Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.758
Peer-review started: November 27, 2020
First decision: December 21, 2020
Revised: December 22, 2020
Accepted: December 30, 2020
Article in press: December 30, 2020
Published online: January 26, 2021
Processing time: 54 Days and 5.4 Hours
The in-stent restenosis (ISR) rates are reportedly inconsistent despite the increased use of second-generation drug eluting stent (DES). Although bioresorbable vascular scaffold (BVS) have substantial advantages with respect to vascular restoration, the rate of scaffold thrombosis is higher with BVS than with DES. Optimal treatment strategies have not been established for DES-ISR to date.
We report on a case of a 60-year-old man patient with acute coronary syndrome. He had a history of ST-segment elevation myocardial infarction associated with very late scaffold thrombosis and treated with a DES. Coronary angiography revealed significant stenosis, suggesting DES-ISR on the previous BVS. Optical coherence tomography (OCT) identified a plaque rupture and a disrupted scaffold strut in the neointimal proliferation of DES. To treat the DES-ISR on the previous BVS, we opted for a drug-coated balloon (DCB) after a balloon angioplasty using a semi-compliant and non-compliant balloon. The patient did not experience adverse cardiovascular events on using a DCB following the use of intensive dual antiplatelet therapy and statin for 24 mo.
This case highlights the importance of OCT as an imaging modality for characterizing the mechanism of target lesion failure. The use of a DCB following the administration of optimal pharmacologic therapy may be an optimal strategy for the treatment and prevention of recurrent BVS thrombosis and DES-ISR.
Core Tip: In-stent restenosis still poses a limitation of percutaneous coronary intervention and bioresorbable vascular scaffold are associated with a high rate of target lesion revascularization We present herein, a rare case of restenosis of a drug eluting stent on the previous bioresorbable vascular scaffold. Optical coherence tomography is an instrumental imaging modality for identifying the mechanisms underlying target lesion failure. Drug-coated balloon following the administration of optimal pharmacologic therapy may be an optimal strategy for the treatment and prevention of recurrent target lesion failure.
- Citation: Jang HG, Kim K, Park HW, Koh JS, Jeong YH, Park JR, Kang MG. Restenosis of a drug eluting stent on the previous bioresorbable vascular scaffold successfully treated with a drug-coated balloon: A case report. World J Clin Cases 2021; 9(3): 758-763
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/758.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.758
Although percutaneous coronary intervention (PCI) with drug eluting stent (DES) is the standard of care for the treatment of coronary artery stenosis, in-stent restenosis (ISR) still poses a limitation[1]. Optimal treatment strategies for DES-ISR have not been established to date[2]. Bioresorbable vascular scaffold (BVS) have substantial advantages with respect to vascular restoration[3,4]. However, BVS are associated with a high rate of target lesion revascularization (TLR)[5] .Optical coherence tomography (OCT) is an instrumental imaging modality for identifying the mechanisms underlying target lesion failure (TLF), especially in cases involving BVS. Here, we present a case of acute coronary syndrome (ACS) caused due to DES-ISR on the previous BVS. To the best our knowledge, this is the first report on ACS caused by restenosis of a second-generation DES on the previous BVS. Patient has provided informed consent for publication of the case.
A 60-year-old man was referred to the emergency room with a complaint of chest pain.
Patient’s symptoms started a 3 d ago with recurrent episodes during exertion, which had been worsened the last 1 h.
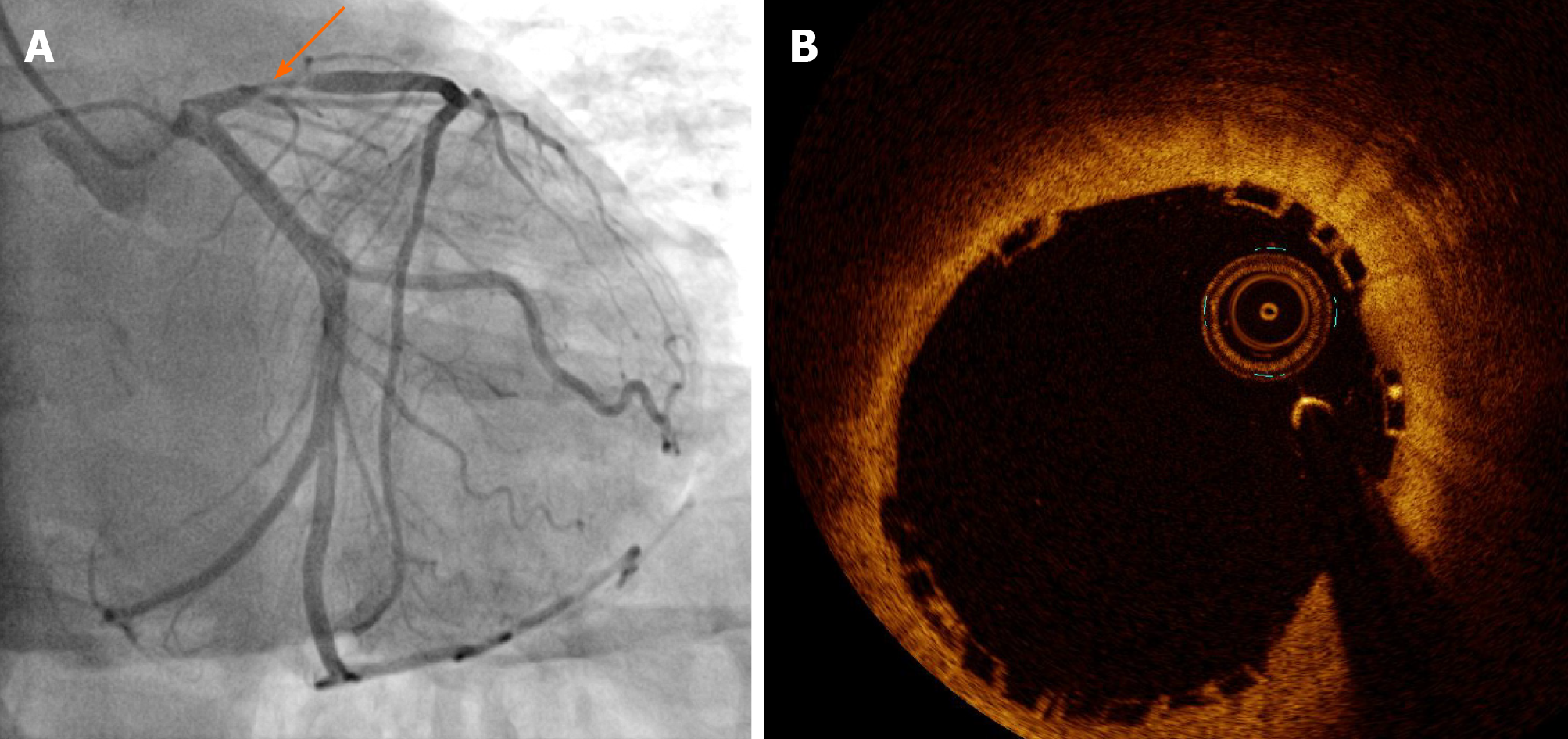
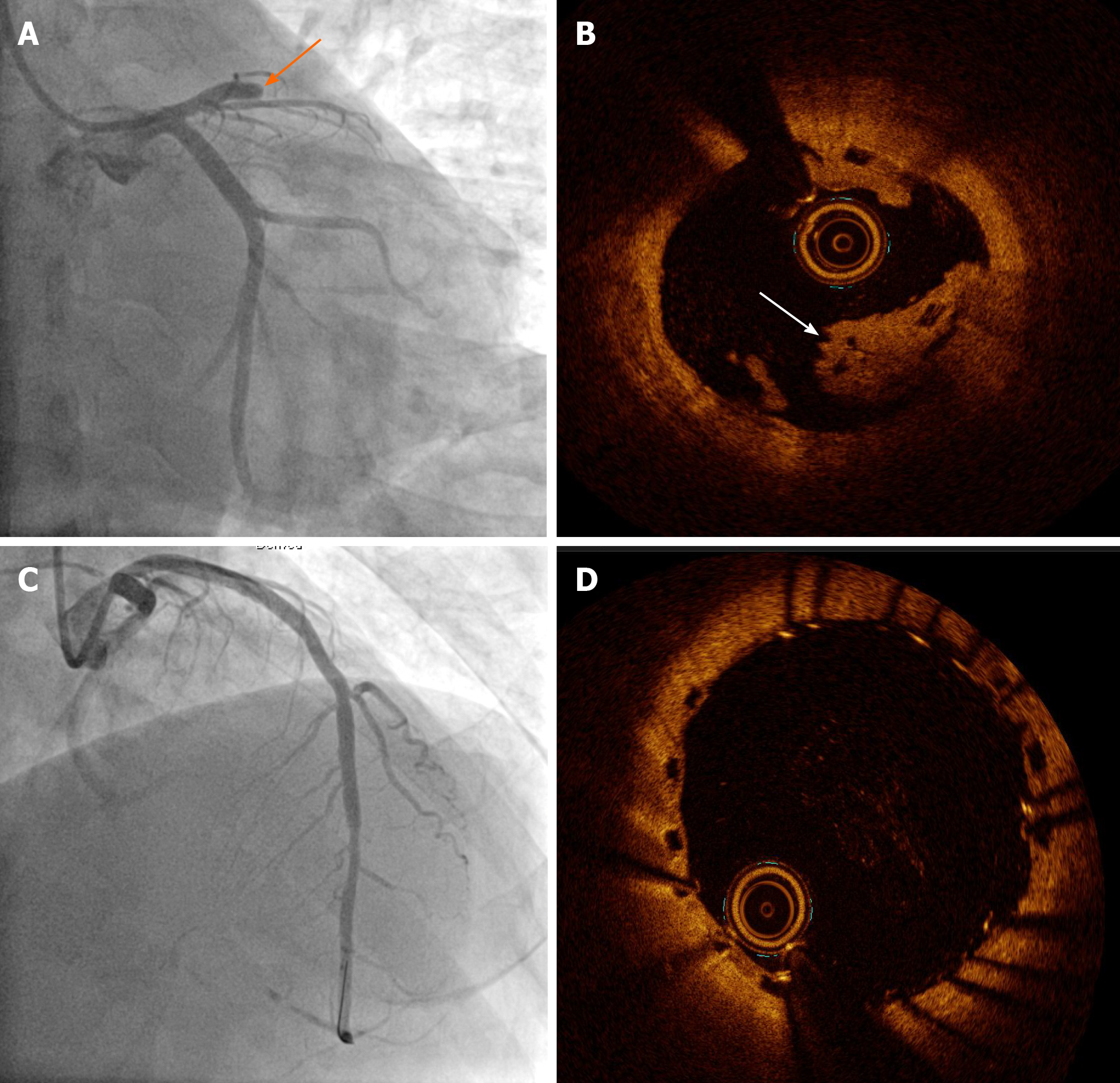
He had a history of unstable angina treated with a BVS (3.5 mm × 18 mm Absorb GT1 everolimus-eluting bioresorbable scaffold, Abbott Vascular, Santa Clara, CA, United States) in the proximal left anterior descending artery (LAD) 36 mo prior to the current presentation (Figure 1). Twenty months ago, he was diagnosed with ST-segment elevation myocardial infarction associated with very late scaffold thrombosis (Figure 2A). A red thrombus associated with disruption of the scaffold strut was identified on OCT (Dragonfly, St. Jude Medical, St. Paul, MN, United States; (Figure 2B). We treated the patient with a DES (3.5 mm × 33 mm XIENCE everolimus-eluting stent, Abbott Vascular, Santa Clara, CA, United States), covering the whole segment of the previous scaffold (Figure 2C and D).
The patient’s blood pressure was 130/80 mmHg, heart rate was 66 bpm, respiratory rate was 14 breaths per minute, temperature was 36.8 °C, and oxygen saturation in room air was 98%.
Baseline electrocardiography showed Q waves in precordial leads and blood test including cardiac enzymes were normal.
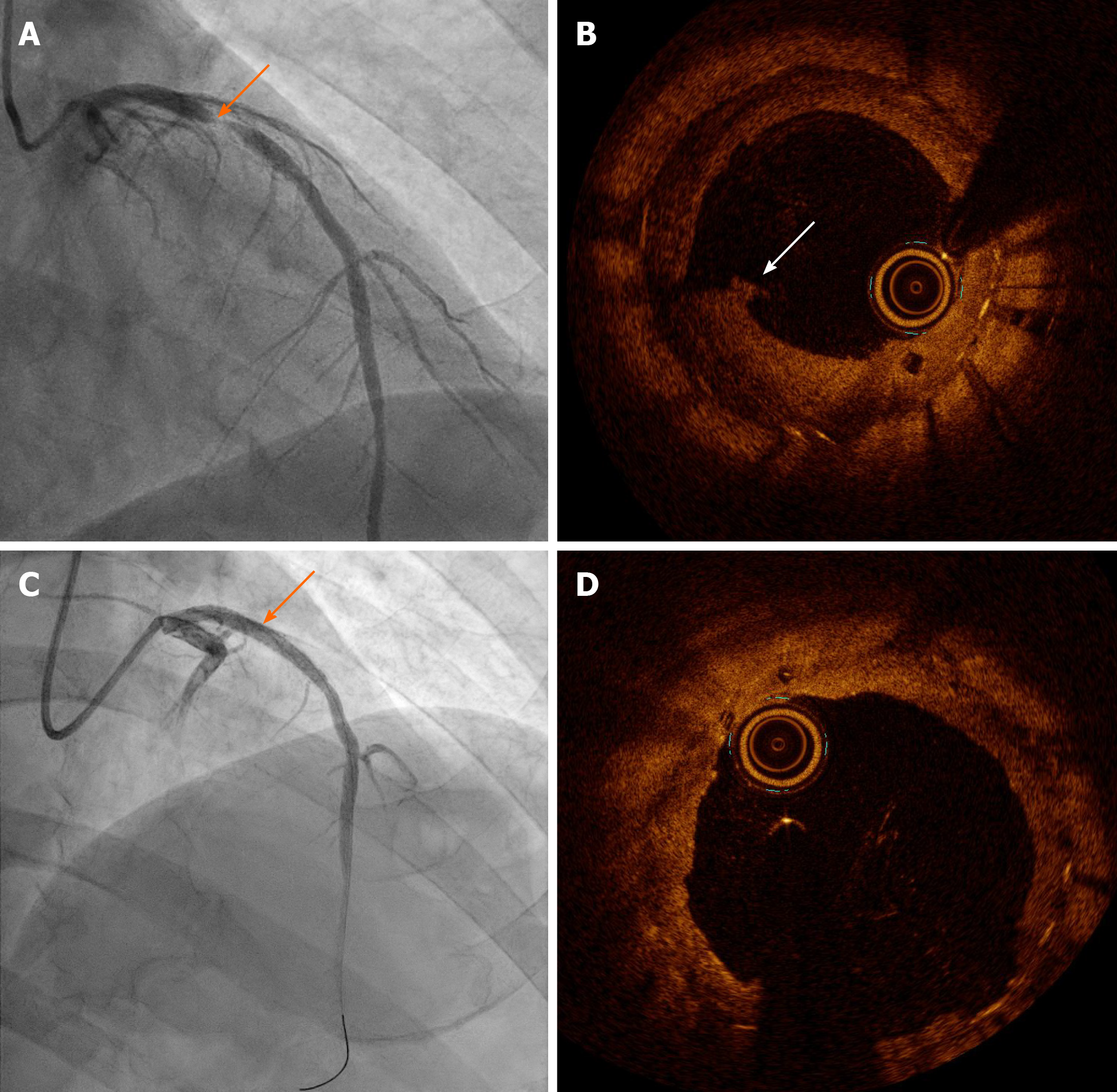
Coronary angiography (CAG) revealed tight stenosis (diameter > 90%) of the proximal LAD, suggesting DES-ISR on the previous BVS (Figure 3A). OCT indicated plaque rupture and a disrupted scaffold strut in the neointimal proliferation of DES (Figure 3B).
The final diagnosis of the presented case is unstable angina associated with restenosis of a DES on the previous BVS.
We performed a balloon angioplasty using a 2.5 mm × 20 mm semi-compliant balloon and a 3.0 mm × 12 mm non-compliant balloon to treat the DES-ISR on the previous BVS. We opted for a drug-coated balloon (DCB) rather than a DES. After placement of the DCB (3.5 mm × 26 mm SeQuent Please paclitaxel-eluting balloon, B. Braun, Melsungen, Germany) at 16 atm (up to diameter 4.0 mm, for 60 min), CAG revealed an acceptable residual stenosis of < 10% (Figure 3C). OCT showed achievement of optimal luminal gain (minimal/mean lumen diameter, 3.25/3.45 mm, minimal lumen area, 9.45 mm2, Figure 3D). He was discharged on the day after the procedure and was prescribed 90 mg ticagrelor twice daily, 100 mg aspirin daily, and 20 mg rosuvastatin daily.
The patient had an uneventful clinical course for 24 mo. At 12 mo follow-up, we deescalated to 60 mg ticagrelor twice daily.
We report a case of ACS caused by restenosis of a second-generation DES on the previous BVS. The patient did not experience adverse cardiovascular events on using a DCB following the use of intensive dual antiplatelet therapy (DAPT) and statin for 12 mo.
In patients with coronary artery stenosis who receive a metallic DES, adverse events, such as late TLF may be related, in part, to the persistent presence of the metallic stent frame in the coronary vessel wall[3] .Theoretically, the use of a BVS was considered a promising treatment for coronary artery disease associated with a favorable tissue response[4]. However, the cumulative 5-year adverse event rates were higher in patients with a BVS than in those with DESs; thus, restricted use of BVSs was noted in current clinical practice[5].
Recent real-world long-term data reported that lesion complexity and more pronounced cardiovascular risk factors were the determinants of TLF of BVS[6]. In this case, very late scaffold thrombosis was associated with peri-strut low-intensity area and strut discontinuity[7]. We used the DES to cover the entire segment of the previous scaffold, following the administration of continuous intensive DAPT with ticagrelor and aspirin. Unfortunately, the patient experienced an emergent revascularization after the procedure, and we performed OCT-guided PCI using a DCB.
In contemporary clinical practice, the management of ISR remains challenging. When selecting a treatment strategy for ISR, the specific advantages and disadvantages of DESs and DCBs should be carefully weighed. Although the use of a DES resulted in superior late angiographic findings in the treatment of ISR[8], repeat implantation of a DES remains, arguably, the most widely used treatment strategy. The use of a DCB for ISR is an effective treatment option; additional stent implantation is hence, not required and the outcomes are similar with those noted with TLR[9]. Thus, the use of a DCB can be an attractive treatment option for patients with recurrent stent or scaffold failure.
To best our knowledge, this case report of ACS by restenosis of second-generation DES on the prior BVS is the earliest in the literature. In this case, the structural deformities associated with the previous BVS such as, disruption of the scaffold strut, were identified as a risk factor for TLF. Hence, tailored strategies involving imaging-guided PCI following intensive medical treatment are necessary to prevent thrombosis and neo-atherosclerosis after the implantation of a BVS and DES[10-12]. To the best of our knowledge, the presented case is the first in the literature describing the evolution of ACS associated with restenosis of a DES on the previous BVS.
This case highlights the importance of OCT as an imaging modality for characterizing the mechanism of target lesion failure. The use of a DCB following the administration of optimal pharmacologic therapy may be an optimal strategy for the treatment and prevention of recurrent BVS thrombosis and DES-ISR.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hussein A S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 603] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 2. | Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez-Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, Jüni P, Windecker S. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 3. | Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW; ABSORB III Investigators. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med. 2015;373:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 4. | Karanasos A, Simsek C, Gnanadesigan M, van Ditzhuijzen NS, Freire R, Dijkstra J, Tu S, Van Mieghem N, van Soest G, de Jaegere P, Serruys PW, Zijlstra F, van Geuns RJ, Regar E. OCT assessment of the long-term vascular healing response 5 years after everolimus-eluting bioresorbable vascular scaffold. J Am Coll Cardiol. 2014;64:2343-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Kereiakes DJ, Ellis SG, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, Tan SH, Ediebah DE, Simonton C, Stone GW; ABSORB III Investigators. Clinical Outcomes Before and After Complete Everolimus-Eluting Bioresorbable Scaffold Resorption: Five-Year Follow-Up From the ABSORB III Trial. Circulation. 2019;140:1895-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Wiebe J, Baquet M, Dörr O, Hoppmann P, Jochheim D, Rheude T, Boeder N, Grundman D, Blachutzik F, Theiss H, Cassese S, Hofmann FJ, Gschwendtner S, Elsässer A, Massberg S, Hamm C, Laugwitz KL, Byrne RA, Mehilli J, Kastrati A, Nef H. Long-term follow-up and predictors of target lesion failure after implantation of everolimus-eluting bioresorbable scaffolds in real-world practice. Int J Cardiol. 2020;312:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Cuculi F, Puricel S, Jamshidi P, Valentin J, Kallinikou Z, Toggweiler S, Weissner M, Münzel T, Cook S, Gori T. Optical Coherence Tomography Findings in Bioresorbable Vascular Scaffolds Thrombosis. Circ Cardiovasc Interv. 2015;8:e002518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, García-Touchard A, López-Minguéz JR, Benedicto A, Masotti M, Zueco J, Iñiguez A, Velázquez M, Moreno R, Mainar V, Domínguez A, Pomar F, Melgares R, Rivero F, Jiménez-Quevedo P, Gonzalo N, Fernández C, Macaya C; RIBS IV Study Investigators (under auspices of Interventional Cardiology Working Group of Spanish Society of Cardiology). A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol. 2015;66:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 9. | Baan J Jr, Claessen BE, Dijk KB, Vendrik J, van der Schaaf RJ, Meuwissen M, van Royen N, Gosselink ATM, van Wely MH, Dirkali A, Arkenbout EK, de Winter RJ, Koch KT, Sjauw KD, Beijk MA, Vis MM, Wykrzykowska JJ, Piek JJ, Tijssen JGP, Henriques JPS. A Randomized Comparison of Paclitaxel-Eluting Balloon Versus Everolimus-Eluting Stent for the Treatment of Any In-Stent Restenosis: The DARE Trial. JACC Cardiovasc Interv. 2018;11:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Moriyama N, Shishido K, Tanaka Y, Yokota S, Hayashi T, Miyashita H, Koike T, Yokoyama H, Takada T, Nishimoto T, Ochiai T, Tobita K, Yamanaka F, Mizuno S, Murakami M, Takahashi S, Saito S. Neoatherosclerosis 5 Years After Bioresorbable Vascular Scaffold Implantation. J Am Coll Cardiol. 2018;71:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; PLATO Investigators; Freij A; Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4952] [Cited by in RCA: 5169] [Article Influence: 323.1] [Reference Citation Analysis (0)] |
| 12. | Gili S, Iannaccone M, Colombo F, Montefusco A, Amabile N, Calcagno S, Capodanno D, Scalone G, Rognoni A, Omedè P, Ugo F, Cavallo E, Mancone M, Mangiameli A, Boccuzzi G, Hiansen J, Motreff P, Toutouzas K, Garbo R, Sardella G, Tamburino C, D'Amico M, Moretti C, Templin C, Gaita F, Souteyrand G, Niccoli G, D'Ascenzo F. Effects of statins on plaque rupture assessed by optical coherence tomography in patients presenting with acute coronary syndromes: insights from the optical coherence tomography (OCT)-FORMIDABLE registry. Eur Heart J Cardiovasc Imaging. 2018;19:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |