Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.632
Peer-review started: July 21, 2020
First decision: November 3, 2020
Revised: November 18, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 26, 2021
Processing time: 181 Days and 21.1 Hours
Lower body positive pressure (LBPP) treadmill has potential applications for improving the gait of patients after stroke, but the related mechanism remains unclear.
A 62-year-old male patient suffered from ischemic stroke with hemiplegic gait. He was referred to our hospital because of a complaint of left limb weakness for 2 years. The LBPP training was performed one session per day and six times per week for 2 wk. The dynamic plantar pressure analysis was taken every 2 d. Meanwhile, three-digital gait analysis and synchronous electromyography as well as clinical assessments were taken before and after LBPP intervention and at the 4-wk follow-up. During LBPP training, our patient not only improved his lower limb muscle strength and walking speed, but more importantly, the symmetry index of various biomechanical indicators improved. Moreover, the patient’s planter pressure transferring from the heel area to toe area among the LBPP training process and the symmetry of lower body biomechanical parameters improved.
In this study, we documented a dynamic improvement of gait performance in a stroke patient under LBPP training, which included lower limb muscle strength, walking speed, and symmetry of lower limb biomechanics. Our study provides some crucial clues about the potential dynamic mechanism for LBPP training on gait and balance improvement, which is related to rebuilding foot pressure distribution and remodeling symmetry of biomechanics of the lower limb.
Core Tip: Lower body positive pressure (LBPP) treadmill has potential applications for improving the gait of patients after a stroke, although the related mechanism remains unclear. To the best of our knowledge, our case report is the first study for the dynamic observation on hemiplegic gait rehabilitation using plantar pressure analysis, which presents with every detail of the changes in gait. Meanwhile, we also presented the macroscopic longitudinal changes using the clinical assessments and three-digital gait analysis before and after LBPP intervention and at a follow-up. The critical clues were found for the potential dynamic mechanism for LBPP training on the gait and balance improvement, which is related to rebuilding foot pressure distribution and remodeling symmetry of biomechanics of the lower limb.
- Citation: Tang HF, Yang B, Lin Q, Liang JJ, Mou ZW. Dynamic biomechanical effect of lower body positive pressure treadmill training for hemiplegic gait rehabilitation after stroke: A case report. World J Clin Cases 2021; 9(3): 632-638
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/632.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.632
Recovery of locomotor function and community mobility is the primary goal of individuals post-stroke and is prioritized during physical rehabilitation[1]. Lower body positive pressure (LBPP) treadmill is one of emerging body weight-supported training technology, and has been used in musculoskeletal and neurological disease rehabilitation[2-4], but the potential mechanism is still unclear and requires further study. The distribution of foot pressure (especially the toe area) has important effects on walking characteristics (velocity and pace), lower limb muscle activation, and joint stability[5,6]. Therefore, this study investigated dynamic plantar pressure distribution changes by plantar pressure analysis and gait pattern, as well as lower limb muscle activity changes by three-dimensional (3D) gait analysis and synchronous electromyography (EMG) during walking to explore the effect of LBPP training on locomotor function rehabilitation in a stroke patient. To our knowledge, no studies have evaluated the effect of LBPP training for stroke patients by dynamic plantar pressure analysis.
A 62-year-old male patient was referred to the rehabilitation department because of a 2-year history of left limb weakness. He suffered from the first right hemorrhagic stroke 27 mo prior. He lived independently in the community and was ambulatory. However, there was residual motor and gait impairment due to his stroke.
He had a history of hypertension for 10 years, and his systolic blood pressure was up to 190 mmHg. Upon presentation, he took oral antihypertensive drugs to control blood pressure (Baixintong, Goteling, Dyvan, and Furosemide), and the blood pressure was controlled at 130-140/80-90 mmHg.
He had a history of smoking for more than 30 years.
The patient denied any family history of hypertension, coronary heart disease, or stroke.
The blood biochemistry and urine analysis were normal.
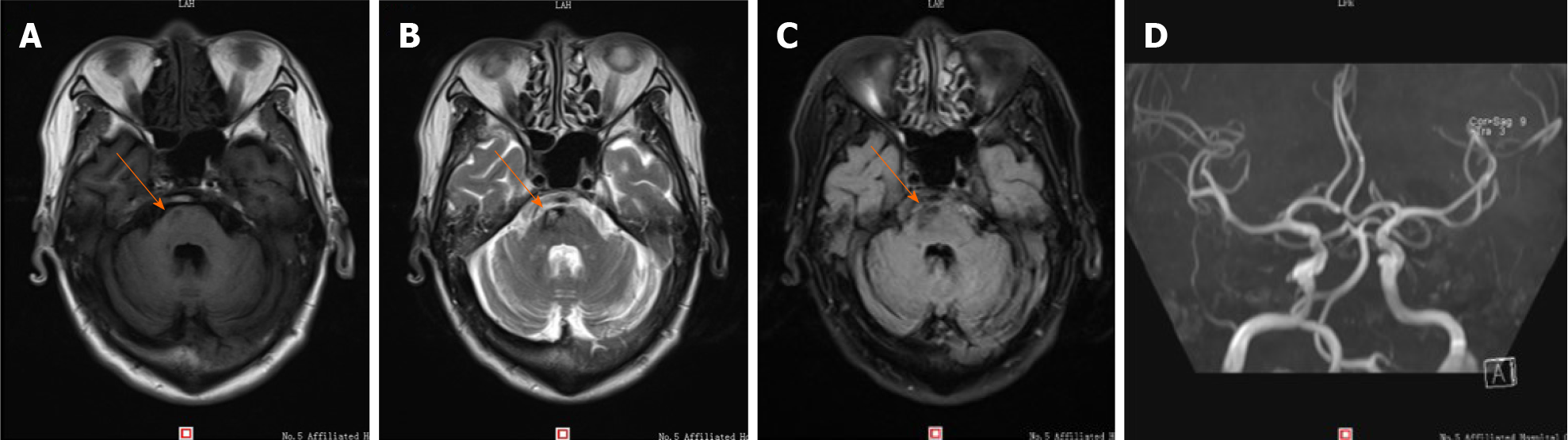
Laboratory findings, including routine blood, coagulation function, kidney function, and liver function tests, were within normal ranges. Magnetic resonance imaging (MRI) findings on December 12, 2018 revealed chronic infarction in the right part of the pons (Figure 1).
The clinical assessments, including lower extremity subscale of the Fugl-Meyer Assessment (FMA-LE)[7], Berg Balance Scale (BBS)[8], and Timed Up and Go test (TUG)[9], were performed at day 1 (baseline/pretreatment), day 14 (posttreatment), and day 28 (4-wk follow-up) by a trained physical therapist.
Further examinations included plantar pressure analysis (FreeStep, Italy) and 3D gait analysis with synchronized EMG (BTS SMART, Italy)[10]. The patient underwent the plantar pressure analysis on days 1, 4, 7, 11, 14, and 28 to detect the dynamic changes of plantar pressure distribution on the affected side and unaffected side during walking. Moreover, the patient underwent 3D gait analysis with EMG on days 1, 14, and 28 to detect gait spatial-temporal parameters (mean velocity and cadence) and muscle activities of the lower limb [root-mean-square of EMG (RMS EMG)], which included the tibialis anterior (TA), gastrocnemius lateralis (GL), and gastrocnemius medialis (GM). The symmetric index (SI) of gait parameters (cadence and stride length) and mean RMS parameters were computed over the stance phase and swing phase. The formula for calculating the symmetry index is as follows[11]: SI = [(Vaffected - Vunaffected)/0.5(Vaffected + Vunaffected)] × 100%, where V represents the parameters put into the formula. The lower the value of SI, the lesser the difference between the affected side and the unaffected side.
Light side hemiparesis, hemorrhages in chronic phase, hypertension, and lumbar disc herniation.
The patient provided informed consent, and all procedures were approved by the Medical Ethics Association of the Fifth Affiliated Hospital of Guangzhou Medical University. This study has been registered at the China Clinical Trial Registration Center (No. ChiCTR1800020253). The timeline of this study is shown in Figure 2.
The patient performed one session of LBPP treadmill walking training per day (provided using AlterG M320 Antigravity Treadmill, California, United States), 6 d per week, for 2 wk [LBPP parameter setting: 70% bodyweight (BW); 1.2 mph peak speed; and 0° incline]. Each session lasted approximately 30 min, including 5 min warmup on the treadmill and 5 min cooling down period. Meanwhile, the patient also undertook a 60-min conventional physical therapy program based on the Guidelines for Adult Stroke Rehabilitation and Recovery from the American Heart Association/American Stroke Association[12] and medication for secondary prevention of stroke. The patient did not show any discomfort after interventions.
The clinical assessments (FMA-LE, BBS, and TUG) are reported in Table 1. Scores on the BBS improved from pretreatment (baseline) to posttreatment (2 wk of training) and the 4-wk follow-up, whereas the scores on the FMA-LE were not changed. The timing on TUG varied slightly from posttreatment to the 4-wk follow-up, but still improved compared with pretreatment.
| FMA-LE (total score) | BBS (total score) | TUGT (s) | |
| Pre-treatment | 18 (34) | 39 (56) | 34.1 |
| Post-treatment | 18 (34) | 43 (56) | 27.1 |
| Follow-up | 18 (34) | 45 (56) | 27.5 |
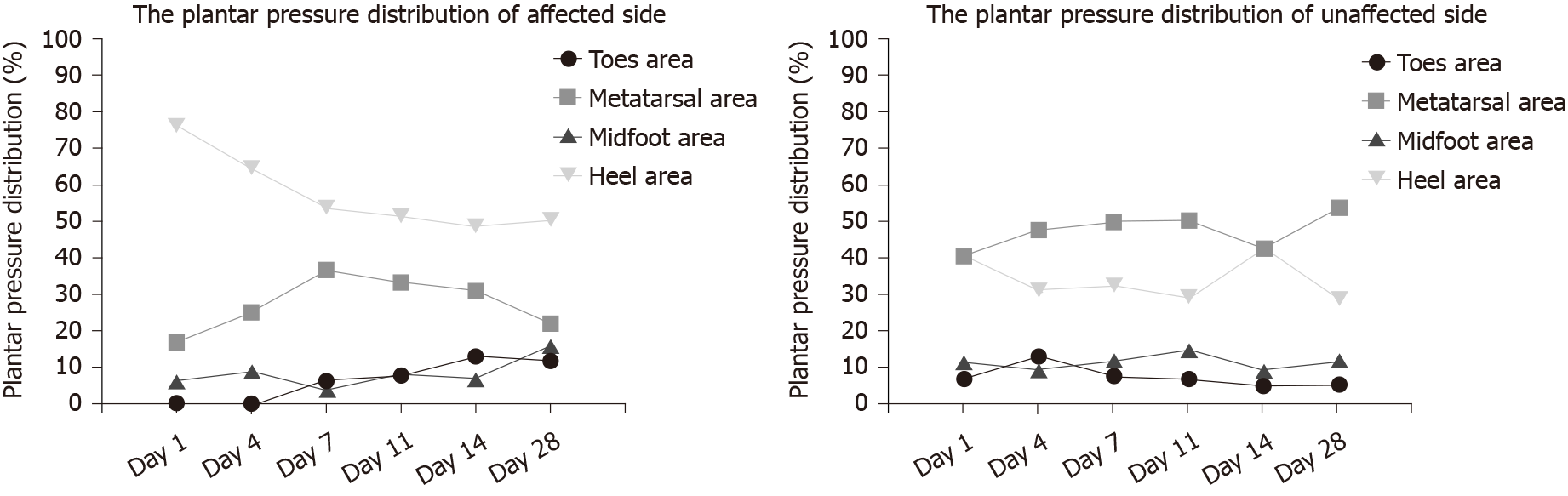
The dynamic plantar pressure distributions on days 1 (baseline/pretreatment), 4, 7, 11, 14 (posttreatment), and 28 (follow-up) on the affected side and unaffected side are shown in Table 2 and Figure 3. The dynamic plantar pressure distributions in four foot areas (including toes, metatarsal, midfoot, and heel area) on the unaffected side presented only slight variation over the six measurements, whereas the plantar pressure distribution in the toes area on the affected side showed 0 values (unloading) on days 1 and 4, and then gradually increased to normal range compared with the unaffected side. The plantar pressure distribution in the heel area on the affected side was gradually decreased over the whole training process, except a slight variation at the follow-up, and was obviously greater than the unaffected side (affected side range from 48.59%-76.1% vs unaffected side range from 28.52%-42.46%). The plantar pressure distribution in the midfoot area and metatarsal area on the affected side showed only slight variation over the whole process, and the values were smaller than those of the unaffected side.
| Toes area | Metatarsal area | Midfoot area | Heel area | |||||
| Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | |
| Day 01 | 0 | 6.92 | 16.93 | 40.63 | 6.47 | 11.43 | 76.1 | 40.77 |
| Day 04 | 0 | 12.98 | 25.06 | 47.62 | 9.04 | 9.42 | 64.48 | 30.96 |
| Day 07 | 6.15 | 7.67 | 36.55 | 49.74 | 3.82 | 11.76 | 53.66 | 32.35 |
| Day 11 | 7.71 | 6.75 | 33.17 | 50.13 | 8.15 | 14.84 | 51.32 | 29.02 |
| Day 14 | 12.9 | 4.92 | 30.8 | 42.43 | 6.87 | 9.23 | 48.59 | 42.46 |
| Day 28 | 11.75 | 5.27 | 22.02 | 53.72 | 15.9 | 11.71 | 50.32 | 28.52 |
The 3D gait analysis results showed that the mean velocity was gradually increased from pretreatment to posttreatment and even at the follow-up. Meanwhile, the SI of stride length (%height) and SI of cadence increased from pretreatment to posttreatment, which indicated a lesser difference between the affected side and unaffected side at posttreatment (Table 3).
| Mean velocity (%height/s) | Cadence (steps/min) | Symmetric index (SI, %) | ||
| SIStride (%height) | SICadence | |||
| Pre-treatment | 16.95 | 73.60 | 125.12 | 193.31 |
| Post-treatment | 18.21 | 81.20 | 126.74 | 193.85 |
| Follow-up | 18.39 | 76.40 | 122.40 | 193.69 |
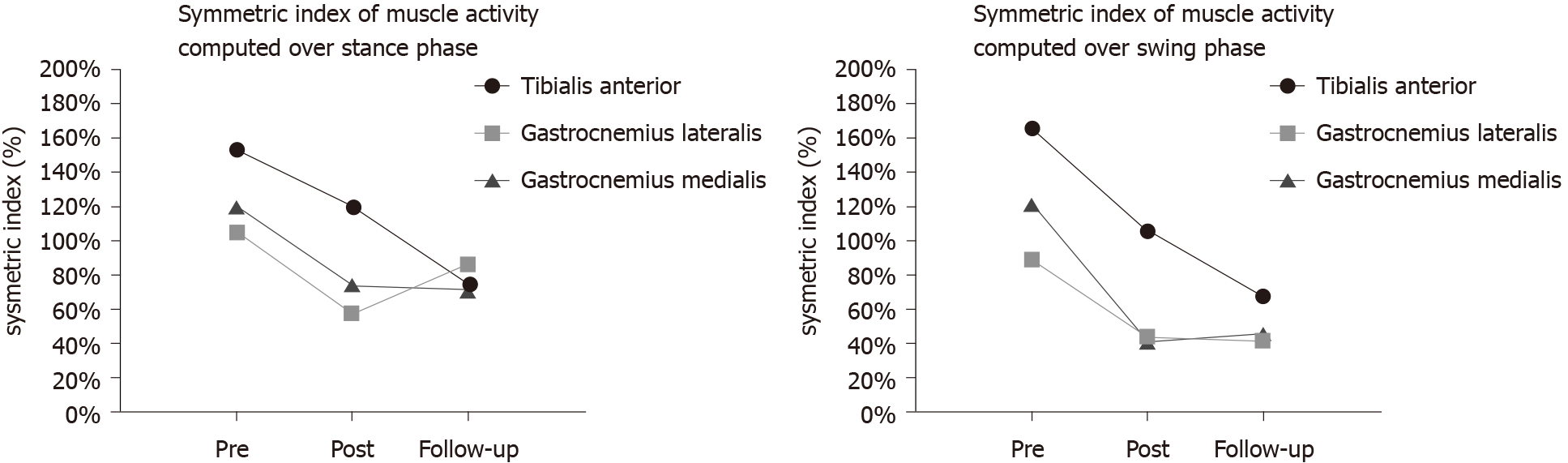
The mean RMS values (microvolt, μV) of the lower limb muscles (including the tibialis anterior, gastrocnemius lateralis, and gastrocnemius medialis) and their SI values over the gait cycle are shown in Table 4 and Figure 4. The values of mean RMS on the affected side gradually increased from pretreatment to follow-up but remained lower than those of the unaffected side. During both the stance phase and swing phase, the posttreatment SI values of the muscle activities were lower than the pretreatment values. The symmetry of gastrocnemius medialis activities varied slightly from posttreatment to follow-up.
| TA | GL | GM | Symmetric index (SI, %) | ||||||
| Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | SITA | SIGL | SIGM | |
| Stance phase | |||||||||
| Pre-treatment | 9.95 | 75.78 | 22.53 | 72.83 | 11.96 | 48.20 | 153.58 | 105.49 | 120.48 |
| Post-treatment | 19.46 | 77.74 | 25.95 | 46.85 | 19.00 | 41.24 | 119.92 | 57.42 | 73.84 |
| Follow-up | 25.54 | 55.85 | 23.05 | 58.38 | 20.41 | 43.24 | 74.48 | 86.77 | 71.74 |
| Swing phase | |||||||||
| Pre-treatment | 8.11 | 88.40 | 20.27 | 52.73 | 9.93 | 41.19 | 166.39 | 88.93 | 122.30 |
| Post-treatment | 20.73 | 67.43 | 22.12 | 34.60 | 17.96 | 27.23 | 105.94 | 44.01 | 41.03 |
| Follow-up | 36.38 | 73.66 | 21.74 | 33.18 | 18.97 | 30.26 | 67.76 | 41.66 | 45.87 |
The LBPP treadmill has potential applications for improving the gait of patients after stroke, but the related mechanism remains unclear[13]. Here we present the rehabilitation effect of LBPP training on gait performance in a stroke patient mainly by plantar pressure analysis and 3D gait analysis with synchronous EMG. During LBPP training, our patient not only improved his lower limb muscle strength and walking speed, but more importantly, the symmetry index of various biomechanical indicators improved. Correspondingly, the scores of clinical balance assessment scales of BBS and TUGT improved, although his score of lower limb function scale FMA did not change. This might be related to one of the functional mechanisms of the LBPP treadmill setting, which is a pressure chamber with air inflation designed to increase support for the patient’s lower limb to restore the patient’s normal gait pattern[14]. Intensive training in constant normal gait mode might be a great help to the recovery of the patient’s balance. Also, through dynamic foot pressure observation, this case report presents the patient’s plantar pressure transferring from the heel area to the toe area during the LBPP training process, and the symmetry of lower body biomechanical parameters improved. Other studies also found that the toe area pressure could affect gait and balance[6,15].
Although this case study reports the details of the LBPP training program and evaluation, and obtained some potential theoretical clues, more cases are required to be included in future studies to further verify the mechanism of LBPP for gait and balance improvement after stroke.
We have documented a dynamic improvement of gait performance in a stroke patient under LBPP training, which includes lower limb muscle strength, walking speed, and symmetry of lower limb biomechanics. Our study provides some clues about the potential mechanism for LBPP training on gait and balance improvement, which is related to rebuilding foot pressure distribution and remodeling symmetry of biomechanics of the lower limb.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Radenovic L S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Schröder J, Truijen S, Van Criekinge T, Saeys W. Feasibility and effectiveness of repetitive gait training early after stroke: A systematic review and meta-analysis. J Rehabil Med. 2019;51:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Liang J, Guo Y, Zheng Y, Lang S, Chen H, You Y, O'Young B, Ou H, Lin Q. The Lower Body Positive Pressure Treadmill for Knee Osteoarthritis Rehabilitation. J Vis Exp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Grabowski AM. Metabolic and biomechanical effects of velocity and weight support using a lower-body positive pressure device during walking. Arch Phys Med Rehabil. 2010;91:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Kristiansen M, Odderskær N, Kristensen DH. Effect of body weight support on muscle activation during walking on a lower body positive pressure treadmill. J Electromyogr Kinesiol. 2019;48:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lee KS, Ko E, Lee SY. Immediate Effect of the Toe Spreader on Tibialis Anterior and Peroneus Longus Muscle Activities: a Pilot Study. J Phys Ther Sci. 2013;25:293-295. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lee KB, Kim BR, Lee KS. Effects of toe spreader on plantar pressure and gait in chronic stroke patients. Technol Health Care. 2018;26:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 463] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 Suppl 2:S7-11. [PubMed] |
| 9. | Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 10. | Temporiti F, Zanotti G, Furone R, Molinari S, Zago M, Loppini M, Galli M, Grappiolo G, Gatti R. Gait analysis in patients after bilateral versus unilateral total hip arthroplasty. Gait Posture. 2019;72:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 12. | Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:e98-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1844] [Article Influence: 204.9] [Reference Citation Analysis (0)] |
| 13. | Masumoto K, Joerger J, Mercer JA. Influence of stride frequency manipulation on muscle activity during running with body weight support. Gait Posture. 2018;61:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hoffman MD, Donaghe HE. Physiological responses to body weight--supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Bonanno DR, Zhang CY, Farrugia RC, Bull MG, Raspovic AM, Bird AR, Landorf KB. The effect of different depths of medial heel skive on plantar pressures. J Foot Ankle Res. 2012;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |