Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9395
Peer-review started: April 7, 2021
First decision: June 27, 2021
Revised: July 16, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: November 6, 2021
Processing time: 204 Days and 19.9 Hours
Many scores have been suggested to assess the severity of acute pancreatitis upon onset. The extrapancreatic necrosis volume is a novel, promising score that appears to be superior to other scores investigated so far.
To evaluate the discriminatory power of extrapancreatic necrosis volume to identify severe cases of acute pancreatitis.
A total of 123 patients diagnosed with acute pancreatitis at Institute of Gastroenterology and Hepatology, St Spiridon Hospital between January 1, 2017 and December 31, 2019 were analyzed retrospectively. Pancreatitis was classified according to the revised Atlanta classification (rAC) as mild, moderate, or severe. Severity was also evaluated by computed tomography and classified according to the computed tomography severity index (CTSI) and the modified CTSI (mCTSI). The results were compared with the extrapancreatic volume necrosis to establish the sensitivity and specificity of each method.
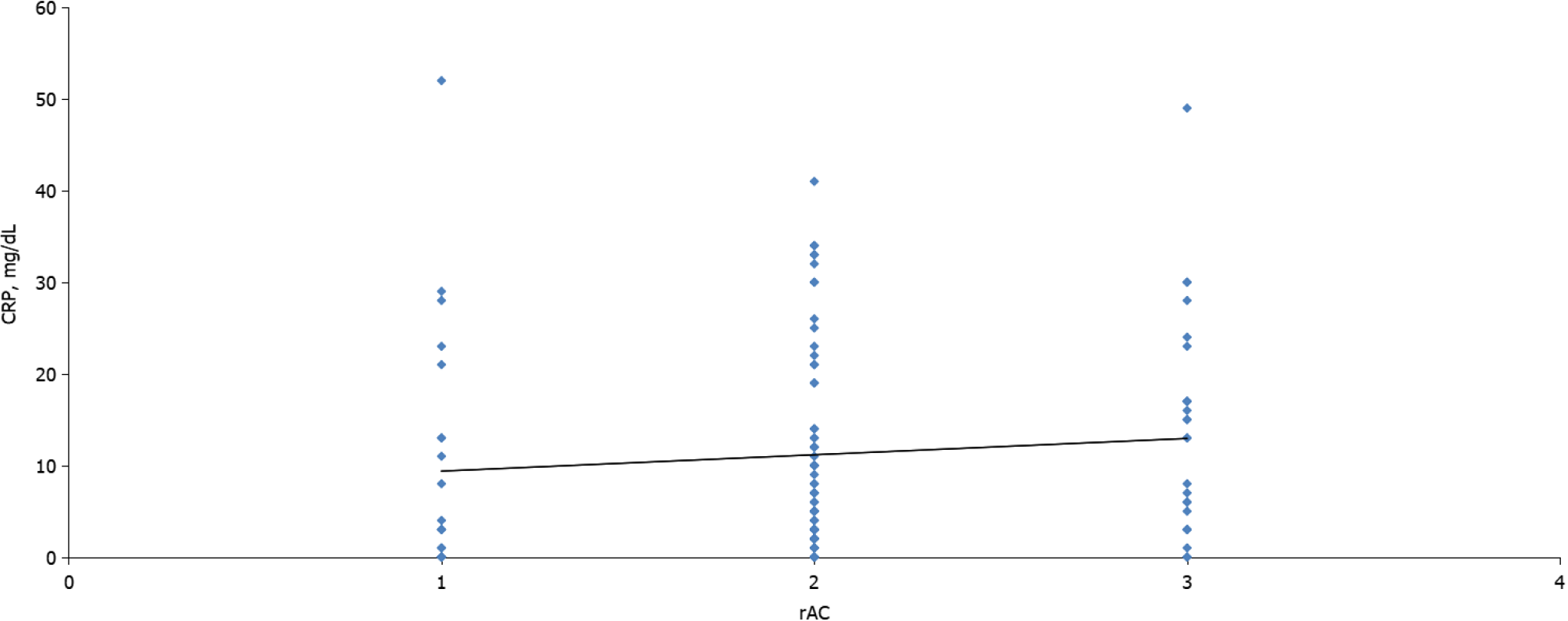
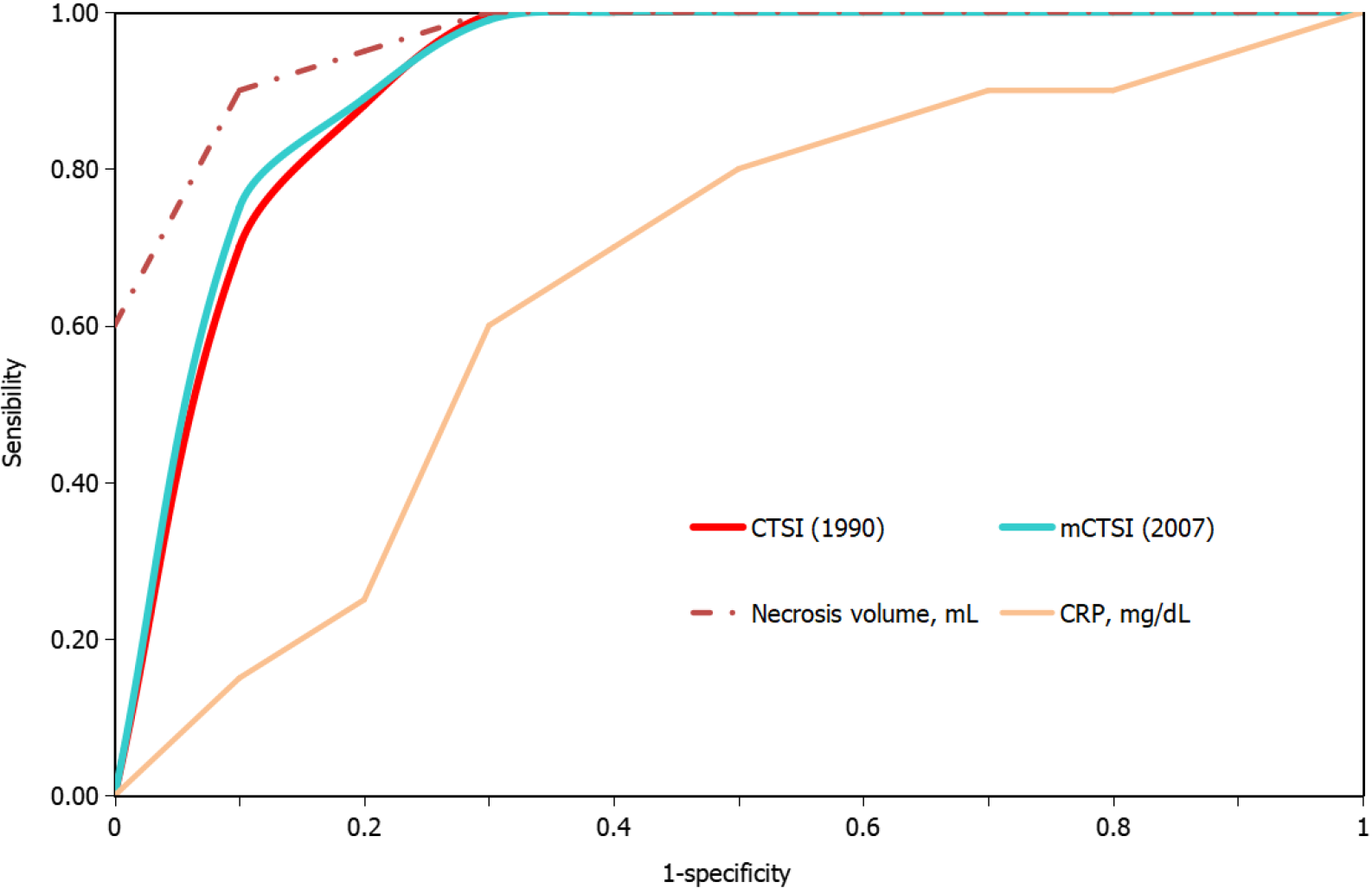
The CTSI and mCTSI imaging scores and the extrapancreatic necrosis volume were highly correlated with the severity of pancreatitis estimated by the rAC (r = 0.926, P < 0.001 and r = 0.950, P < 0.001; r = 0.784, P < 0.001, respectively). The correlation of C-reactive protein with severity was positive but not as strong, and was not significant (r = 0.133, P = 0.154). The best predictor for the assessment of severe pancreatitis was the extrapancreatic necrosis volume [area under the curve (AUC) = 0.993; 95% confidence interval (CI): 0.981-1.005], with a 99.5% sensitivity and 99.0% specificity at a cutoff value of 167 mL, followed by the mCTSI 2007 score (AUC = 0.972; 95%CI: 0.946-0.999), with a 98.0% sensitivity and 96.5% specificity, and the CTSI 1990 score (AUC = 0.969; 95%CI: 0.941-0.998), with a 97.0% sensitivity and 95.0% specificity.
Radiological severity scores correlate strongly and positively with disease activity. Extrapancreatic necrosis volume shows the best diagnostic accuracy for severe cases.
Core Tip: This retrospective study evaluated the role of extrapancreatic necrosis volume in the evaluation of acute pancreatitis. The patients were evaluated by established computed tomography scores, the computed tomography severity index (CTSI), and the modified CTSI, as well as a new method using the extrapancreatic necrosis volume. Although all the imaging scores had a strong correlation with the severity of acute pancreatitis, the extrapancreatic necrosis volume had the best diagnostic accuracy for the severe form.
- Citation: Cucuteanu B, Negru D, Gavrilescu O, Popa IV, Floria M, Mihai C, Cijevschi Prelipcean C, Dranga M. Extrapancreatic necrosis volume: A new tool in acute pancreatitis severity assessment? World J Clin Cases 2021; 9(31): 9395-9405
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9395.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9395
The incidence of acute pancreatitis has increased in the past two decades and poses a serious health threat[1]. The mild and moderate forms have a benign evolution with rapid resolution of symptoms, while the severe form is a therapeutic challenge and has a high mortality caused by life-threatening complications[2]. Under such circumstances, rapid identification of patients with acute pancreatitis and severe prognosis could lead to timely and more effective therapeutic strategies and reduced morbidity and mortality. Many scores that include clinical, biological, and imagistic markers have been suggested to assess the severity of pancreatitis at onset, for example, the Ranson, acute physiology and chronic health evaluation (APACHE II), and Glasgow scores[3-5]. The computed tomography severity index (CTSI)[6] and the modified CTSI (mCTSI)[7] remain the most widely used imaging scores for assessing the severity of pancreatitis.
Since their development, there have been attempts to design predictive imaging scores, but none has proven better. Extrapancreatic necrosis volume is a recent, promising predictor of severe acute pancreatitis. The Atlanta classification (AC), which was introduced in 1992[8], improved clinical diagnosis, computed tomography (CT) evaluation, and the criteria of disease progression by dividing acute pancreatitis into two morphological subtypes: Mild and severe. In 2012, the AC was revised to create three subtypes: Mild, moderate, and severe[9]. This study aimed to: (1) Evaluate the discriminatory power of the extrapancreatic necrosis volume to identify cases of severe acute pancreatitis; (2) Demonstrate a correlation between the extrapancreatic necrosis volume and the severity of acute pancreatitis; and (3) Improve the existing level of evidence supporting the performance of the extrapancreatic necrosis volume in detecting severe acute pancreatitis, paving the way for better and safer management of patients at risk.
This retrospective study included 123 patients, hospitalized at the Institute of Gastroenterology and Hepatology in Iaşi, Romania between January 1, 2017 and December 31, 2019. The diagnosis of pancreatitis was established in patients with two of the following three revised AC (rAC) protocol criteria: (1) A clinically significant picture; (2) An increase in pancreatic enzymes of > 3 times normal; and (3) Ra
Cardiovascular failure was defined as hypotension requiring the administration of vasoactive medications. Renal failure required a serum creatinine level > 1.3 mg/dL, or the need for hemo- or peritoneal dialysis. Respiratory failure required a partial oxygen pressure of 60 mmHg or ventilatory support. Neurological failure required a Glasgow coma score of < 6 in the absence of sedation. Hematological failure required a platelet count ≤ 100000/mm3. Baseline patient data were collected on admission and included age, sex, and C-reactive protein (CRP; normal value < 0.5 mg/dL). Radio
Contrast-enhanced CT examinations were performed using a Siemens Somatom Emotion 16 system (Erlangen, Germany) with a 70-s scanning delay after intravenous injection of 100 mL of iopromide (370 mg I2/mL of Ultravist 370; Berlex Laboratories, Wayne, NJ, United States) at a rate of 3 mL/s. The volume was adjusted to the patient’s mass, to a maximum 1 mL/kg body weight. CT was performed at 130 kVp, with a maximum current of 300 mAs, 16 mm × 1.5 mm collimation, Care4Dose dose modulation, 3 mm thick reconstructed images, 1.5 mm increment, and a B41s filter. The field of view was 42 cm and the matrix was 512 × 512. CT studies were retrospectively reviewed with a Syngo CT 2007E picture archiving and communication system workstation (Siemens).
The CTSI (1990) and mCTSI (2007) scores were calculated. Areas of extrapancreatic necrosis were determined by peripancreatic and retropancreatic fat necrosis and collection of intra-abdominal fluid or fluid including solid components. Pancreatic ascites was excluded from the measurement. Peritoneal spaces that normally accu
Statistical analyses were performed with PASW (SPSS) Statistics for Windows, Version 18.0 (IBM Corp., Armonk, NY, United States). Because of the non-Gaussian data distribution, indicated by the distance of the mean from the median dataset values, the correlations between parameters were analyzed using the Spearman rank correlation coefficient. P < 0.05 was considered to indicate a statistically significant difference. A receiver operating characteristic (ROC) curve was constructed to determine the optimal threshold of extrapancreatic necrosis volume to predict severe acute pancreatitis. ROC curves were also constructed for the CTSI 1990 and mCTSI 2007 scores. The area under the ROC curve (AUC) was calculated and used as a measure of diagnostic accuracy.
The study was approved by the local ethics committee. All the patients enrolled in the study gave their written informed consent.
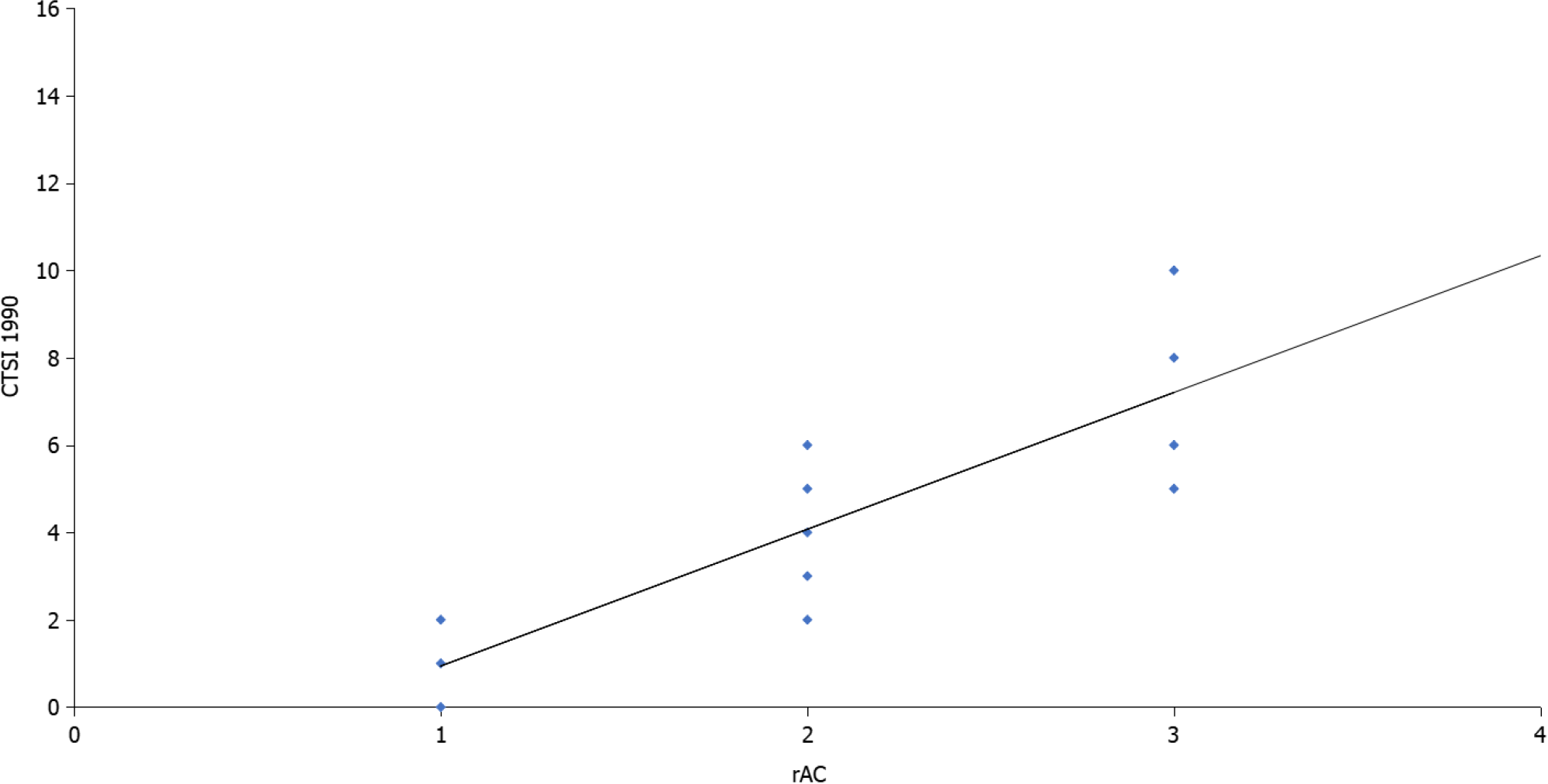
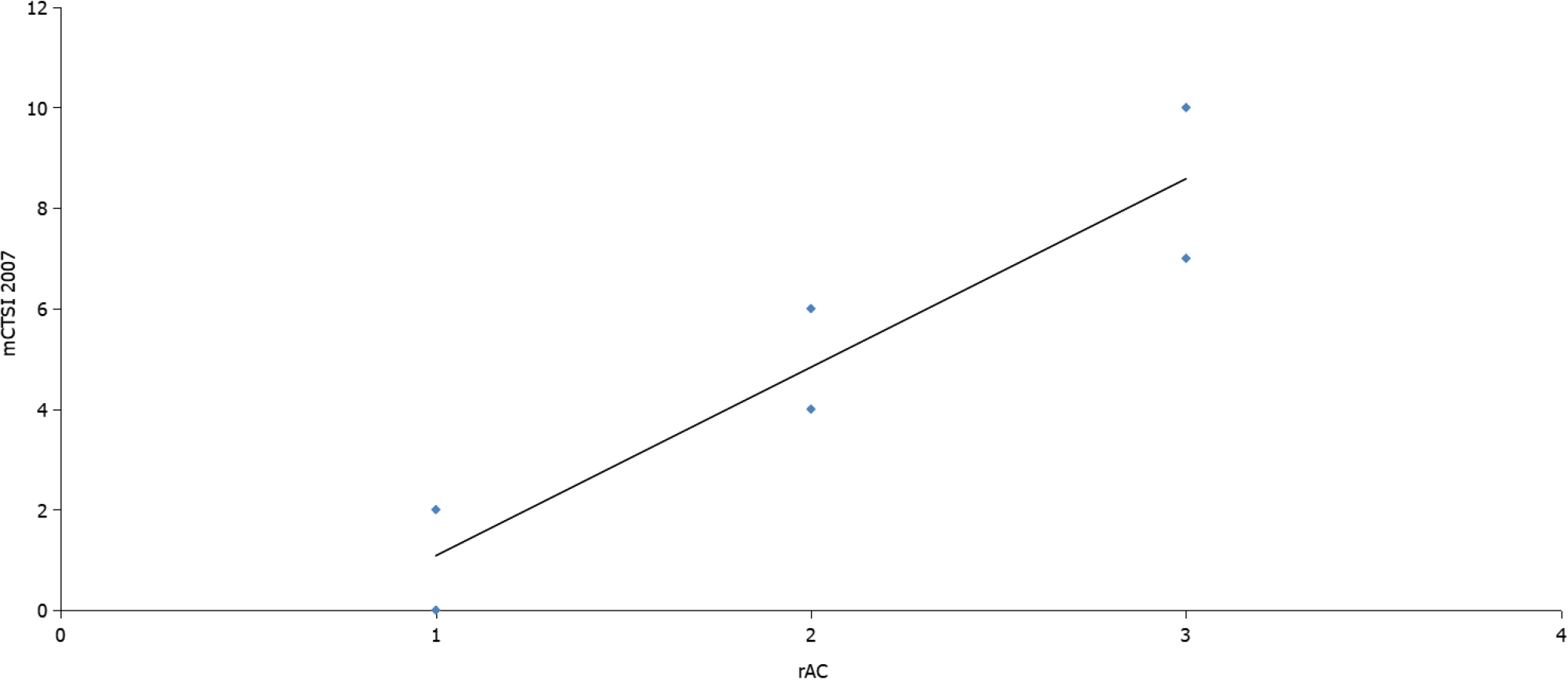
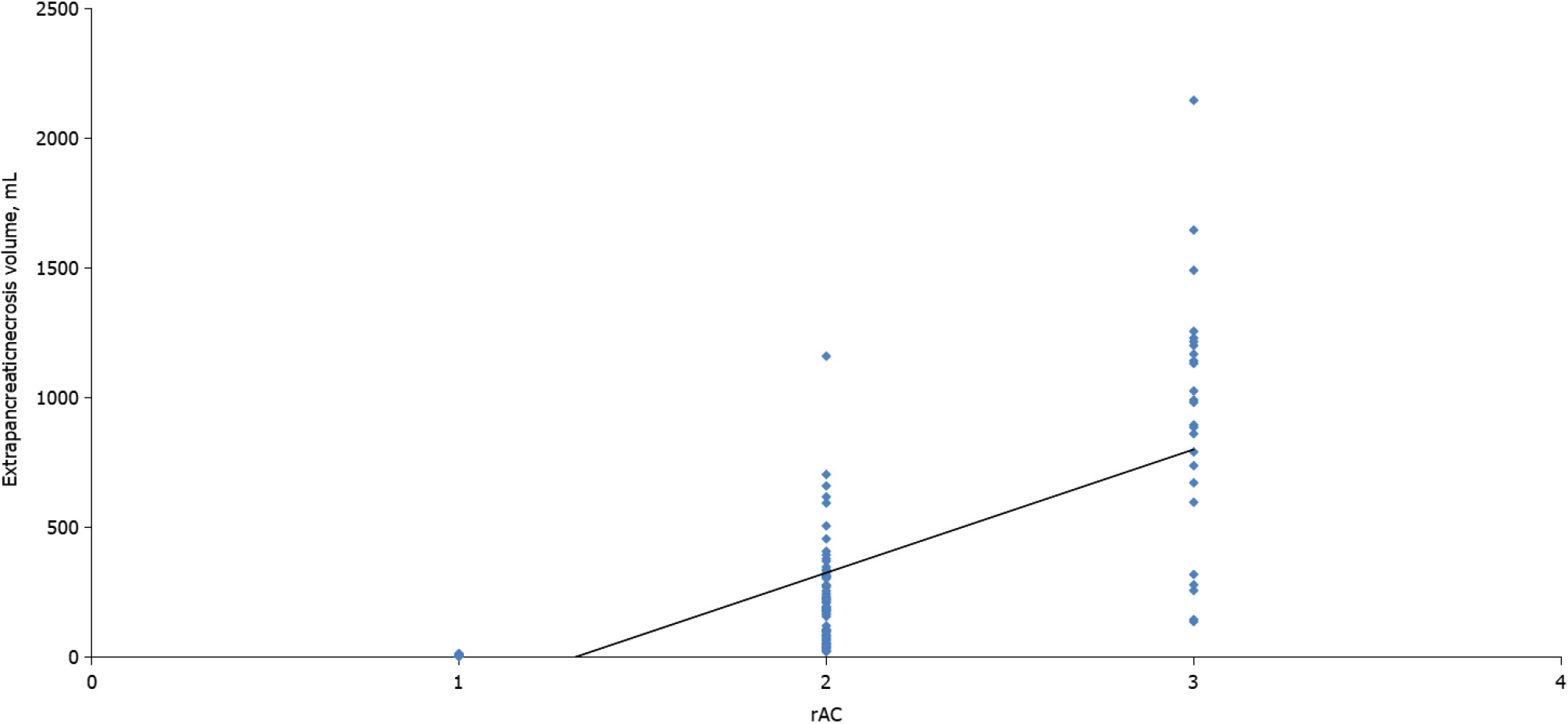
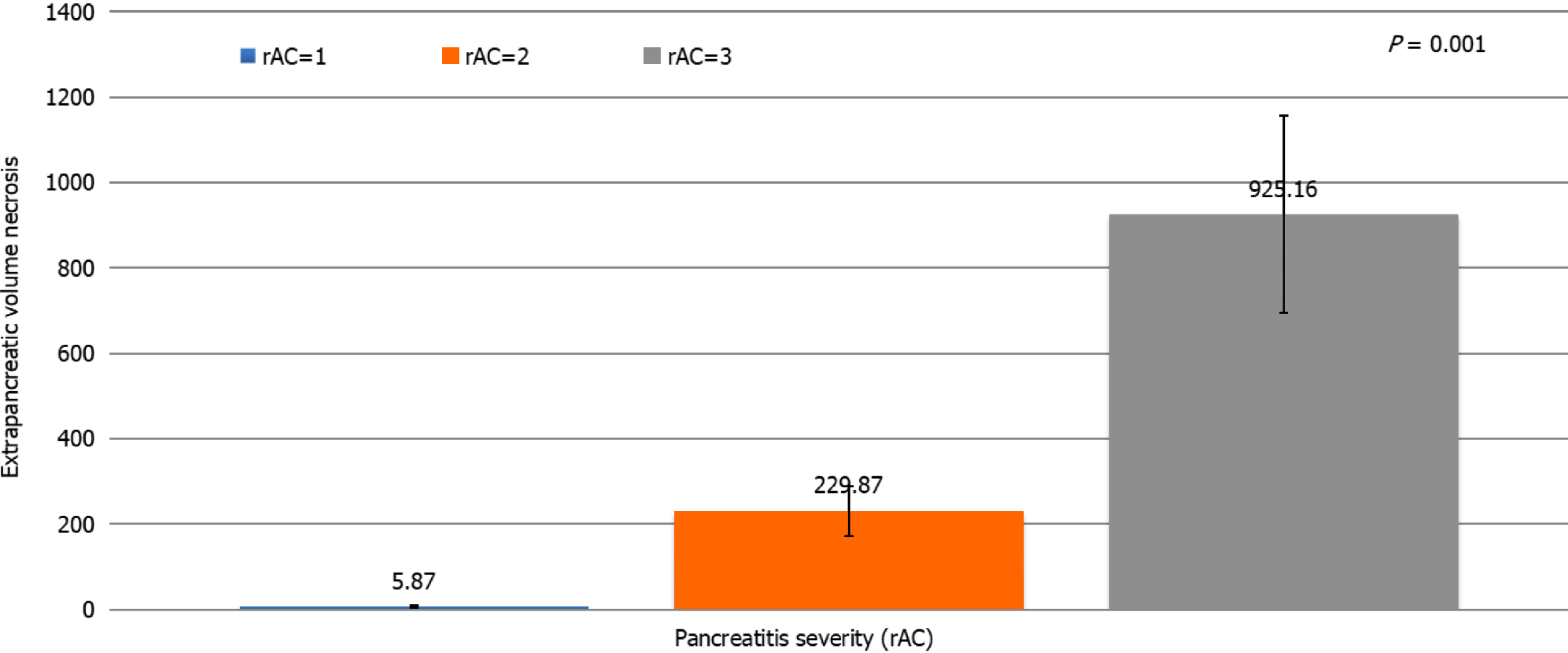
Most patients were men (66.8%) and more than half (56.1%) had a moderate form of acute peritonitis according to the rAC. Extrapancreatic necrosis was absent in 12 patients with mild pancreatitis. The patient characteristics are shown in Table 1. Highly significant correlations were observed between the CTSI (r = 0.926, P < 0.001), mCTSI (r = 0.950, P < 0.001), and extrapancreatic necrosis volume (r = 0.784, P < 0.001) imaging scores and the severity of pancreatitis estimated by the rAC (Figures 1-3), with mild, moderate, and severe disease indicated by values of 1, 2, and 3, respec
| Parameter | Pancreatitis sample, n = 123 |
| Sex | |
| Male, n (%) | 84 (68.3) |
| Female, n (%) | 39 (31.7) |
| Male/female ratio | 2.15 (84/39) |
| Age | |
| mean ± SD (min-max) | 50.38 ± 15.66 (20-92) |
| CTSI (1990) score (%) | |
| Mild: 0-3 | 41 (33.3) |
| Moderate: 4-6 | 75 (61) |
| Severe: 7-10 | 7 (5.7) |
| mCTSI (2007) score (%) | |
| Mild: 0-3 | 29 (23.6) |
| Moderate: 4-6 | 71 (57.7) |
| Severe: 7-10 | 22 (17.8) |
| rAC score (%) | |
| Mild | 29 (23.6) |
| Moderate | 69 (56.1) |
| Severe | 25 (20.3) |
| Necrosis volume in mL | |
| mean ± SD (min-max) | 352.38 ± 39.85 (5-2143) |
| CRP in mg/dL | |
| mean ± SD (min-max) | 11.62 ± 11.59 (0.02-51.60) |
Differences between the mean volumes of extrapancreatic necrosis observed at each level of rAC-defined severity were significant (Figure 5). The best predictor of severe acute pancreatitis was the necrosis volume [AUC = 0.993; 95% confidence interval (CI): 0.981-1.005)], with a 99.5% sensitivity, 99.0% specificity, and cutoff value of 167 mL, followed by the mCTSI (2007) score (AUC = 0.972; 95%CI: 0.946-0.999), with a 98.0% sensitivity and 96.5% specificity, and the CTSI (1990) score (AUC = 0.969; 95%CI: 0.941-0.998), with a 97.0% sensitivity and 95.0% specificity (Figure 6). CRP was a good predictor of severity (AUC = 0.613; 95%CI: 0.489-0.737) but with a lower sensitivity (62.5%) and specificity (50.0%) and a cutoff value of 7 mg/dL (Figure 6).
Since the early 1970s, attempts have been made to develop pancreatitis activity scoring systems that can identify acute pancreatitis with the potential to evolve to a severe condition, organ failure, and a need for intensive therapy. One of the first systems to use radiological methods (CT) was described by Balthazar et al[10] in 1985 and is widely used. It assessed the size of the pancreas and the inflammatory changes, peripancreatic fat, and peripancreatic collection. Its major disadvantage was that it did not assess pancreatic and peripancreatic necrosis. In 1990, the same investigator developed the CTSI, which combined the initial classification system with the presence and extent of pancreatic necrosis. The CTSI score had better diagnostic and prognostic accuracy than the initial Balthazar score[6], but it also has disadvantages. The score is not significantly correlated with the subsequent development of organ failure, extrapancreatic, parenchymal, or vascular complications[11]. In addition, Lecesne et al[12] reported low interobserver agreement between classes C and D and between classes D and E of the Balthazar subscore. Finally, the estimation of the degree of necrosis by the CTSI score is subjective and may be inaccurate for minor necrosis of less than 30%, or between 30% and 50%[7]. The CTSI score has been found to correlate with severe acute pancreatitis. Leung et al[13] reported that the CTSI score was superior to the APACHE II and Ranson scores in assessing severe pancreatitis in a sample of 121 patients[13]. They also found a strong correlation between the CTSI score and the development of systemic complications, including multiple organ failure and mortality[13]. Gürleyik et al[14] found that the CTSI score was better than clinical scores or CRP values in a sample of 55 patients with acute pancreatitis, but other studies do not support those observations. De Waele et al[15] did not find a significant association between CTSI and mortality, and Triantopoulou et al[16] did not find a correlation between severe acute pancreatitis and the CTSI score. In the present study, the CTSI score had a significant positive correlation with the severity of acute pancreatitis, and the ROC curve analysis confirmed a high 97% sensitivity and 95% specificity for predicting severe acute pancreatitis.
Given its limitations, Mortele et al[7] published a modified CTSI score in 2004. The mCTSI is easier to calculate than the CTSI and correlates more accurately with the patient’s progression. It was more accurate in predicting the length of hospital stay, the need for surgery, the risks of infection, and mortality than the CTSI developed by Balthazar. It combines three subscores that assess inflammation of the pancreas and peripancreatic fat, pancreatic or peripancreatic fluid accumulation, pancreatic necrosis of less than 30% or more than 30%, and extrapancreatic complications such as pleurisy, ascites, and vascular or gastrointestinal complications[7]. A recent study by Shaikh et al[17] reported that the mCTSI score had a 100% sensitivity and 87% specificity in distinguishing severe forms of pancreatitis and its complications. The accuracy of the two scores in discriminating the severe forms of pancreatitis has been evaluated in several studies[18,19]. In 2012, Bollen et al[18] did not report a difference in the performance of the two scores in assessing acute pancreatitis, and from a study by Sahu et al[19] published in 2017, the authors found that both scores were highly predictive of moderate-severe acute pancreatitis, with mCTSI being more sensitive (100% vs 97.1%) but less specific (92.3% vs 100%) compared with CTSI. Raghuwanshi et al[20] reported that the mCTSI score was easier to calculate and less operator-dependent than CTSI and that mCTSI had a stronger correlation with clinical pa
CRP is widely used in clinical practice and its benefit is significant if evaluated more than 48 h after the onset of symptoms. De Waele et al[15] reported that CRP was 84.6% sensitive and 73.8% specific with a cutoff value of 150 mg/L, and De la Peña et al[21] reported that with cutoff values of < 100 mg/L, CRP was 100% sensitive and 86% specific in predicting severe acute pancreatitis. Meyrignac et al[22] found that CRP had a sensitivity of 75% and specificity of only 67% in predicting organ failure with a cutoff value of 199 mg/L. Slight increases in sensitivity and specificity were seen when the cutoff was lowered to 150 mg/L. This study found that CRP was predictive of pancreatitis severity (AUC = 0.613; 95%CI: 0.489-0.737), but with a low sensitivity (62.5%) and specificity (50%) and a cutoff value of 70 mg/L. CRP was positively correlated with the severity of pancreatitis, but not significantly (r = 0.133, P = 0.154). These low cutoff values have been a consequence of the assessment of CRP in most patients at the time of diagnosis, which could also explain the reduced accuracy of CRP and its weaker correlations with disease activity[21].
Over time, several authors have noticed that patients with extensive extrapancreatic necrosis had a poorer prognosis in terms of organ failure, need for surgery, or mortality[23,24]. Kitamura et al[25] reported that low enhancement in early CT of the pancreatic parenchyma in the head and tail was independently associated with increased mortality in severe acute pancreatitis. Recently, attempts have been made to determine a cutoff value from which the volume of pancreatic necrosis can accurately assess severe pancreatitis. In 2015, Meyrignac et al[22] evaluated the correlation between the volume of extrapancreatic necrosis and the severity of acute pancreatitis and found that with an extrapancreatic necrosis cutoff volume of 114 mL, the occurrence of multiple organ failure was estimated with a sensitivity of 95% and specificity of 83%. In 2020, Çakar et al[26] reported that an average volume of 246.4 mL (median: 120.24 mL; range: 2-2135 mL) accurately predicted severe acute pancreatitis. In the present study, the volume of extrapancreatic necrosis and the severity of pancreatitis, quantified by the rAC, were correlated but less strongly than the CTSI and mCTSI scores (r = 0.784, P < 0.001). We consider that the extrapancreatic necrosis volume can accurately assess severe pancreatitis but less accurately than the mild and moderate forms. Comparative analysis of the volume of extrapancreatic necrosis showed significant differences (P < 0.01) with each degree of severity of acute pancreatitis. ROC curve analysis of the radiological scores and CRP found that the best predictor for the assessment of severe pancreatitis was the necrosis volume (AUC = 0.993; 95%CI: 0.981-1.005), with a sensitivity of 99.5%, specificity of 99.0%, and cutoff value of 167 mL. The advantage of necrosis volume is that it is a computer-based evaluation, which excludes the subjective parameters included in the Balthazar and mCTSI scores. Another advantage is that the assessment does not require the injection of contrast agents, which makes it particularly useful in patients with severe acute pancreatitis associated with acute renal failure. A disadvantage is that although there were significant differences in average necrosis volume calculated for each degree of severity, it appears that the correlation of necrosis volume was less strong than that seen with the CTSI and mCTSI scores. We consider that it occurred because of a weaker correlation with the mild and moderate forms, in which the necrosis volume can be very low or even absent.
The limitations of this study include the relatively small number of cases of severe acute pancreatitis (n = 20), which requires studies conducted in larger patient samples to validate a cutoff value. However, the observed differences reached statistical significance and we believe that it is of practical value, considering the fact that the percentage of cases of severe pancreatitis (25%) was clinically significant. Second, early CT images underestimate the extension of the pancreatic necrosis lesions. Therefore, CT examinations were performed 48-72 h after the disease onset. Third, the atypical distribution of severity in our study, with a low proportion of mild cases and frequent occurrence of moderate AP, may have resulted from a particularity of our center, as mild acute pancreatitis does not have a clinical indication for CT assessment. There
To conclude, the radiological severity scores correlated strongly and directly with disease activity. The best correlation was seen for the CTSI score (r = 0.926, P < 0.001). The extrapancreatic necrosis volume had the highest diagnostic accuracy for the severe form, with a 99.5% sensitivity and 99.0% specificity for a cutoff value of 167 mL. Extensive studies are required to validate the result. This study has increased the overall quality of evidence, compared with widely used imaging sores, for the inclusion of this score in clinical practice to predict severe pancreatitis. The difference between the study data reported by others consists in finding different cutoff values. Many additional studies and subjects are necessary to identify a common cutoff value that can be used in clinical practice.
Acute pancreatitis has increased in frequency over the past two decades and poses serious health threats. Mild and moderate forms have a benign evolution with rapid resolution of symptoms. Severe forms are a major therapeutic challenge and have a high mortality because of life-threatening complications. Rapid identification of patients with acute pancreatitis and a severe prognosis could lead to timely and more effective treatment and reduced morbidity and mortality.
Many scores derived from clinical, biological, and imaging markers have been proposed to assess the severity of pancreatitis at onset, including the Ranson score, APACHE II, and the Glasgow criteria. The computed tomography severity index (CTSI) and the modified CTSI (mCTSI) are the most widely used imaging scores for assessing the severity of pancreatitis. Since their development, there have been several attempts to design predictive imaging scores, but none has proven better. A recently studied indicator, the extrapancreatic necrosis volume, has shown promise.
The study aimed to: (1) Evaluate the discriminatory power of the extrapancreatic necrosis volume to identify severe acute pancreatitis; (2) Demonstrate a correlation between the extrapancreatic necrosis volume and the severity of acute pancreatitis; and (3) Improve the existing level of evidence regarding the performance of the extrapancreatic necrosis volume to detect severe acute pancreatitis and to pave the way for better and safer management of patients at risk.
We conducted a retrospective study of 123 patients, hospitalized at the Institute of Gastroenterology and Hepatology, Iaşi, Romania between January 1, 2017 and December 31, 2019. The pancreatitis was diagnosed by the revised Atlanta protocol in patients with two of the three following criteria: A clinically significant picture, a significant increase of pancreatic enzymes to at least three times the normal level, and significant radiological findings. The patient characteristics included age, sex, and C-reactive protein on hospital admission (normal value is < 0.5 mg/dL). Radiological scores (CTSI, mCTSI, and extrapancreatic necrosis volume) were calculated following computed tomography examination and within 48-72 h after the onset of symptoms.
Highly significant correlations were noticed between the imaging scores (CTSI, mCTSI, and extrapancreatic necrosis volume) and the severity of pancreatitis es
To conclude, the correlations of radiological severity scores with disease activity were positive and significant. The best correlation was seen for the CTSI score (r = 0.926, P < 0.001). The extrapancreatic necrosis volume had the highest diagnostic accuracy for severe pancreatitis, with a 99.5% sensitivity and 99.0% specificity for a cutoff value of 167 mL. The study increases the overall quality of evidence in support of the inclusion of this score in current practice, as it was more useful in predicting severe forms of pancreatitis compared with widely used imaging scores.
The results have significant impact and value. The relationship of severity with mortality has been documented in numerous evidence-based studies. Disease severity is the single most important indicator of a negative prognosis. Early recognition of severe disease and intervention could be lifesaving. Extensive study is required to validate the superiority of extrapancreatic necrosis volume to assess the severity of acute pancreatitis.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Berger Z, Kitamura K, Xiao B S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Bollen TL. Imaging of acute pancreatitis: update of the revised Atlanta classification. Radiol Clin North Am. 2012;50:429-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Delrue LJ, De Waele JJ, Duyck PO. Acute pancreatitis: radiologic scores in predicting severity and outcome. Abdom Imaging. 2010;35:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81. [PubMed] |
| 4. | Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 517] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 960] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 7. | Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, Perez A, vanSonnenberg E, Ros PR, Banks PA, Silverman SG. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004;183:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1735] [Article Influence: 54.2] [Reference Citation Analysis (1)] |
| 9. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4334] [Article Influence: 361.2] [Reference Citation Analysis (45)] |
| 10. | Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 341] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Ishikawa K, Idoguchi K, Tanaka H, Tohma Y, Ukai I, Watanabe H, Matsuoka T, Yokota J, Sugimoto T. Classification of acute pancreatitis based on retroperitoneal extension: application of the concept of interfascial planes. Eur J Radiol. 2006;60:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Leung TK, Lee CM, Lin SY, Chen HC, Wang HJ, Shen LK, Chen YY. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World J Gastroenterol. 2005;11:6049-6052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Gürleyik G, Emir S, Kiliçoglu G, Arman A, Saglam A. Computed tomography severity index, APACHE II score, and serum CRP concentration for predicting the severity of acute pancreatitis. JOP. 2005;6:562-567. [PubMed] |
| 15. | De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: evaluation of a new scoring system. Pancreas. 2007;34:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Triantopoulou C, Lytras D, Maniatis P, Chrysovergis D, Manes K, Siafas I, Papailiou J, Dervenis C. Computed tomography vs Acute Physiology and Chronic Health Evaluation II score in predicting severity of acute pancreatitis: a prospective, comparative study with statistical evaluation. Pancreas. 2007;35:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Shaikh R, Naz N, Zaheer S, Qadri Z, Khan A, Rana H. Role of Modified CT Severity Index in assessment of Acute pancreatitis in tertiary care Hospital. JIMDC. 2018;7:189-194. |
| 18. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Sahu B, Abbey P, Anand R, Kumar A, Tomer S, Malik E. Severity assessment of acute pancreatitis using CT severity index and modified CT severity index: Correlation with clinical outcomes and severity grading as per the Revised Atlanta Classification. Indian J Radiol Imaging. 2017;27:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Raghuwanshi S, Gupta R, Vyas MM, Sharma R. CT Evaluation of Acute Pancreatitis and its Prognostic Correlation with CT Severity Index. J Clin Diagn Res. 2016;10:TC06-TC11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 21. | De la Peña J, De las Heras G, Galo Peralta F, Casafont F, Pons Romero F. [Prospective study of the prognostic value of C reactive protein, alpha 1-antitrypsin and alpha 1-acid glycoprotein in acute pancreatitis]. Rev Esp Enferm Dig. 1991;79:337-340. [PubMed] |
| 22. | Meyrignac O, Lagarde S, Bournet B, Mokrane FZ, Buscail L, Rousseau H, Otal P. Acute Pancreatitis: Extrapancreatic Necrosis Volume as Early Predictor of Severity. Radiology. 2015;276:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Bakker OJ, van Santvoort H, Besselink MG, Boermeester MA, van Eijck C, Dejong K, van Goor H, Hofker S, Ahmed Ali U, Gooszen HG, Bollen TL; Dutch Pancreatitis Study Group. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Rana SS, Sharma V, Sharma RK, Chhabra P, Gupta R, Bhasin DK. Clinical significance of presence and extent of extrapancreatic necrosis in acute pancreatitis. J Gastroenterol Hepatol. 2015;30:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Kitamura K, Horibe M, Sanui M, Sasaki M, Yamamiya A, Ishii Y, Yoshida H, Sawano H, Goto T, Ikeura T, Hamada T, Oda T, Yasuda H, Ogura Y, Miyazaki D, Hirose K, Chiba N, Ozaki T, Yamashita T, Koinuma T, Oshima T, Yamamoto T, Hirota M, Azumi Y, Nagata K, Saito N, Sato M, Miyamoto K, Iwasaki E, Kanai T, Mayumi T. The Prognosis of Severe Acute Pancreatitis Varies According to the Segment Presenting With Low Enhanced Pancreatic Parenchyma on Early Contrast-Enhanced Computed Tomography: A Multicenter Cohort Study. Pancreas. 2017;46:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Çakar İ, Keven A, Eseroğlu E, Çubuk SM. Role of extrapancreatic necrosis volume in determining early prognosis in patients with acute pancreatitis. Abdom Radiol (NY). 2020;45:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |