Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.748
Peer-review started: November 5, 2020
First decision: November 23, 2020
Revised: December 2, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: January 26, 2021
Processing time: 75 Days and 15.1 Hours
Post-transplant lymphoproliferative disease (PTLD) is a heterogeneous group of diseases that develop after solid organ and hematopoietic stem cells transplantation related to intensive immunosuppression regimen, T-cell depletion and Epstein-Barr virus infection. Despite the improvement in the management of PTLD, the prognosis remains poor. Here we report the management of two transplanted patients with PTLD and infections during immunochemotherapy (ICTH).
Of 65-year-old woman 11 years after kidney transplantation (first case) presented with diffuse large B-cell lymphoma (DLBCL) CS III and started ICHT according to R-CHOP protocol. Despite the secondary prevention of neutropenic fever, the patient developed grade 4 neutropenia with urinary and pulmonary tract infections after the fifth cycle. ICTH was continued in reduced doses up to 7 cycles followed by involved-field radiation therapy of the residual disease. The second case presents a 49-year-old man, 8 years after liver transplantation due to cirrhosis in the course of chronic hepatitis B, who started ICTH for DLBCL Burkitt-like CS IV. The patient received four cycles of ICTH according to R-CODOX/R-IVAC protocol, with reduced doses. In both cases initially undertaken reduction of immunosuppression was ineffective to prevent infectious complications. Despite one incomplete ICHT treatment due to recurrent infections, both our patients remain in complete remission.
Reduction of immunosuppression and the doses of chemotherapeutics may be insufficient to prevent infectious complications during ICTH in PTLD patients.
Core Tip: Post-transplant lymphoproliferative disease (PTLD) is a heterogeneous group of diseases in transplantated patients related to immunosuppression regimen, T-cell depletion and Epstein-Barr virus infection. Immunochemotherapy (ICHT) increases already high incidence of bacterial infections in transplanted patients related to the immunosuppression therapy. We report the successful management of two solid organ transplanted patients with PTLD and urinary and pulmonary tract infections infections during ICTH that developed regardless of the reduction of immunosuppression therapy, doses of chemotherapeutics and GCS-F used in the prevention of neutropenic fever. We show that all these interventions may be insufficient to prevent infectious complications, but they are manageable.
- Citation: Gładyś A, Kozak S, Wdowiak K, Winder M, Chudek J. Infectious complications during immunochemotherapy of post-transplantation lymphoproliferative disease–can we decrease the risk? Two case reports and review of literature. World J Clin Cases 2021; 9(3): 748-757
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/748.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.748
Post-transplant lymphoproliferative disease (PTLD) is a heterogeneous group of diseases that develop after solid-organ and hematopoietic stem cells transplant[1]. 2016 WHO classification distinguished several subcategories: Plasmacytic hyperplasia, infectious mononucleosis, florid follicular hyperplasia, polymorphic (P-PTLD), monomorphic (M-PTLD) and classical Hodgkin lymphoma PTLD[2]. Most P-PTLD cases are EBV positive and develop shortly after transplantation. About half of M-PTLD is associated with EBV infection, and most cases are large B-cell lymphoma (DLBCL), less often Burkitt's lymphoma (BL)[3]. The highest rate of PTLD occurs after small bowel (20%), lung (10%), and heart (6%) transplant. Liver and kidney transplantations are associated with much lower risk, accounted for 2.8% and 2.3%[4].
The main risk factors for PTLD are the intensity of immunosuppression regimen, T-cell depletion and Epstein-Barr virus (EBV) infection[1]. Transplant patients who are EBV-negative before transplantation have a higher risk of PTLD. Both primary and reactivated EBV infections are potent risk factors for PTLD[5]. The infections are often asymptomatic, with a high rate of latency[5].
Clinically, PTLD is hardly distinguishable from aggressive B-cell lymphoma[6]. Immunosuppression reduction (IR) is the first step in PTLD management with overall response rate (ORR) of 45%, in the retrospective analysis[7]. The prospective study has shown response in 1 out of 16 patients (ORR-6%)[7]. Due to the rare occurrence of PTLD, there are currently no guidelines based on hard scientific evidence for further management of patients with PTLD.
Here we report the management of two patients with PTLD and infections during immunochemotherapy (ICTH).
Case 1: A 65-year-old woman with an extensive medical history including end-stage kidney disease, subtotal parathyroid gland removal for severe hyperparathyroidism and subtotal thyroidectomy, renal osteodystrophy, hypertension and atherosclerosis; received a kidney transplant from a deceased donor after four years of hemodialysis therapy (2006). The donor Epstein-Barr virus (EBV) status was unknown (pre-2008 donors and recipients were not routinely screened for EBV serostatus)[8]. The immunosuppressive regimen consisted of mycophenolate mofetil and cyclosporine A.
Case 2: Forty-nine-year old man 8 years after liver transplantation for end-stage chronic hepatitis B, treated with tacrolimus and mycophenolate mofetil, was admitted to the neurological ward due to severe headache on the right side with nausea and vomiting, accompanied by the numbness of his cheek, which lasted for two weeks (October 2015). The patient reported a weight loss of 8-10 kg during the last 6 mo and presented with fever.
Case 1: Eleven years since transplantation (2017) the patient was referred to the transplant ward with persisting night sweats and fatigue. There was no fever and weight loss.
Case 2: The patient was admitted to neurological ward due to the cheek numbness. Head magnetic resonance imaging revealed the infiltration of right pterygoideus medialis muscle. He was then referred to the transplantat ward, with the suspicion of neoplastic changes. After histological examination, the patient was referred to the oncology department.
Case 1: The patient had a history of end-stage kidney disease with hemodialysis treatment started in 2002 and bilateral nephrectomy in 2004 followed by kidney transplantation two years later.
Case 2: HBV infection, which led to chronic B hepatitis and cirrhosis, followed by liver transplantation in 2006.
Case 1: There was long-lasting history of hypertension. Family history was unremarkable.
Case 2: The patient had hypertension and reported Helicobacter pylori stomach infection. Family history was unremarkable.
Case 1: There was no lymadenopathy in physical examination. Liver and spleen were not enlarged.
Case 2: Physical examination revealed pleural effusion in the right cavity and symmetric ankles oedema.
Case 1: Serologic tests for EBV were positive for IgG-class and EBNA, IgM-class was negative. The fine needle biopsy of the submandibular gland revealed monomorphic subtype of PTLD-DLBCL, the most frequent type of PTLD[9]. The patient was referred to the oncological ward for further diagnostic work-up. The blood tests showed leukocytosis 21.65 G/L without thrombocytopenia, mild anaemia (Hb 10.6 g/dL) increased activity of LDH (291 IU/L), and high concentration of β2-microglobulin (13.5 mg/L). The graft function was impaired (eGFR 24 mL/min/1.73m2 without proteinuria).
Case 2: Histological examination of stomach biopsy gave the diagnosis of aggressive (Burkitt-like) DLBCL with CD20 (+), CD10 (+), bcl-6 (+), bcl-2 (-), MUM-1 (+/-), cyclin D1 (-), CD30 (-), CD5 (-), Ki67–almost 100%. Location of the lesions on both sides of the diaphragm in symptomatic patients (loss of weight), was corresponding to the clinical stage was IVB (IPI 3/5). HBV DNA in the blood was not detected. The patient was IgM seronegative but IgG seropositive for both cytomegalovirus (CMV) and EBV. The patients’ immunosuppression regiment was modified-mycophenolate mofetil was switched to everolimus for its anticancer properties but did not prevent DLBCL progression.
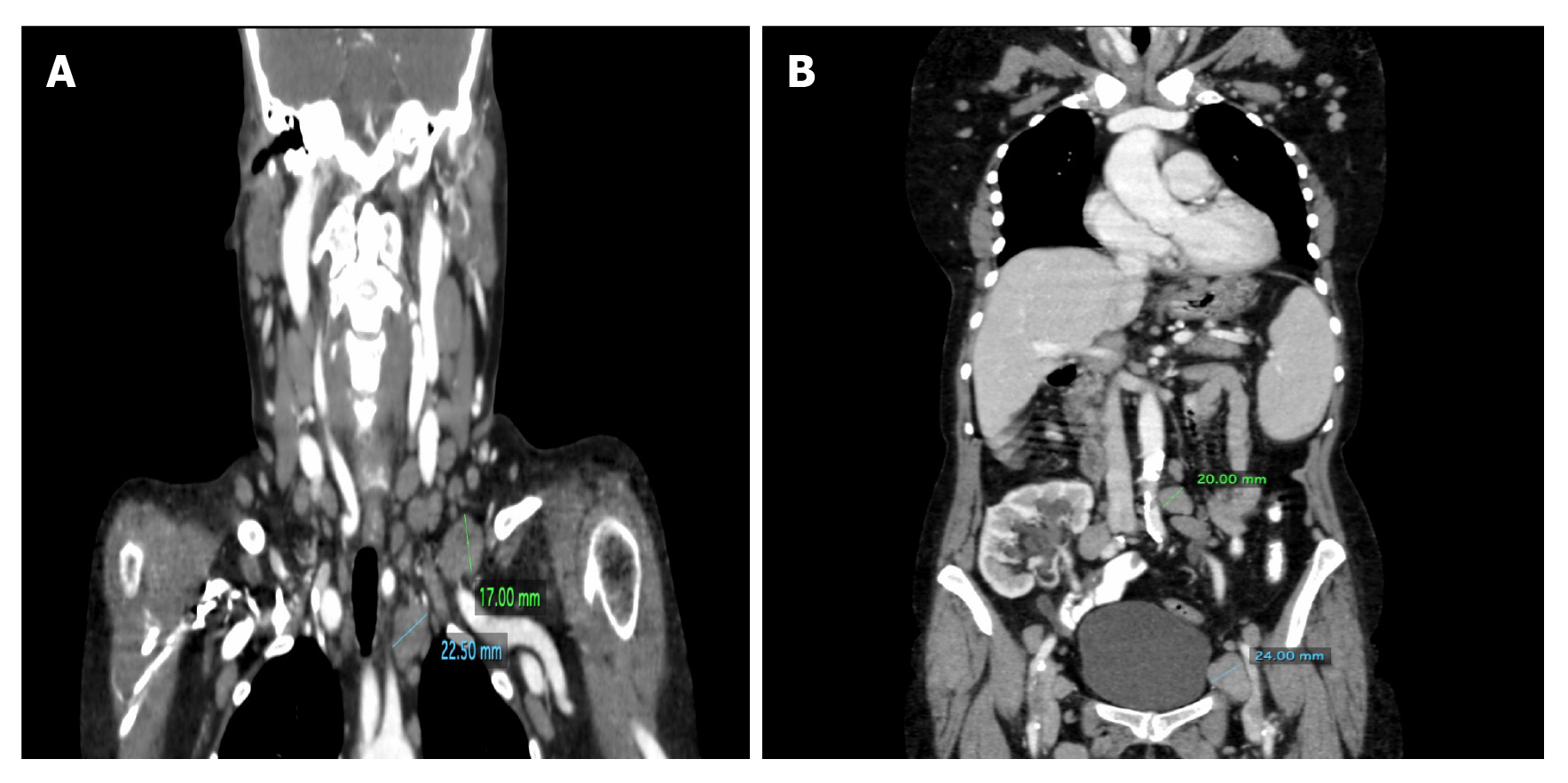
Case 1: The computed tomography (CT) examinations revealed numerous enlarged cervical, mediastinal, axillary, retroperitoneal and inguinal lymph nodes on both sides with a predominance on the left (Figure 1) as well as slight hepatosplenomegaly.
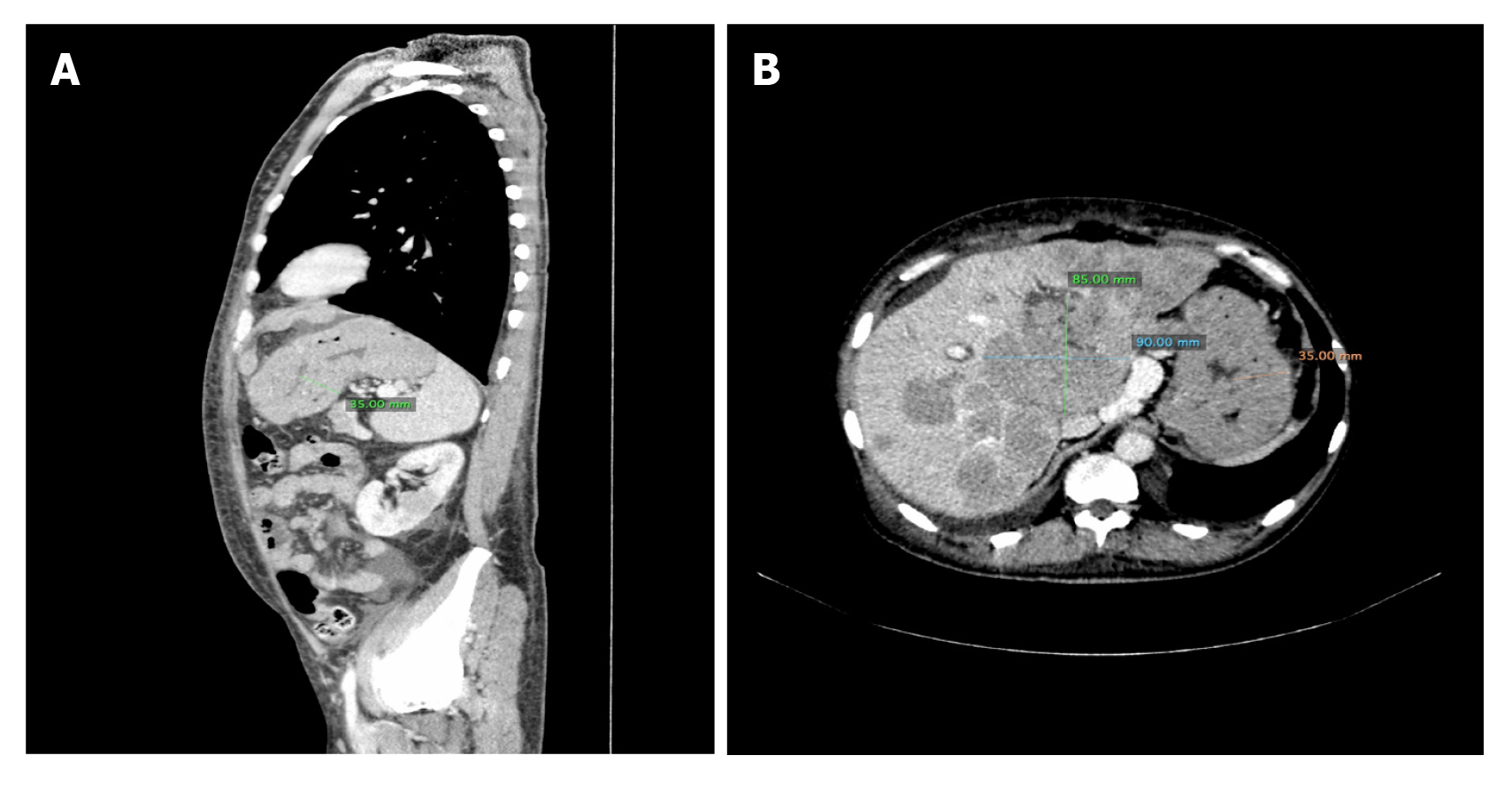
Case 2: CT scan of the brain was normal, while lumbar puncture revealed elevated LDL activity in cerebrospinal fluid. The patient was referred to the transplantation ward with suspicion of neoplastic infiltration. CT scan of the thorax and abdomen revealed numerous hypovascular structures in the liver and kidneys, massive infiltration of mucosa of the stomach and two nodule changes close to the left subclavian vein, below the sternocleidomastoid muscle (Figure 2).
Case 1: Taking into account all the symptoms and tests, a diagnosis of PLTD was made.
Case 2: Taking into account all the symptoms and tests, a diagnosis of PLTD was made.
Case 1: The patient signed informed consent for ICTH and started R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), as initial rituximab monotherapy increases the risk of recurrence and usually does not bring complete remission in monomorphic PTLD[9]. Beyond slight, local upper limbs oedema, the patient tolerated the applied ICTH quite well. Night sweats subsided after the second R-CHOP cycle. Neutropenia and anaemia (requiring transient blood transfusion) were manageable. An episode of atrial fibrillation occurred after the second cycle. The immunosuppression, as well as the doses of chemotherapeutics, were reduced (mycophenolate mofetil was discontinued) due to episodes of prolonged pancytopenia. The G-CSF treatment (filgrastim) in the secondary prevention of neutropenic fever (NF) was used. Regardless of the use of GCS-F and Pneumocystis jiroveci prophylaxis (with co-trimoxazole), after the fifth cycle, the patient developed grade 4 neutropenia with symptoms of febrile neutropenia. There was an increase in the concentration of inflammatory markers: CRP 217 mg/L and procalcitonin 40.1 ng/mL. Empirical antibiotic therapy with ciprofloxacin was started. After one week, regardless of neutrophil recovery, inflammatory parameters were still elevated and the symptoms (fever, weakness and dysuria) persisted. Blood culture showed a growth of Staphylococcus aureus (sensitive to ciprofloxacin) and Klebsiella pneumoniae ESBL (+) (resistant to ciprofloxacin and co-trimoxazole, but sensitive to carbapenems and aminoglycosides). Broad-spectrum antibiotic therapy with meropenem (1 g for every 12 h) and amikacin (500 mg once a day) was administered. Three days later, there was a worsening of dyspnea, cough and decreased oxygen saturation of 88%-90%, without an increase in the concentration of inflammatory markers. Pneumocystis jiroveci infection was suspected and a smear from the epiglottis was taken for the examination. Additionally, co-trimoxazole was administered intravenously at a dose of 960 mg twice daily. Few days later, a significant improvement of the patient's clinical condition with a decrease of inflammatory markers concentration (CRP 14.8 mg/L and PCT 0.4 ng/mL) was observed. The patient recovered, however with persisting impaired graft function. The doses of ICTH were reduced by 30% in the next 2 cycles. Despite dose reduction, the therapy had been terminated because of grade 4 neutropenia.
PET-CT performed after the seventh cycle revealed active lesions in sub- and infraclavicular area with the avidity of 4 points in the Deauville five-point scale. The patient started involved-field radiation therapy (3D-IMRT, Dc = 30 Gy/df = 3) of the residual disease.
Case 2: The patient signed informed consent for ICTH with 4 cycles of R-CODOX (rituximab, cyclophosphamide doxorubicin, vincristine, cytarabine) followed by R-IVAC scheme (rituximab, ifosfamide, cytarabine, methotrexate) and started the treatment. After the 1st cycle of R-CODOX therapy, symptoms of neutropenic fever appeared. The presence of multi-drug-resistant bacterias was found in the blood cultures. The first blood culture revealed Stenotrophomonas maltophilia and ESBL Escherichia coli (resistant to beta-lactams, cephalosporins and co-trimoxazole but sensitive to carbapenems) and 5 d later the second blood culture showed MRCNS Staphylococcus lentus and MRCNS Staphylococcus hominis (resistant to beta-lactams, carbapenems, cephalosporins but sensitive to aminoglycosides). CRP level was 164 mg/L. After therapy with meropenem (1 g every 8 h) and amicin (500 mg daily) for ten days, CRP level decreased to 6.0 mg/L. Subsequently, ICTH was continued with reduced doses and after the second R-IVAC cycle, neutropenia developed with negative blood cultures (performed 3 times). Because of the clinical symptoms (fever, dyspnoea, weakness) and high CRP level 173 ng/mL intravenous therapy with vancomycin (2 g every 8 h) was started. As there was no clinical improvement (repeated negative blood cultures), after 7 d, colistin was administered intravenously (2 mL units every 8 h) with a good clinical response (CRP 15.8 mg/L). During the whole ICTH the patient received red blood cells transfusions (14 units in total) because of grade 4 anaemia.
Case 1: The treatment was complicated by neutropenia and anemia, but followed by complete remission (CR). After two years the patient remains in CR with stable, however poor allograft function (eGFR 21.8 mL/min/1.73 m2). The patient remains under the care of an oncologist and a clinical transplantolgist. The timeline of the information presented in this case report is presented in Table 1.
| Date | Procedure |
| November 1, 2002 | Dialysis initiation |
| August 2, 2004 | Laparotomy with bilateral nephrectomy |
| July 18, 2006 | Kidney transplantation |
| October 5, 2006 | Thyroid and parathyroid resection |
| July 28 to August 10, 2017 | Hospital stay at the nephrology ward |
| July 31, 2017 | Retrieval of the submandibular node |
| August 7, 2017 | Chest CT scan |
| August 28 to September 8, 2017 | 1st hospital stay at the oncology department |
| August 31, 2017 | Abdominal CT scan |
| September 4 and 5, 2017 | Chemotherapy I |
| September 25 to October 9, 2017 | 2nd hospital stay at the oncology department |
| September 25 and 26, 2017 | Chemotherapy II |
| September 29, 2017 | Blood transfusion-2 units |
| October 16 to 25, 2017 | 3rd hospital stay at the oncology department |
| October 18 and 19, 2017 | Chemotherapy III |
| October 19, 2017 | Abdominal CT scan |
| November 8 to 24, 2017 | 4th hospital stay at the oncology department |
| October 8 and November 8, 2017 | Chemotherapy IV |
| November 11, 2017 | Chest CT scan |
| December 15, 2017 to January 24, 2018 | 5th hospital stay at the oncology department |
| December 15 and 16, 2017 | Chemotherapy V |
| January 23, 2018 | Chemotherapy VI |
| February 28 to March 7, 2018 | 6th hospital stay at the oncology department |
| March 1, 2018 | Chemotherapy VII |
| April 10, 2018 | Positron emission tomography (PET scan) |
| April 12 to 27, 2018 | 7th hospital stay at the oncology department |
| June 28 to July 11, 2018 | Radiation therapy |
| July 12 to 17, 2018 | 8th hospital stay at the oncology department |
Case 2: ICTH was complicated by neutropenia and anemia, but followed by CR in CT scans of the neck, thorax, abdomen and pelvis as well as trephine biopsy (March 2016). After 4 years the patient remains in CR with regular check-up every 6 mo in the oncology outpatient clinic. Table 2 presents the information from this case report organized into a timeline.
| Date | Procedure |
| 2006 | Liver transplantation |
| October 24, 2015 | Head CT scan |
| October 24, 2015 | Lumbar puncture |
| October 26, 2015 | Head MRI scan |
| October 27, 2015 | Discharge from the neurological ward |
| October 27 to November 9, 2015 | Hospital stay at the transplantation ward |
| October 29, 2015 | Chest CT scan |
| November 3, 2015 | Stomach biopsy |
| November 9, 2015 to March 14, 2016 | Hospital stay at the oncology department |
| November 10, 2015 to Feburary 18, 2016 | Chemotherapy |
| March 9, 2016 | Chest CT scan |
| March 11, 2016 | Trephine biopsy |
According to 2019 NCCN guidelines[10], the first-line treatment always includes the reduction of immunosuppressive drug regime. Further management depends on PTLD subtype and patient’s response to changes in the immunosuppression. In both reported cases the reduction of immunosuppression regiment was ineffective, however safe, and was not followed by acute rejection or progressive deterioration in graft function[9]. Currently, the standard of care in case of this type of lymphomas is the initial administration of rituximab as monotherapy and depending on the response to treatment, its continuation or the implementation of CHOP chemotherapy[9-11]. These recommendations are based on the results of the largest prospective study so far–PTLD-1. According to this trial, about 25% of PTLD patients may not require chemotherapy[11]. Also, after sequential therapy low treatment mortality rate was observed (13%) in comparison to upfront CHOP chemotherapy (26%) described in the literature[12]. Because of the fast progression of the disease and no response to IR we decided to start ICTH upfront but with reduced doses. Besides single-centre experience reports, there is no clinical trial with BL after solid organ transplantation. The role of CNS prophylaxis is unclear.
Despite the immunosuppression therapy reduction, both our patients developed severe infections during ICTH. Immunosuppression therapy in transplanted patients per se is associated with an increased incidence of bacterial infections of the urinary and respiratory tracts. Urinary tract infections were reported to occur almost twice more frequently than in non-transplant patients (7% vs 4.4%)[13]. The occurrence of pneumonia depends on transplanted organs and ranges from 7.3% in the kidney[14], 22% in the liver[15], up to 36% in the lung graft recipients[16] during the first post-transplant year. Infectious complications in kidney transplant patients are associated with significant morbidity and mortality[17], highest in developing countries associated with low socioeconomic profile[18-20], and severity of immunosuppression regimen[21]. Prophylactic use of antibiotics decreased the prevalence from 6.3% to 2.2% at the end of the first year after transplantation[22]. Trappe et al[9] noticed grade 3 and grade 4 infections in about 41% of patients and graderade 3 and grade 4 leukopenia in 68% of patients during PTLD chemotherapy[7].
Neutropenic fever is a common adverse event during R-CHOP treatment of DLBCL with the incidence estimated at 5%-40%[22-24], usually occurring after the first cycle of ICTH[25]. There is no data on the frequency of NF in PTLD patients. Our kidney transplant patient presented with NF in the fifth cycle despite using G-CSF prophylaxis, which is a standard in NF prevention[26]. NF was successfully treated with antibiotics, however, associated with deterioration of the kidney graft function.
We confirm that Pneumocystis carinii prophylaxis should be utilized in each PTLD patients during ICHT and consisted of 960 mg co-trimoxazole orally 3 times a week[8]. The prophylaxis is recommended as the infection occurs in one-third of treated PTLD patients[9].
Despite the improvement in the management of PTLD, related mainly to the introduction of rituximab in B-cell lymphoproliferative disorders[27], the prognosis remains generally poor[1,28], with 55% 3-year overall survival[1], median OS 64 mo[29]. Until now both our patients remain in clinical remission, despite one of the patients had not received complete ICHT treatment due to recurrent infections.
It is worth to be mentioned that PTLD may be linked to other viruses presence, such as HCV, HHV-8 and CMV[1]. Although it has been reported that after liver transplant, HBV reactivation plays an important role in PTLD occurrence[30], even 12 years after transplantation[31]. In the case of our patient, the disease onset was late (8 years after liver transplant), and the patient HBV DNA remains undetectable, therefore the PTLD (DLBCL) seems unrelated to hepatotropic viruses.
In summary, ICTH on the tope of immunosuppression therapy increases the risk of infectious complications in PTLD patients. Reduction of immunosuppression and the doses of chemotherapeutics may be insufficient to diminish the risk of infectious complications during ICTH.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society for Medical Oncology, No. 473843.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zheng R S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
| 1. | Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5427] [Article Influence: 603.0] [Reference Citation Analysis (0)] |
| 3. | Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Singavi AK, Harrington AM, Fenske TS. Post-transplant lymphoproliferative disorders. Cancer Treat Res. 2015;165:305-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Martinez OM, Krams SM. The Immune Response to Epstein Barr Virus and Implications for Posttransplant Lymphoproliferative Disorder. Transplantation. 2017;101:2009-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Menter T, Dickenmann M, Juskevicius D, Steiger J, Dirnhofer S, Tzankov A. Comprehensive phenotypic characterization of PTLD reveals potential reliance on EBV or NF-κB signalling instead of B-cell receptor signalling. Hematol Oncol. 2017;35:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematology Am Soc Hematol Educ Program. 2013;2013:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Morton M, Coupes B, Roberts SA, Klapper PE, Byers RJ, Vallely PJ, Ryan K, Picton ML. Epidemiology of posttransplantation lymphoproliferative disorder in adult renal transplant recipients. Transplantation. 2013;95:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, Morschhauser F, Jaccard A, Lamy T, Leithäuser M, Zimmermann H, Anagnostopoulos I, Raphael M, Riess H, Choquet S; German PTLD Study Group; European PTLD Network. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, Chang JE, Chavez JC, Christian B, Fayad LE, Glenn MJ, Habermann TM, Lee Harris N, Hernandez-Ilizaliturri F, Kaminski MS, Kelsey CR, Khan N, Krivacic S, LaCasce AS, Mehta A, Nademanee A, Rabinovitch R, Reddy N, Reid E, Roberts KB, Smith SD, Snyder ED, Swinnen LJ, Vose JM, Dwyer MA, Sundar H. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw. 2019;17:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. 2017;35:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, Olthoff KM, Schuster SJ, Nasta SD, Stadtmauer EA, Tsai DE. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Vidal E, Torre-Cisneros J, Blanes M, Montejo M, Cervera C, Aguado JM, Len O, Carratalá J, Cordero E, Bou G, Muñoz P, Ramos A, Gurguí M, Borrell N, Fortún J; Spanish Network for Research in Infectious Diseases (REIPI). Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl Infect Dis. 2012;14:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Kupeli E, Ulubay G, Colak T, Ozdemirel TS, Ozyurek BA, Akcay S, Haberal M. Pulmonary complications in renal recipients after transplantation. Transplant Proc. 2011;43:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Levesque E, Hoti E, Azoulay D, Honore I, Guignard B, Vibert E, Ichai P, Antoun F, Saliba F, Samuel D. Pulmonary complications after elective liver transplantation-incidence, risk factors, and outcome. Transplantation. 2012;94:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Aguilar-Guisado M, Givaldá J, Ussetti P, Ramos A, Morales P, Blanes M, Bou G, de la Torre-Cisneros J, Román A, Borro JM, Lama R, Cisneros JM; RESITRA cohort. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007;7:1989-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand MR. Infectious complications after kidney transplantation: a single-center experience. Transpl Infect Dis. 2007;9:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Snydman DR. Infection in solid organ transplantation. Transpl Infect Dis. 1999;1:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Fishman JA. Infection in renal transplant recipients. Semin Nephrol. 2007;27:445-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Sousa SR, Galante NZ, Barbosa DA, Pestana JO. [Incidence of infectious complications and their risk factors in the first year after renal transplantation]. J Bras Nefrol. 2010;32:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kumar MS, Cridge P, Molavi A, Stephan R, Abouna GM. Infectious complications in the first 100 days after renal transplantation. Transplant Proc. 1995;27:2705-2706. [PubMed] |
| 23. | Park S, Kang CI, Chung DR, Peck KR, Kim WS, Kim SJ. Clinical Significance of Non-neutropenic Fever in the Management of Diffuse Large B-Cell Lymphoma Patients Treated with Rituximab-CHOP: Comparison with Febrile Neutropenia and Risk Factor Analysis. Cancer Res Treat. 2015;47:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Bartlett NL, Wilson WH, Jung SH, Hsi ED, Maurer MJ, Pederson LD, Polley MC, Pitcher BN, Cheson BD, Kahl BS, Friedberg JW, Staudt LM, Wagner-Johnston ND, Blum KA, Abramson JS, Reddy NM, Winter JN, Chang JE, Gopal AK, Chadburn A, Mathew S, Fisher RI, Richards KL, Schöder H, Zelenetz AD, Leonard JP. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol. 2019;37:1790-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 291] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 25. | Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A, Davies J, Turner D, Kruger A, Johnson P, Gambell J, Linch D. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 396] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 26. | Crawford J, Allen J, Armitage J, Blayney DW, Cataland SR, Heaney ML, Htoy S, Hudock S, Kloth DD, Kuter DJ, Lyman GH, McMahon B, Steensma DP, Vadhan-Raj S, Westervelt P, Westmoreland M; National Comprehensive Cancer Network. Myeloid growth factors. J Natl Compr Canc Netw. 2011;9:914-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Salles G, Barrett M, Foà R, Maurer J, O'Brien S, Valente N, Wenger M, Maloney DG. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther. 2017;34:2232-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 429] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 28. | Liu M, Husain S, Famure O, Li Y, Kim SJ. Incidence, Risk Factors, Clinical Management, and Outcomes of Posttransplant Lymphoproliferative Disorder in Kidney Transplant Recipients. Prog Transplant. 2019;29:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Martínez-Calle N, Alfonso A, Rifón J, Herrero I, Errasti P, Rábago G, Merino J, Panizo Á, Pardo J, Prósper F, García-Muñoz R, Lecumberri R, Panizo C. First-line use of rituximab correlates with increased overall survival in late post-transplant lymphoproliferative disorders: retrospective, single-centre study. Eur J Haematol. 2017;98:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Zhang A, Zhang M, Shen Y, Wang W, Zheng S. Hepatitis B virus reactivation is a risk factor for development of post-transplant lymphoproliferative disease after liver transplantation. Clin Transplant. 2009;23:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Yu F, Huang Y, Wang Y, Yu Z, Li X, Dong J. Very late onset post-transplant diffuse large B cell lymphoma in a liver transplant recipient with hepatitis B: A case report. Medicine (Baltimore). 2018;97:e13063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |