely seen in clinical work, mostly presented with a liver mass discovered by a radiography or imaging test, and usually been misdiagnosed as other hepatic malignancies. In our study, we discovered that patients with elevated lactate dehydrogenase and liver functions abnormality tend to have shorter progression-free survival, and biopsy before treatment along normal tumor markers has most essential clinical significance, and immunoincompetent patients were must susceptible to this aliment.
- Citation: Hai T, Zou LQ. Clinical management and susceptibility of primary hepatic lymphoma: A cases-based retrospective study. World J Clin Cases 2021; 9(31): 9417-9430
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9417.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9417
Primary hepatic lymphoma (PHL) or liver lymphoma, first described by Lei[1], is roughly defined as a pathologically confirmed lymphoproliferative disorder that is confined to the liver without lymphatic organs[1,2]. The prevalence of PHL is extremely rare it comprises 0.16% of newly diagnosed lymphoma[3] and therefore raises several challenges about the differentiation between PHL and other hepatic malignancies, such as primary hepatocellular carcinoma (HCC) and metastatic liver cancer. Because of the aggressive nature of malignant lymphoma and misdiagnosis of this aliment, an optimal scheme for the diagnosis of PHL patients should be properly developed.
While the pathogenesis remains unclear, some studies have shown that an immunosuppressive status and chronic inflammatory process be involved in the occurrence of PHL[3,4]. Upper abdominal malaise and aminotransferase are the most frequent manifestations in these patients, and other presentations include fatigue, jaundice, nausea, and B symptoms such as loss of weight, fever, and night sweat. Laboratory tests are considered insignificant except for elevated lactate dehydrogenase (LDH), alkaline phosphatase, and tumor markers. Researchers have failed to create a reliable diagnostic pattern to distinguish PHL from other liver malignancies by comparing numerous computed tomography (CT) or magnetic resonance imaging (MRI) images[5,6]. The only definitive diagnosis of PHL is biopsy by laparotomy or image-guided needle aspiration. The most prevalent types are diffuse large B-cell lymphoma (DLBCL)[2]. Chemotherapy represents a major part of lymphoma treatment, and surgery is critical for hepatic malignancy. Because of the indistinguishable diagnostic imaging of PHL, these two treatment methods are usually used in combination even if surgery operations are unnecessary under some circumstances.
We have searched many cases published online or offline in the past few decades. In 2017, researchers from France (Cesaretti et al[2]) analyzed 147 PHL patients (DLBCL type only) without immunosuppressive situations from 1976–2015 and concluded that patients with resectable lesions had a better prognosis[2]. However, some questions, including treatment choice or susceptibility of immunoincompetent patients, remain disputable in the rituximab era. A large population-level study conducted using the SEER database concluded that the incidence of PHL has been steadily increasing[2,7], but failed to more practically elucidate PHL. Due to the lack of large-scale clinical trials, PHL patients are treated empirically.
For the first time, we combined all different subtypes of PHL cases into a statistical pool to optimize the treatment modality selection process, especially for patients with resectable lesions, and further explored the likely pathogenesis, prognostic factors, and survival trends of PHL.
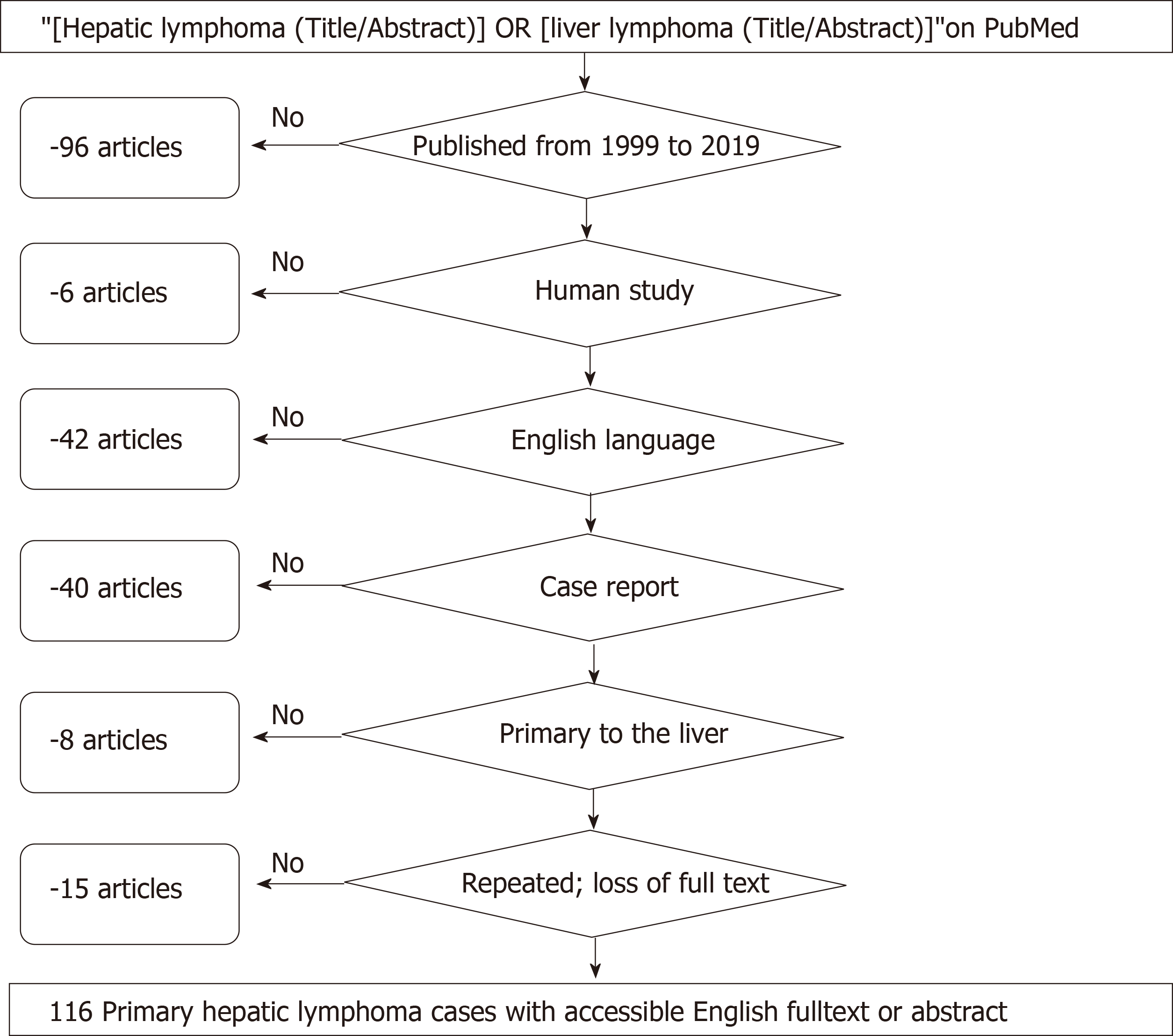
First, open published cases with the terms “hepatic lymphoma” or “liver lymphoma” in their titles or abstracts on PubMed from 1999 to 2019 were extracted to establish a study set. After the exclusion process, a review of the remaining full texts was performed again by researchers to enrich the existing data. The baseline characteristics of PHL patients were mostly based on the original reports; reasonable supplementary information, which was reviewed from the original context both textually and graphically by a senior oncologist, pathologist, radiologist, and surgeon, was taken into consideration at the same time.
One solitary lesion with a size less than 10 cm in diameter was categorized as a single lesion, and lesions over 10 cm in diameter were categorized as bulky lesions. Multiple lesions were defined as more than 2 detectable lesions that were similar in size. Patients without obvious or resectable lesions on diagnostic imaging were classified as having diffuse lesions. At least one imaging study was required, such as upper abdominal CT, MRI, ultrasonography (US) or positron emission tomography (PET). Dynamic liver function tests of patients were not uncommon, but we only took the highest records as valid data. Liver function abnormality (LFA), including existing hepatitis, asymptomatic aminotransferase elevation, or bilirubin elevation of any degree, was also classified as LFA. LDH levels were divided into two groups: Elevated and normal, as was alkaline phosphatase (ALP). Hepatitis B virus (HBV)- or hepatitis C virus (HCV)-infected patients were listed regardless of whether the antivirals were taken. The invasive process can be separated into two parts: (1) Presurgery biopsy, including ultrasound-guided, CT-guided, transjugular, and laparoscopic liver biopsy without removal of the liver parenchyma; and (2) Surgery, including partial hepatectomy, sectionectomy, segmentectomy, and hemihepatectomy. Liver samples were obtained by surgery or presurgery biopsy, and the diagnosis of PHL completely relied on later pathological results. Patients in the chemotherapy group took at least one cycle of designated regimens regardless of whether it worked; for the chemo-free group, the text must have specifically stated that no chemotherapy was given before or after surgery, otherwise it was considered not specified. The exact location of a well-demarcated liver mass was depicted in some reports and was described in the form of liver segmentation, which is often presented in Roman numerals.
Liver specimens were immunohistochemically stained to confirm PHL diagnosis, and positive results were recorded in this article. Misdiagnosis, which PHL patients frequently encounter, includes primary hepatic tumor, intrahepatic cholangiocellular carcinoma, cirrhosis, second liver metastasis, cholecystitis, abscess, hemangioma, inflammatory pseudotumor, etc. Virus-infected [HBV, HCV, Human immunodeficiency virus (HIV), Epstein-Barr virus (EBV)] patients had a definite medical history before being admitted or were strictly confirmed by later blood tests. Immunocompromised patients were those: (1) Who had autoimmune diseases such as rheumatoid arthritis (RA), Sjogren's syndrome (SS), autoimmune hemolytic anemia, and primary biliary cirrhosis; (2) Were persistently receiving immunosuppressive drugs such as methotrexate, azathioprine, Infliximab, and steroids; and (3) With diagnosis of HIV infection before or after admission. There was at least 24 mo of follow-up for all patients, except for those who died during hospitalization or relapsed at less than 24 mo. The following demographic and clinical variables were extracted from the original reports: age, sex, region, chief complaint, B symptoms, remarkable personal history, radiotherapy, subtype of PHL, hepatomegaly, and splenomegaly.
As mentioned above, clinically valuable textual information varied greatly among reports, and thus we tried to make the data deficiency less apparent. First, we made direct contact with the original corresponding authors by e-mailing them at the address provided in these reports and asked (1) What is his or her status now? (2) How long does the follow-up last? (3) Is the patient receiving other treatment targeted to PHL? and (4) Other questions that were concerned. Second, seniors from the radiology, pathology and hepatic surgery department again reviewed the text and graphics to provide additional information such as (1) What kind of imaging tests did they do? (2) Which liver segments are the masses located in? and (3) What subtype of lymphoma was it? All the supplemental materials needed to be more identifiable and obvious, but were not written down in the reports. They had to be strictly scrutinized and rejected if they were not by the original materials or might be turned against the integrity of existing data.
Progression-free survival (PFS) was defined as the interval from surgery or PHL targeted treatment that was completed to the last follow-up, first documentation of tumor recurrence or death. The Kaplan–Meier method and a log-rank test were used to construct a PHL survival curve and analyze PFS. Cox regression analysis was conducted to identify risk factors for PHL patient survival. Statistical analysis was performed with GraphPad 8.0.2. Differences with P < 0.05 were considered statistically significant (α = 0.05).
From 1999 to 2019 (Figure 1), articles on the PubMed website were searched using the MeSH terms “[hepatic lymphoma (Title/Abstract)] or [liver lymphoma (Title/
| Characteristics | Patient numbers (%) (n = 116) |
| Age, median (range) | 58.5 (19-92) |
| Years of diagnosis | |
| 1999-2009 | 36 (31.8) |
| 2009-2019 | 80 (68.2) |
| Sex | |
| Male | 72 (62.1) |
| Female | 44 (37.9) |
| Presentation | |
| Abdominal pain | 58 (50.0) |
| Fatigue | 21 (18.1) |
| Nausea and vomiting | 13 (11.2) |
| Anorexia | 7 (6.0) |
| Hepatomegaly | 53 (45.6) |
| Splenomegaly | 9 (7.7) |
| Asymptomatic | 14 (12.0) |
| Lesion | |
| Single/multiple/diffused/bulky | 58 (50.0)/23 (19.8)/33 (28.4)/29 (25.0) |
| Lactate dehydrogenase | |
| Elevated/normal | 55 (47.4)/58 (52.6) |
| B symptoms | |
| Loss of weight/fever/night sweat | 39 (33.6)/36 (31.0)/9 (7.7) |
| Infection | |
| HBV/HCV/EBV | 15 (12.9)/12 (10.3)/9 (7.7) |
| Immunosuppressive status | |
| Autoimmune disease | 13 (11.2) |
| Rheumatic Arthritis | 7 (6.0) |
| HIV | 7 (6.0) |
| Differential diagnosis | |
| HCC | 21 (18.1) |
| Metastasis | 18 (15.5) |
| Abscess or infection | 12 (10.3) |
| ICC | 10 (8.6) |
| Cholecystitis | 5 (4.3) |
| Pseudolymphoma | 4 (3.4) |
| Hemangioma | 4 (3.4) |
| Treatment | |
| Surgery | 36 (31.0) |
| Chemotherapy/R-CHOP/CHOP | 86 (74.1)/55(47.4)/10(8.6) |
| Radiotherapy | 6 (5.1) |
| PHL subtypes | |
| B cell lymphoma | 99 (85.3) |
| DLBCL/MALT/HL/BL/FL/HGBCL | 72 (62.1)/14(12.0)/4(3.4)/3(2.5)/3(2.5)/3(2.5) |
| T cell lymphoma | 7 (6.0) |
| Other unclassified1 | 10 (11.6) |
| Region | |
| Asia | 72 (62.1) |
| Japan/China/India/South Korea | 24 (20.6)/17(14.6)/16(13.7)/8(6.8) |
| Other | 7 (6.0) |
| Europe | 20 (17.2) |
| North America | 15 (12.9) |
At diagnosis, among the 116 patients, the male to female ratio was 1.67:1, with a median age of 58.5 years (range, 19 to 92). The number of patients diagnosed from 2009 to 2019 was more than twice the number of patients diagnosed from 1999 to 2009 (2.22:1). Only 3 cases specifically reported ethnic groups, and therefore regional information about PHL patients was recorded according to the initial researchers’ institutions. The majority of patients originated from Asia (72/116), with Japan contributing most (24/116), followed by China (17/116), India (16/116), and South Korea (8/116). Europe accounted for 17.2% (20/116), while North America accounted for 12.9% (15/116).
As mentioned in the literature previously, most patients presented with nonspecific symptoms such as abdominal pain (50%), fatigue (18.1%), nausea and vomiting (11.2%), and anorexia (6%). Fourteen (12.0%) patients were asymptomatic but radiographically indicated. Hepatomegaly (45.6%) and splenomegaly (7.7%) were also detected on CT imaging or palpable liver by physical examination. In the PHL setting, B symptoms of lymphoma included loss of weight (33.6%), fever (31.0%), and night sweat (7.7%). HBV, HCV, and EBV infections were identified in a blood test or recorded in the history of 12.9%, 10.3%, and 7.7% of patients, respectively. Meanwhile, single lesions comprised 50% of all PHL cases, multiple 19.8%, diffused 28.4%, and bulky 25%.
Patients with LFAs, which were defined as an elevation of aspartate trans
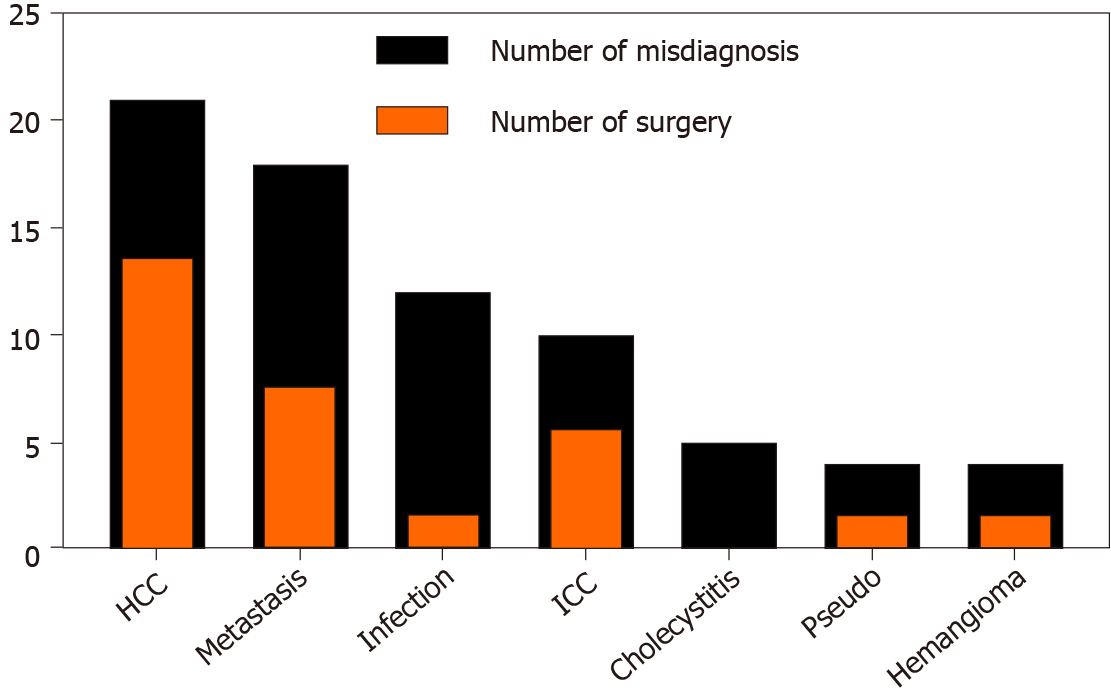
Differential diagnosis or first considered diagnosis in this context was as follows, sorted from most to least: HCC (21/116), metastatic tumor (18/116), abscess or infection (12/116), intrahepatic cholangiocarcinoma (10/116), cholecystitis (5/116), pseudolymphoma (4/116), and hemangioma (4/116). The first discovery of liver lesions usually originated from a diagnostic imaging test or routine physical examination. In total, 110 patients underwent CT, while 66 had US, 46 had MRI, and 38 had PET; others included 3 magnetic resonance cholangiopancreatography and 3 angiographies.
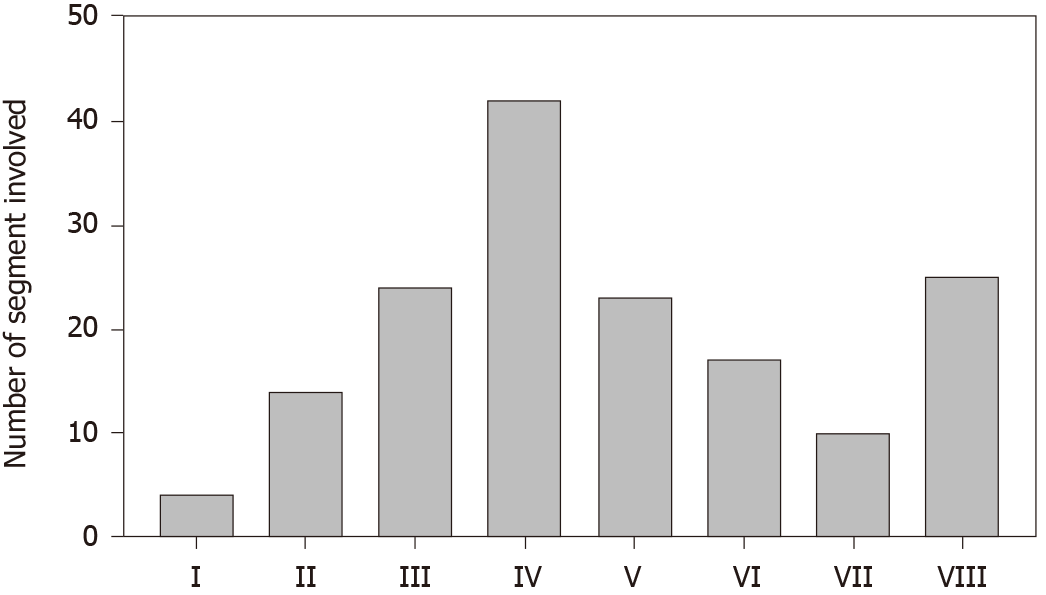
A histogram of the liver lesion location (Figure 3) was drawn to create a clear vision of which part of the hepatic segment was heavily loaded with PHL. Because the diffuse lesions revealed no visible liver masses on imaging tests, they were excluded from the statistics. Most single lesions, bulky lesions, or multiple lesions infiltrate more than one segment, and thus the frequency of every single infiltrated segment was based on the sum of all PHL patients assembled. The histogram resembles a normal distribution with segment IV (42) at the peak, and the remaining segments, I (4), II (14), III (24), V (23), VI (17), and VII (10), were almost evenly distributed on both sides, while segment VIII (25) did not follow the trend statistically.
Among all 106 patients with definite PHL subtype diagnosis (10 unclassified), DLBCL remained the most common subtype, with 72 in number, and the occurrence of the other subtypes differed from the normal prevalence of lymphoma subtypes: 14 mucosa-associated lymphoid tissue lymphomas (MALT), 7 T-cell lymphomas, 4 Hodgkin’s lymphomas (HL), 3 Burkitt lymphomas (BL), 3 follicular lymphomas (FL), and 3 high-grade B cell lymphomas; there was only 1 case each of primary plasmacytic hepatic lymphoma and anaplastic large cell lymphoma. CD20 immunohistochemical staining exhibited positive expression in 81 patients.
A total of 36 patients were hepatectomized; 27 of these were performed before biopsy, 7 were performed after biopsy negativity, and 2 were performed therapeutically after a confirmed PHL diagnosis. Among them, if a surgery was performed based on the first considered diagnosis mentioned above, then the data were altered and shown as in (Figure 4): HCC (14/21), metastatic tumor (8/18), intrahepatic cholangiocarcinoma (6/10), abscess or infection (2/12), pseudolymphoma (2/4), and hemangioma (2/4). At least 86 patients had undergone diagnostic biopsy before receiving any treatment, and there were 69, 10, 4, and 3 ultrasound-guided, CT-guided, transjugular, and laparoscopic liver biopsies, respectively (Table 2). Although the majority of these patients were definitively diagnosed after biopsy, there were 6 negative results, which mostly ended up having surgery.
Regardless of surgery, 83 patients had at least one cycle of various chemotherapy regimens; 47.4% of patients received the R-CHOP regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and dose-reduced R-CHOP regimen (55/116), while 8.6% (10/116) patients were treated with CHOP only. In addition, the total number of patients who used rituximab alone or in combination was 62. It was not specified in 4 patients. Despite the high rate of chemotherapy usage, 30 patients did not receive any kind of chemotherapy, and 8 neither received chemo nor surgery, mostly due to early death resulting from a critical condition such as fulminant hepatitis or serious sepsis. Interestingly, 8 immunosuppressive drug users spontaneously entered the remission stage after surgery or chemotherapy if the discontinuation of immunosuppressive drugs that were perpetually used for pre-existing conditions was started. Radiotherapy was not a conventional treatment option as only 6 patients received it.
Twenty immunocompromised patients are listed in Table 3. Based on predescribed immunosuppressive criteria, 13 PHL patients had concomitant autoimmune disease and received immunosuppressive drugs. The most common ailment was rheumatic arthritis (7), which was consistently treated with methotrexate. Meanwhile, there were 7 HIV-positive patients, and antiviral drugs were taken by four of them. The median time from the first treatment of those concomitant conditions to the diagnosis of PHL was 8 years, and the latency ranged from 1 to 38 years. According to the records, all concomitants were basically under good control. Methotrexate (8) and steroids (6) were the most frequent medications used. Only 2 received surgery, and another 2 did not receive any treatment at all but still had a good response.
| Age | Latency (years) | HIV | Autoimmune disease | Immunosuppressive agents | Subtype | Treatment |
| 73 | 8 | - | RA | MTX; Prednisone; Cyclophosphamide | DLBCL | Chemo + DOD |
| 89 | - | - | PBC | - | MALT | Chemo |
| 55 | 11 | + | - | - | HL | Surgery + Chemo |
| 65 | 7 | - | RA | MTX | DLBCL | Surgery + DOD |
| 65 | - | - | CTD | Azathioprine; Prednisone; Hydroxychloroquine | DLBCL | Chemo |
| 63 | 10 | - | RA | MTX | DLBCL | DOD |
| 32 | - | + | - | - | DLBCL | Chemo |
| 56 | 7 | - | RA | MTX | DLBCL | Chemo + DOD |
| 64 | 2 | - | RA | MTX; Infliximab | DLBCL | Chemo + DOD |
| 67 | 38 | - | RA | MTX; Prednisone | DLBCL | Chemo + DOD |
| 36 | 10 | - | ITP | MTX; Azathioprine; Steroid | DLBCL | Chemo |
| 45 | 8 | - | Renal transplanted | Tacrolimus; Azathioprine; Prednisone | DLBCL | - |
| 56 | - | + | - | - | Unclassified | - |
| 64 | 3 | - | AIHA | Corticosteroids; Rituximab; Alemtuzumab | DLBCL | Chemo |
| 48 | 4 | - | RA | MTX | DLBCL | DOD |
| 27 | - | + | - | - | HGBCL | - |
| 43 | - | + | - | - | ALCL | - |
| 34 | 11 | + | - | - | DLBCL | Chemo |
| 62 | 18 | - | SS | Cyclophosphamide; Prednisolone | DLBCL | Chemo + DOD |
| 32 | 1 | + | - | - | BL | Chemo |
After excluding 23 patients with invalid follow-up times, the mean follow-up time was 21.5 mo (range: 1–128). As we can see in the picture (Figure 2), patients with elevated LDH [Hazard ratio (HR): 3.076, 95% confidence interval (CI): 1.207–7.840, P = 0.018] or abnormal liver function (HR: 2.909, 95%CI: 1.135–7.452, P = 0.026) had a much worse prognosis than those who without. The survival curve did not significantly differ between patients aged over 60 years and younger than 60 years (P = 0.809), and the patients in the surgery group did not exhibit statistically improved long-term survival (P = 0.142). Univariate Cox regression analysis was conducted to identify 5 risk factors (LDH, liver function, B symptoms, hepatosplenomegaly, and lesions), but multivariate Cox analysis did not produce similar results (Table 4).
| Variables | Univariate | Multivariate | ||||
| HR | 95%CI | P value | Adjusted HR | 95%CI | P value | |
| Sex | ||||||
| Female | Ref. | |||||
| Male | 1.442 | 0.582-3.574 | 0.429 | NA | NA | NA |
| Age (years) | ||||||
| < 60 | Ref. | |||||
| ≥ 60 | 1.101 | 0.468-2.593 | 0.825 | NA | NA | NA |
| Virus infection | ||||||
| Unknown | Ref. | |||||
| Yes | 1.009 | 0.370-2.756 | 0.985 | NA | NA | NA |
| LDH | ||||||
| Normal | Ref. | Ref. | ||||
| Elevated | 3.023 | 1.172-7.797 | 0.022 | 1.901 | 0.667-5.416 | 0.229 |
| Liver function | ||||||
| Normal | Ref. | Ref. | ||||
| Abnormal | 2.747 | 1.108-6.813 | 0.030 | 1.333 | 0.477-3.730 | 0.584 |
| B symptom | ||||||
| No | Ref. | Ref. | ||||
| Yes | 2.810 | 1.090-7.246 | 0.032 | 1.785 | 0.654-4.872 | 0.258 |
| Hepatosplenomegaly | ||||||
| No | Ref. | Ref. | ||||
| Yes | 2.971 | 1.152-7.663 | 0.024 | 1.925 | 0.697-5.466 | 0.203 |
| Lesion | ||||||
| Single | Ref. | Ref. | ||||
| Multiple or diffused | 2.743 | 1.064-7.075 | 0.037 | 1.992 | 0.753-5.276 | 0.165 |
Twenty deceased patients were profoundly associated with LFA (16/20), elevated LDH (16/20), and severe clinical symptoms on admission. Meanwhile, 18 had diffuse, multiple, and bulky lesions, demonstrating that they had a much higher disease burden.
The reported rare primary locations of lymphoma include the heart, liver, lung, kidney, adrenal gland, bone, and breast, which comprise less than 1% of all non-Hodgkin lymphomas. All of these affected organs have unique clinical manifestations, and these patients face misdiagnosis of the most common malignancies of related primary sites as well.
It is hard to explain why Asians were more common than people in other regions. One of the possible explanations might have been the higher prevalence of HBV and HCV in the Western Pacific region[9] or East Asia[10], as the etiological factor of PHL has been reported to have a connection to the chronic inflammatory reaction caused by virus infection[4,11]. The number of PHL cases from 2009 to 2019 was double that from 1999 to 2019 (2.22:1), suggesting that the prevalence of PHL has increased in the past few decades, at least statistically speaking. It might have something to do with the overwhelming amount of easily accessible literature online over the years. Technically, PHL may be soaring due to advancing diagnostic technology and awareness.
Because the clinical management and outcome of lymphoma cannot be more different from those of other malignancies, early diagnosis of PHL is critical to launching treatment. Nearly every patient had CT; therefore, there is no point in further discussing its diagnostic significance. Imaging studies have shown that no MRI features appear to be specific in differentiation, but vessel encasement is a helpful sign in the diagnosis of PHL[4]. Despite that, radiologists Abe et al[12] and Rajesh et al[6] intended to separate PHL from other malignancies, but the remaining overlapping imaging findings could not be separated from each other; thus, they failed[6,12]. Changing HCC patient outcomes is limited by PET/CT[13], yet they play a major role in evaluating the response of DLBCL. In the appropriate clinical context, such as B symptoms, LDH elevation and an immunocompromised status combining imaging and laboratory features can be meaningful in approaching the correct diagnosis or calling for diagnostic biopsy. We first reported that segment IV of the liver most frequently exhibits PHL.
Clinical manifestations might be insignificant, but after the onset of acute liver failure, the patient’s condition could easily collapse. Researchers have reported that when a patient experiences jaundice, general weakness or acute liver failure that leads to hepatic encephalopathy, they could die[14,15]. In our study, deceased patients enduring heavy tumor burden had more severe symptoms on admission and then deteriorated very quickly within months. Their poor physical condition made them no candidate for surgery, let alone cytotoxic chemotherapy. Eight died after chemo
PHL is still the least considered differential diagnosis of undecided liver masses. Among all patients, 21 were first clinically diagnosed with HCC, 18 with metastatic tumor, 12 with abscess or infection, and 10 with intrahepatic cholangiocarcinoma; 30 were hepatectomized accordingly. In a previously published case review, a group of selected PHL-DLBCL patients with resectable lesions who underwent early surgery combined with postoperative chemotherapy had better outcome[6,16]; however, the data gathered in our study showed that the survival rate of an aggressive surgical approach did not outstrip that of the chemotherapy group. We suggest that surgery should be carefully discussed by a multidisciplinary team if a patient with a large resectable liver lesion is admitted[17].
The idea of biopsy prior to the implementation of surgery should be taken seriously when knowledge of a specific diagnosis is likely to alter the management plan[18]. Nonspecific symptoms of PHL along with radiological characteristics lead to a misdiagnosis, considering its rarity. Imaging-guided or transjugular liver biopsy resulted in an excellent outcome, with only 2 negatives among 83 patients. The less invasive process was easy to accept, and PHL patients seemed well tolerated.
Accounting for 62.1% (72/116) of all patients, DLBCL was the most common subtype of PHL. The standard front-line treatment for DLBCL is the R-CHOP/CHOP regimen, which can produce a 50% to 70% cure rate[19], and 65 patients receive it. While follicular lymphoma is the second most common lymphoma in general[20], the MALT lymphoma subtype of PHL outnumbered the FL, probably because MALT lymphoma is more common in the digestive system. Watchful waiting before any indication of intervention should be initiated, and an active approach to eradicating pathogenic factors such as Helicobacter pylori infection should commence. The clinical physician also suggests a follow-up of 6 to 8 wk before starting chemotherapy, as regression of the tumor after MTX withdrawal was rapid[21]. Other subtypes were too rare to draw any conclusions, and thus they were treated empirically.
The International Prognostic Index (IPI), age-adjusted IPI, or revised NCCN-IPI has been the basis for determining prognosis in aggressive non-Hodgkin lymphoma patients, which comprises at least five components: Age > 60 years, ECOG performance status ≥ 2, Ann Arbor stage III-IV, extranodal disease ≥ 2, and elevated LDH[22]. ECOG performance status, stage, and extranodal disease could not be appraised because only a few have been reported. According to the survival curve, age > 60 years was not identified as predictive of PHL or of deceased patients (10 vs 10). By IPI, patients with elevated LDH levels tended to have shorter PFS. In clinical practice, patients with LFAs often experience treatment delays, and they are less tolerable to antitumor regimens; thus, LFAs became an exclusive independent prognostic factor for PHL in our study. Univariate Cox regression analysis suggested that LDH, liver function, B symptoms, hepatosplenomegaly, and lesions were significantly associated with PHL patient survival (P < 0.05), but multivariate Cox regression analysis failed to identify independent risk factors. This is probably due to the data impairment.
Only 4 patients had slightly higher tumor markers; therefore, we could conclude that a blood test for PHL is not only a predictive tool but also decisive in the exclusion of PHL if tumor markers such as AFP, CA19–9, and CA15–3 exceed the upper limit. LDH elevation is usually accompanied by an increase in ALP, and it was not strongly associated with survival. A review by Vallet et al[23] demonstrated that hypercalcemia was independently associated with poor PFS and OS[23]. Many researchers have reported that 40% of PHL patients have serum calcium abnormality[24,25], but there were only 6 patients diagnosed with hypercalcemia in our study; hence, more data are needed to elucidate its significance in the PHL setting.
The etiology of PHL occurrence still needs to be fully unveiled, and there are two vigorously debated theories: infection and compromised immunity. The prevalence of HBV or HCV infection in PHL patients is higher than that in the general population, and therefore researchers believe there may be causality. Interestingly, two retrospective analyses regarding PHL conducted by Yang et al[16] and Bronowicki et al[26] back in 2010 and 2003 showed 33.3% and 21% HBV or HCV infection, respectively, which then dropped to 23.3% and 10.6% in 2017[6,26,27], and were at 12.9% and 10.3% in our study. It appears that as PHL increases, the relationship weakens accordingly, and a threshold might be reached in the future. EBV and its etiological link to a range of lymphoproliferative lesions and malignant lymphomas have been broadly learned; 9 patients were EBV positive by a blood test or in situ hybridization, but we assume that the real prevalence rate was diluted because many patients did not undergo EBV testing. Although the definition of the immunocompromised patient may be disputable, MTX and other immunosuppressants used to mitigate RA for many years carry a risk of lymphoproliferative disorders[28], and remission after drug withdrawal or reduction was observed in PHL. At the same time, non-Hodgkin lymphoma is the most common cancer in HIV-infected patients[29]. While virus infection and compromised immunity along with PHL development have been bound together since they were first reported in the 1970s, the vast number of noninfected and immunocompetent patients makes these two theories controversial.
All pre-existing data and supplementary information collected from case reports involving PHL can be checked online. As a retrospective study, the authenticity and integrity of the data mainly rely on case providers whose publications have an interval of almost 20 years, which makes this study permanently suffer from an unavoidable defect. A series of reviews results in slightly contradictory results. Possible remedies to this problem were already attempted, including direct contact with the original provider and seniors’ consultation, and it was still an utterly inadequate measure. Owing to the limited number of patients and the fact that it is nearly impossible to fully grasp how long they have survived due to its rarity, our research is a descriptive study rather than solid guidance. More patients are required to further explore this disease.
Even if uncertainty remains, diagnostic liver biopsy before curative treatment implementation is strongly recommended, and double sampling is recommended if necessary. PHL should be taken into consideration for differential diagnosis of an image-indicated atypical liver mass along with elevated LDH in an immunoincompetent patient. PHL patients with elevated LDH and LFAs tend to have shorter PFS. PHL could be excluded with confidence if tumor markers, including AFP, CA19–9, and CA15–3, are far beyond the upper limit. As the upward prevalence of PHL is expected to continue in the foreseeable future, a retrospective review of PHL should be performed.
In clinical practice, primary hepatic lymphoma (PHL) was is the least considered differential diagnosis of undecided liver masses due to its’ rarity(less than 1% of all diagnosed lymphoma patients), and most of the time, they were misdiagnosed as hepatic cell carcinoma or metastatic malignancies. Surgery was performed based on initial diagnosis, and we believe that proper biopsy before the aggressive operation was better for PHL patients.
The large-scale clinical trial was not suitable for PHL so that a retrospective analysis was needed to address emerged problems.
This study aimed to investigate the clinical characteristics of patients with PHL to avoid misdiagnosis and its’ risk factors.
All data was available online. In this retrospective study, baseline characteristics, test results, and follow-up time of 116 patients with PHL were summarized and underwent survival analysis. Statistical analysis was performed using Graphpad 8.0.2 (P < 0.05 were considered statistically significant).
The diffuse large B-cell lymphoma (62.1%) was the most common subtype. Patients’ survival was significantly shortened by elevated lactate dehydrogenase and liver function abnormality. Heavy disease burden was observed in deceased patients. A few PHL patients (3.4%) have slightly higher tumor markers. Univariate Cox regression also identified B symptom, hepatosplenomegaly, and lesion were risk factors for PHL patients.
Proper biopsy before treatment in undecided patients with no tumor markers exceeds upper limits is essential to avoid misdiagnosis, especially in immunoincompetent patients.
The incidence of PHL is increasing according to data from the National Institute of Health. Researchers long discussed the etiology of PHL, and they believed that virus infection, autoimmune diseases (often accompanied with MTX consumption) were most likely to be related to PHL incidence. We are highly second that and suggested that every 5 or 10 years a retrospective review of PHL should be properly done.
We sincerely thank Dr. Koichiro Kaneko, Dr. Vishnu Prasad, Dr. Irappa Madabhavi, Professor Young-Dong Yu, Dr. Venkatesh Rangarajan, Dr. Cezar Betianu, and many others for kindly providing additional unpublished follow-up information.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tzeng IS S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Lei KI. Primary non-Hodgkin's lymphoma of the liver. Leuk Lymphoma. 1998;29:293-299. [RCA] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Cesaretti M, Loustau M, Robba C, Senescende L, Zarzavadjian Le Bian A. Reappraisal of primary hepatic lymphoma: Is surgical resection underestimated? Crit Rev Oncol Hematol. 2018;123:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Padhan RK, Das P, Shalimar. Primary hepatic lymphoma. Trop Gastroenterol. 2015;36:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Kikuma K, Watanabe J, Oshiro Y, Shimogama T, Honda Y, Okamura S, Higaki K, Uike N, Soda T, Momosaki S, Yokota T, Toyoshima S, Takeshita M. Etiological factors in primary hepatic B-cell lymphoma. Virchows Arch. 2012;460:379-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Semelka RC, Nimojan N, Chandana S, Ramalho M, Palmer SL, DeMulder D, Parada Villavicencio C, Woosley J, Garon BL, Jha RC, Miller FH, Altun E. MRI features of primary rare malignancies of the liver: A report from four university centres. Eur Radiol. 2018;28:1529-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Rajesh S, Bansal K, Sureka B, Patidar Y, Bihari C, Arora A. The imaging conundrum of hepatic lymphoma revisited. Insights Imaging. 2015;6:679-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Zhang SL, Chen C, Rao QW, Guo Z, Wang X, Wang ZM, Wang LS. Incidence, Prognostic Factors and Survival Outcome in Patients With Primary Hepatic Lymphoma. Front Oncol. 2020;10:750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87:146-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 9. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1986] [Article Influence: 198.6] [Reference Citation Analysis (3)] |
| 10. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1142] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 11. | Alves AMA, Torres US, Velloni FG, Ribeiro BJ, Tiferes DA, D'Ippolito G. The many faces of primary and secondary hepatic lymphoma: imaging manifestations and diagnostic approach. Radiol Bras. 2019;52:325-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Abe H, Kamimura K, Kawai H, Kamimura H, Domori K, Kobayashi Y, Nomoto M, Aoyagi Y. Diagnostic imaging of hepatic lymphoma. Clin Res Hepatol Gastroenterol. 2015;39:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Hayakawa N, Nakamoto Y, Nakatani K, Hatano E, Seo S, Higashi T, Saga T, Uemoto S, Togashi K. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int J Clin Oncol. 2014;19:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Williams MO, Akhondi H, Khan O. Primary Hepatic Follicular Lymphoma Presenting as Sub-acute Liver Failure: A Case Report and Review of the Literature. Clin Pathol. 2019;12:2632010X19829261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Farag F, Morcus R, Ramachandran P, Pasrija UR, Wang JC. Fever of Unknown Origin due to Primary Hepatic Diffuse Large B-cell Lymphoma: A Case Report. Cureus. 2019;11:e4220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Yang XW, Tan WF, Yu WL, Shi S, Wang Y, Zhang YL, Zhang YJ, Wu MC. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16:6016-6019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 17. | Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 390] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 18. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1568] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 19. | Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc Hematol Educ Program. 2016;2016:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 21. | Soubrier M, Arrestier S, Bouloudian S, Dubost JJ, Ristori JM. Epstein-Barr virus infection associated hepatic lymphoma in a patient treated with methotrexate for rheumatoid arthritis. Joint Bone Spine. 2006;73:218-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 653] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 23. | Vallet N, Ertault M, Delaye JB, Chalopin T, Villate A, Drieu La Rochelle L, Lejeune J, Foucault A, Eloit M, Barin-Le Guellec C, Hérault O, Colombat P, Gyan E. Hypercalcemia is associated with a poor prognosis in lymphoma a retrospective monocentric matched-control study and extensive review of published reported cases. Ann Hematol. 2020;99:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Hsu A, Gagnier M, Ryer E, Salhab M, Rosmarin AG. Hypercalcemia due to Primary Hepatic Lymphoma. Case Rep Hematol. 2016;2016:1876901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Nan DN, Fernández-Ayala M, Terán E, Parra JA, Fariñas MC. Severe hypercalcemia and solitary hepatic mass as initial manifestation of primary hepatic lymphoma. Liver. 2001;21:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, Rousselet MC, Dharancy S, Gaulard P, Flejou JF, Cazals-Hatem D, Labouyrie E. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. 2003;37:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Peng Y, Qing AC, Cai J, Yue C, French SW, Qing X. Lymphoma of the liver: Clinicopathological features of 19 patients. Exp Mol Pathol. 2016;100:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kurita D, Miyoshi H, Ichikawa A, Kato K, Imaizumi Y, Seki R, Sato K, Sasaki Y, Kawamoto K, Shimono J, Yamada K, Muto R, Kizaki M, Nagafuji K, Tamaru JI, Tokuhira M, Ohshima K. Methotrexate-associated Lymphoproliferative Disorders in Patients With Rheumatoid Arthritis: Clinicopathologic Features and Prognostic Factors. Am J Surg Pathol. 2019;43:869-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Noy A. Optimizing treatment of HIV-associated lymphoma. Blood. 2019;134:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |