Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.707
Peer-review started: September 15, 2020
First decision: November 14, 2020
Revised: November 23, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 26, 2021
Processing time: 126 Days and 22.2 Hours
Systemic lupus erythematosus (SLE) and antineutrophil cytoplasmic antibody-associated vasculitis (AAV) are classically thought to cause renal impairment and small vessel vasculitis with different pathophysiologies. Their overlap constitutes a rare rheumatologic disease. To date, only dozens of such cases with biopsy-proven glomerulonephritis have been reported worldwide typically in women of childbearing age. Here, we present a unique clinical case due to its rarity and individualized treatment of a Chinese man in his eighth decade of life.
A 77-year-old man was admitted to several hospitals for shortness of breath and received nonspecific treatments over the past 3 years. As his symptoms were not completely relieved, he visited our hospital for further treatment. Laboratory examinations revealed kidney dysfunction, severe anaemia, hypocom-plementemia, glomerular proteinuria, and microscopic haematuria. Antinuclear antibodies, as well as anti-dsDNA antibodies, were positive. Computed tomography of the chest showed right pleural effusion. Renal biopsy was performed, and histology suggested crescentic glomerulonephritis, pauci-immune type. After treatment with plasmapheresis, glucocorticoid, and cyclo-phosphamide, the disease was in remission, and the patient remained in a stable condition for over 3 years post-hospital discharge.
Due to its complexity and rarity, SLE and AAV overlap syndrome is easily misdiagnosed. An accurate diagnosis and treatment at the earliest stage may significantly improve the condition and reduce irreversible organ injury.
Core Tip: Systemic lupus erythematosus and associated vasculitis overlap syndrome was first described in 1997, and only dozens of such cases have been reported to date worldwide. We report an elderly male patient who was not diagnosed with this disease in a timely manner due to hidden features. After treatment with plasmapheresis, glucocorticoid, and cyclophosphamide, the patient’s condition improved, even at his advanced age.
- Citation: Xu ZG, Li WL, Wang X, Zhang SY, Zhang YW, Wei X, Li CD, Zeng P, Luan SD. Systemic lupus erythematosus and antineutrophil cytoplasmic antibody-associated vasculitis overlap syndrome in a 77-year-old man: A case report. World J Clin Cases 2021; 9(3): 707-713
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/707.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.707
Systemic lupus erythematosus (SLE) and associated vasculitis (AAV) overlap syndrome, first reported in 1997[1], is a rare disease characterized by simultaneous presentation of serological marker positivity, severe clinical manifestations (rapidly progressive glomerulonephritis and frequent pulmonary involvement)[2], or histopathology of SLE and AAV in the same patient, with the case fulfilling the classification criteria for both diseases. The disease is commonly seen in women of childbearing age. Those who have both overlapping syndromes commonly present with severe renal disease[3].
This case report describes the simultaneous manifestation of SLE and AAV. Based on our literature search, this overlap has rarely been reported in older men with biopsy-proven glomerulonephritis (GN).
This case report involves a 77-year-old Chinese man admitted to our hospital for recurrent shortness of breath for more than 9 mo.
The man was admitted to a local hospital for shortness of breath since February 2017. No clear diagnosis was made, and he was treated (the medication is unknown), but the symptom was recurrent. He felt worse and went to our hospital 2 mo later and was successively admitted to the Department of Cardiology and Respiratory. Laboratory examination showed mild renal insufficiency with serum creatinine (Scr) at 113 µmol/L. Anaemia with haemoglobin at 83 g/L, was present. Urinalysis revealed microscopic haematuria, but the urine protein was negative. Computed tomography (CT) of the chest showed right pleural effusion. For a correct diagnosis, the patient subsequently underwent thoracentesis and pleural biopsy. The hydrothorax was exudative, and the histological examination showed chronic inflammation. On the 14th day after admission, immunological tests revealed antinuclear antibodies (ANA) and anti-dsDNA antibody positivity, and myeloperoxidase (MPO)-antineutrophil cytoplasmic antibody (ANCA) was detected at 133 AU/mL using chemiluminescence. For further treatment, the patient went to a chest hospital and received nonspecific therapies. As his symptoms were not completely relieved in November 2017, he came to our hospital again.
He had no history of coronary heart disease, metabolic disease, rheumatic disease, or liver disease, but had over 50 years of water pipes smoking history.
He has no special personal and family history.
His blood pressure, heart rate, and temperature were normal. A moderate anaemic appearance and decreased breathing sounds in the right lower lung were noted. Heart auscultation and abdominal examination were normal. No pitting oedema in the extremities was detected. He had no skin involvement, lymphadenopathy, or synovitis.
On admission, serum laboratory results revealed the following values: Haemoglobin (Hb), 57 g/L; white blood cells, 5.9 × 109/L, platelets, 156 × 109/L; Scr, 310 µmol/L; serum albumin, 39 g/L; complement C3, 0.72 g/L; C4, 0.17 g/L; C-reactive protein, 32.1 mg/L. Liver function was normal. The patient was positive for ANA and anti-dsDNA antibodies. MPO-ANCA was detected at 180.62 AU/mL using chemiluminescence, and cANCA was positive by indirect immunofluorescence. Proteinase (PR3)-ANCA and pANCA were normal. We detected no anti-glomerular basement membrane or anti-phospholipase A2 receptor antibodies. Urinalysis revealed glomerular proteinuria (24-h urine protein, 0.887 g/d) and microscopic haematuria.
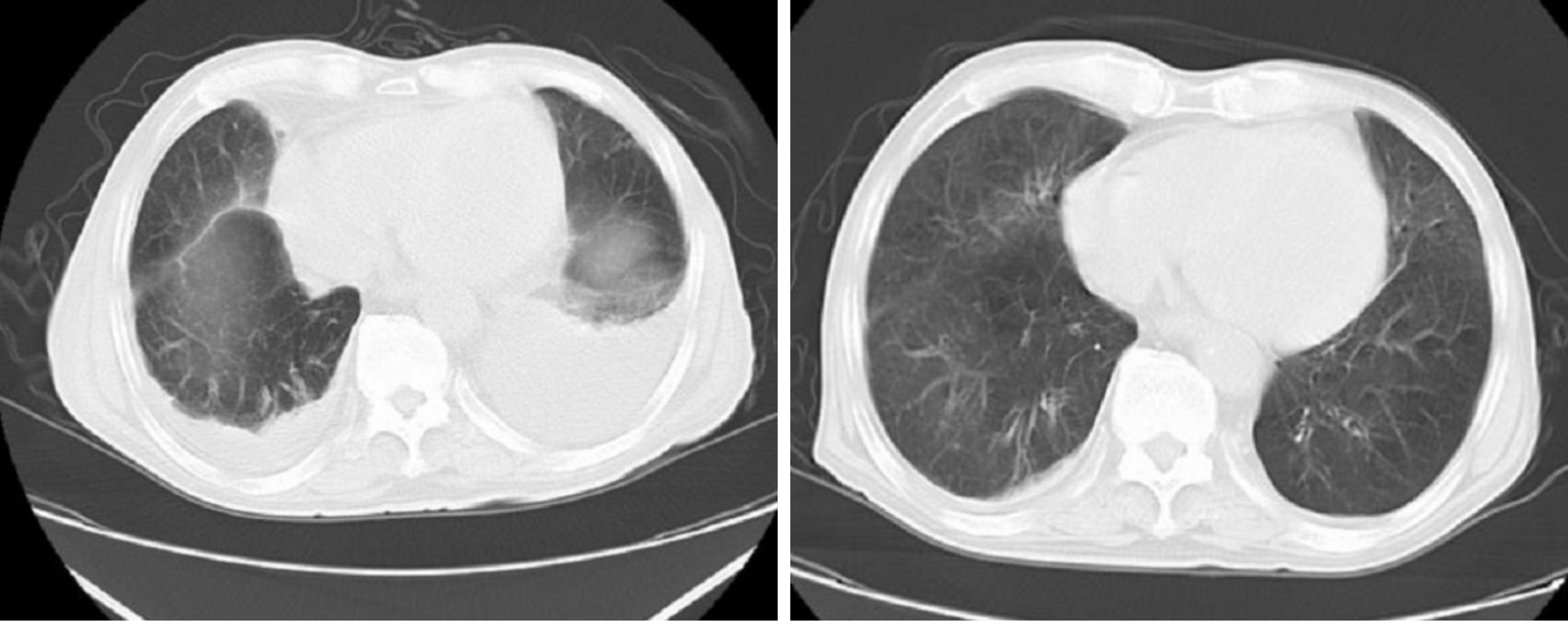
CT of the chest showed right pleural effusion, and Doppler echocardiography indicated decreased left ventricular diastolic dysfunction and moderate pulmonary hypertension. Kidney ultrasound examination revealed normal-sized kidneys, renal cysts, and right kidney stones, with obstruction excluded.
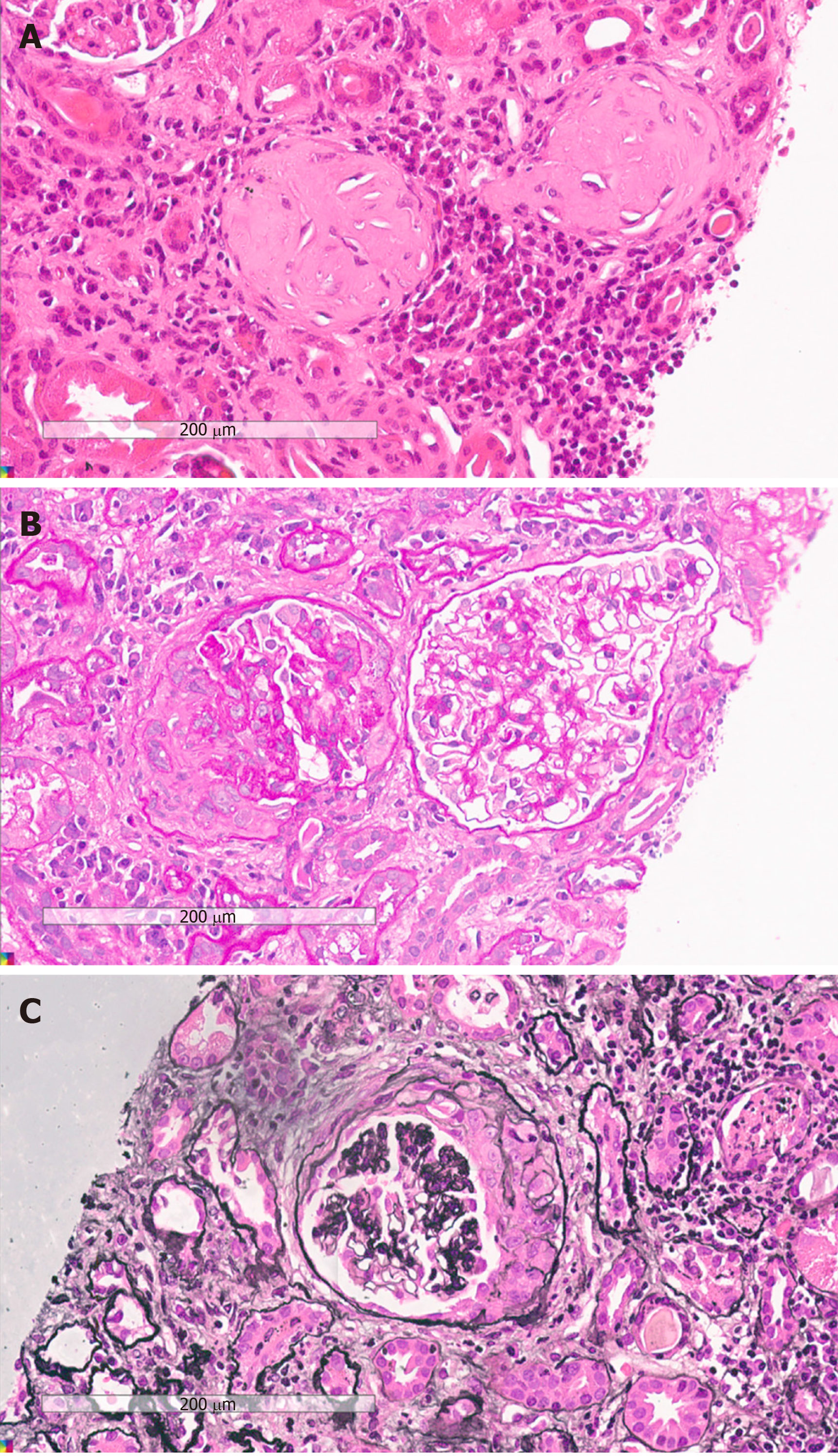
Lymph nodes or AVV was considered, leading us to perform a renal biopsy. Histological examination of the renal biopsy demonstrated 36 glomeruli, of which 13 were globally sclerotic and 14 were crescent, including 3 cellular, 1 fibro-cellular, 8 fibrous, 1 small cellular, and 1 small fibro-cellular, with some lesions of capillary fibrinoid necrosis (Figure 1). No marked mesangial cell proliferation, matrix proliferation, or fuchsinophilic protein deposits were found in the remaining glomeruli. Part of the interstitial region was infiltrated with inflammatory cells. Immunohistology showed negativity for immunoglobulin (Ig) M (+), IgA, IgG, C3, and C1q. Vacuolar degeneration in endothelial cells was observed by electron microscopy, and part of the glomerular capillary loops were compressed.
We corrected the previous diagnosis result to SLE and AAV overlap syndrome.
As the diagnosis was clear, we started injections of methylprednisolone (500 mg/d, 3 d in total), cyclophosphamide (CTX) (0.4 g/d, 2 d in total), and γ-globin (15 g/d, 5 d in total). Plasmapheresis (double plasmapheresis, exchange dosage: 800 mL fresh frozen plasma) and continuous renal replacement therapy were immediately initiated. Although MPO-ANCA did not decrease apparently after five plasmapheresis sessions, the patient’s condition improved, the reexamination image was also evidently promoted, and the value of Scr was stable at approximately 270 µmol/L.
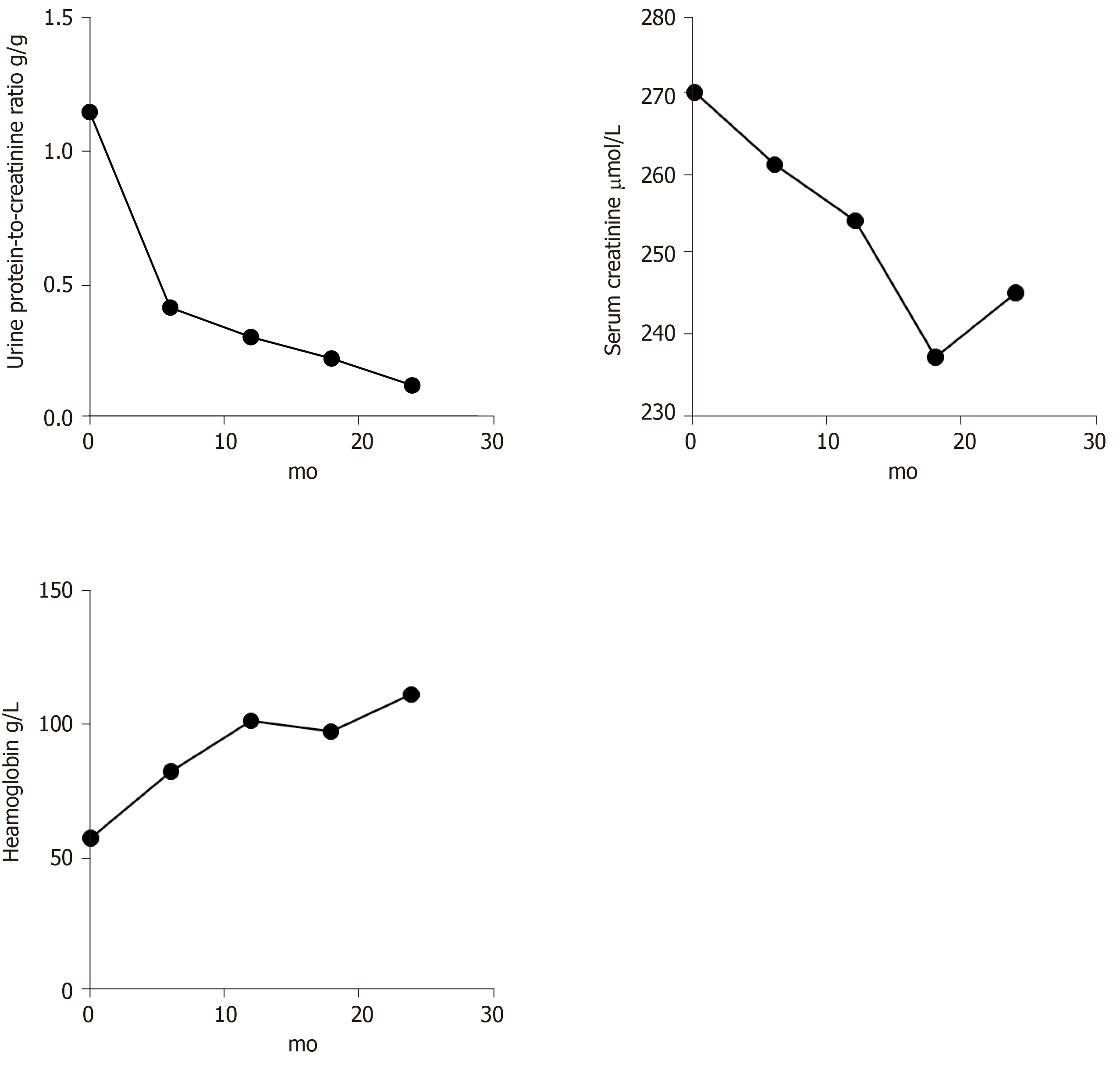
After being discharged, the patient was maintained on tapering PO doses of prednisone that was eventually suspended one year later. He was administered nine courses of 800 mg intravenous CTX (7.2 g in total) and was transitioned to oral daily azathioprine (100 mg), which continues to date. Further results, including urine protein-to-creatinine ratio, urinary erythrocyte, Scr, Hb, and images, revealed effective relief (Figures 2 and 3).
SLE and AAV are classically thought to cause renal impairment and small vessel vasculitis with different pathophysiologies. Both affect the kidneys, but the mechanism of SLE involves massive deposition of immune complexes under the endothelium of glomerular capillaries. It is a classical immune complex-mediated kidney injury[4]. In contrast, AAV leads to necrotizing and crescentic glomerulonephritis without immune complex deposition[5].
SLE and AAV overlap syndrome was first described in 1997. It is typically seen in women of childbearing age. Our case is unique because to our knowledge, this overlap is rarely observed in older men with biopsy-proven GN worldwide. The clinical manifestations of this patient were multisystem involvement, the associated autoantibodies were positive, and the histological examination of the renal biopsy revealed AAV-renal impairment. After assessment, the case fulfilled both SLE and AAV classification criteria.
Many factors have been known to cause SLE, including smoking, alcohol[6], ultraviolet light[7], virus infection[8], certain drugs[9], silica[10], etc. In this case, the patient was not exposed to intense ultraviolet light. There was no evidence to suggest that he was infected with viruses before the onset of this overlap syndrome. Procainamide, hydralazine, phenytoin sodium, isoniazid, propylthiouracil, penicillamine, and other special drugs have not been taken recently. However, given his water pipes smoking history, we suspect that it might be a potential trigger for SLE. Like SLE, there are a number of factors that contribute to serum ANCA positivity. Staphylococcus aureus is a common bacterium that has been implicated in the genesis of ANCAs[11]. Cigarette smoking and drugs that we mentioned above are also involved in inducing ANCA production[12,13]. There was no evidence that this patient had been infected with bacteria according to the physical examination upon admission. Therefore, in this case, water pipes smoking seems to be the common cause of SLE and ANCAs.
Corticosteroids, immunosuppressive agents, and plasmapheresis are recommended as regular therapies for this overlap. In this case, a high dose of glucocorticoid (GC) and CTX were given to diminish inflammation and prevent permanent organ damage. After we detected ANCA in the patient’s circulation, plasma exchange was used to rapidly remove the pathogenic autoantibodies and prevent the deterioration of renal function. When he was in complete remission, azathioprine was used after CTX withdrawal due to its lower toxicity. Further results, including urine protein, Scr, Hb, and images, showed effective relief in 3 years.
This case reveals that early diagnosis and timely treatment for this overlap syndrome are critical. The patient had been misdiagnosed and received nonspecific treatments for 6 mo before visiting our department. Thus, early diagnosis and proper treatments are crucial for renal function preservation. In addition, it is generally believed that elderly patients are vulnerable to the adverse effects of the GC to treat autoimmunological diseases. In a recent study, Sada et al[14] divided the elderly AAV patients into three groups according to initial GC dose. They found that there were no statistically significant intergroup differences in remission or relapse, but serious infection developed more frequently in the high-dose group (prednisolone ≥ 0.8 mg/kg per day). Considering that the patient was elderly and prone to GC-related side effects such as serious infection and gastrointestinal bleeding, we treated him with only 0.5 g methylprednisolone QD for 3 d and achieved satisfactory results. Thus, a conservative dose for GC therapy may be an appropriate option for the elderly.
In conclusion, SLE and AAV overlap syndrome is a rare autoimmune disease involving multiple systems. It usually damages small vessels and causes renal impairment. Due to the complexity of this disease, it is easily misdiagnosed. An accurate diagnosis and treatment at the earliest stage may significantly improve the patient’s condition and reduce irreversible organ injury.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanaka H S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Marshall S, Dressler R, D'Agati V. Membranous lupus nephritis with antineutrophil cytoplasmic antibody-associated segmental necrotizing and crescentic glomerulonephritis. Am J Kidney Dis. 1997;29:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Jarrot PA, Chiche L, Hervier B, Daniel L, Vuiblet V, Bardin N, Bertin D, Terrier B, Amoura Z, Andrés E, Rondeau E, Hamidou M, Pennaforte JL, Halfon P, Daugas E, Dussol B, Puéchal X, Kaplanski G, Jourde-Chiche N. Systemic Lupus Erythematosus and Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Overlap Syndrome in Patients With Biopsy-Proven Glomerulonephritis. Medicine (Baltimore). 2016;95:e3748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Hervier B, Hamidou M, Haroche J, Durant C, Mathian A, Amoura Z. Systemic lupus erythematosus associated with ANCA-associated vasculitis: an overlapping syndrome? Rheumatol Int. 2012;32:3285-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Kewalramani R, Singh AK. Immunopathogenesis of lupus and lupus nephritis: recent insights. Curr Opin Nephrol Hypertens. 2002;11:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Takvorian SU, Merola JF, Costenbader KH. Cigarette smoking, alcohol consumption and risk of systemic lupus erythematosus. Lupus. 2014;23:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Simard JF, Costenbader KH, Liang MH, Karlson EW, Mittleman MA. Exposure to maternal smoking and incident SLE in a prospective cohort study. Lupus. 2009;18:431-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Poole BD, Scofield RH, Harley JB, James JA. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Hess E. Drug-related lupus. N Engl J Med. 1988;318:1460-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 128] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Parks CG, De Roos AJ. Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus. Lupus. 2014;23:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 463] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | McDermott G, Fu X, Stone JH, Wallwork R, Zhang Y, Choi HK, Wallace ZS. Association of Cigarette Smoking With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. JAMA Intern Med. 2020;180:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Radić M, Martinović Kaliterna D, Radić J. Drug-induced vasculitis: a clinical and pathological review. Neth J Med. 2012;70:12-17. [PubMed] |
| 14. | Sada KE, Ohashi K, Asano Y, Hayashi K, Morishita M, Watanabe H, Matsumoto Y, Fujimoto S, Takasaki Y, Yamagata K, Banno S, Dobashi H, Amano K, Harigai M, Arimura Y, Makino H; Japan Research Committee of the Ministry of Health; Labour; and Welfare for Intractable Vasculitis (JPVAS) and the Research Committee of Intractable Renal Disease of the Ministry of Health; Labour; and Welfare of Japan. Treatment-related damage in elderly-onset ANCA-associated vasculitis: safety outcome analysis of two nationwide prospective cohort studies. Arthritis Res Ther. 2020;22:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |