Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.614
Peer-review started: June 3, 2020
First decision: November 14, 2020
Revised: November 25, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: January 26, 2021
Processing time: 230 Days and 5 Hours
Hematopoietic stem cell transplantation (HSCT) is widely used in the treatment of hematological diseases. However, complications after transplantation, such as acute and chronic graft-vs-host disease (GVHD), still seriously affect the quality of life and even threaten the lives of patients. There is evidence that glomerular diseases can manifest as GVHD. However, GVHD should not occur as a result of syngeneic HSCT.
A 20-year-old male diagnosed with T lymphoblastic lymphoma (stage IIIA, aaIPI 1) in September 2013 was treated with six cycles of hyper-CVAD and achieved complete remission. He underwent syngeneic HSCT in June 2014, and had no kidney disease history before the transplant. However, nephrotic syndrome occurred 24 mo later in the patient after syngeneic HSCT. Renal biopsy was performed, which led to a diagnosis of atypical membranous nephropathy. After treatment with glucocorticoids combined with cyclophosphamide and cyclosporine, the nephrotic syndrome was completely relieved.
We report a case of delayed nephrotic syndrome after syngeneic HSCT. Antibody-mediated autoimmune glomerular disease may be the underlying mechanism. After treatment with immunosuppressive agents, the nephrotic syndrome was completely relieved but further long-term follow-up is still needed.
Core Tip: Postoperative complications such as acute and chronic graft-vs-host disease (GVHD) seriously affect the quality of life of patients after transplantation and even threaten patients’ lives. There is evidence that glomerular diseases may manifest as GVHD. However, GVHD should not occur as a result of syngeneic hematopoietic stem cell transplantation (HSCT). We report a rare case of delayed nephrotic syndrome following syngeneic HSCT for treatment of T lymphoblastic lymphoma. Nephrotic syndrome occurred 24 mo later. Renal biopsy was performed, which led to a diagnosis of atypical membranous nephropathy. After treatment with glucocorticoids combined with cyclophosphamide and cyclosporine, the nephrotic syndrome was completely relieved. The specific pathogenesis of nephrotic syndrome in this case is unclear but some findings may support the role of induction of the autoimmune response after transplantation.
- Citation: Bai MC, Wu JJ, Miao KR, Zhu JF, Mao HJ. Nephrotic syndrome in syngeneic hematopoietic stem cell transplantation recipients: A case report. World J Clin Cases 2021; 9(3): 614-622
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/614.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.614
Hematopoietic stem cell transplantation (HSCT) is often complicated by kidney damage, which can be characterized by acute kidney injury and nephrotic syndrome and may develop into chronic kidney disease or even end-stage renal disease[1,2]. The percentage of HSCT recipients with glomerular lesions is very small, accounting for only 1%-6% of allogeneic HSCT patients[3,4]. HSCT recipients usually present with membranous nephropathy (MN) (64%), minimally pathological nephropathy (19%), and other glomerular diseases, including focal segmental glomerulosclerosis (5%), IgA nephropathy (5%), proliferative glomerular disease (4%), mesangial proliferative glomerular disease (1%), and anti-neutrophil cytoplasmic antibody-associated glomerulonephritis (1%)[5]. There is evidence that these glomerular diseases are a manifestation of graft-vs-host disease (GVHD)[2]. Compared with that after allogenic HSCT, GVHD after syngeneic HSCT is rarely encountered in the clinic. The tissue lesions have been mainly located in the gastrointestinal tract and liver, especially in the distal colon[4], and there is no definite diagnostic basis. Nephrotic syndrome associated with syngeneic HSCT has not yet been reported.
We describe herein the first published case of delayed nephrotic syndrome occurring in the stable phase after syngeneic HSCT and attempt to explore its development.
A 20-year-old male presented to the Nephrology Department of our hospital complaining of limb soreness, and after 5 mo, the patient began to develop proteinuria and edema in both lower extremities.
In February 2016, the patient developed limb soreness. The initial laboratory data were as follows: hemoglobin of 128 g/L and a platelet count of 398 × 109/L. Other values were as follows: Serum albumin was 29.4 g/L, alanine aminotransferase was 246.6 U/L, aspartate aminotransferase was 314.8 U/L, and creatine kinase was 4612.9 U/L. We considered that chronic rejection after transplantation resulted in myositis and liver damage, so the patient was treated with corticoid therapy by administration of oral methylprednisolone (32 mg/d). The outcome was favorable. However, after 5 mo, the patient began to develop proteinuria and edema in both lower extremities.
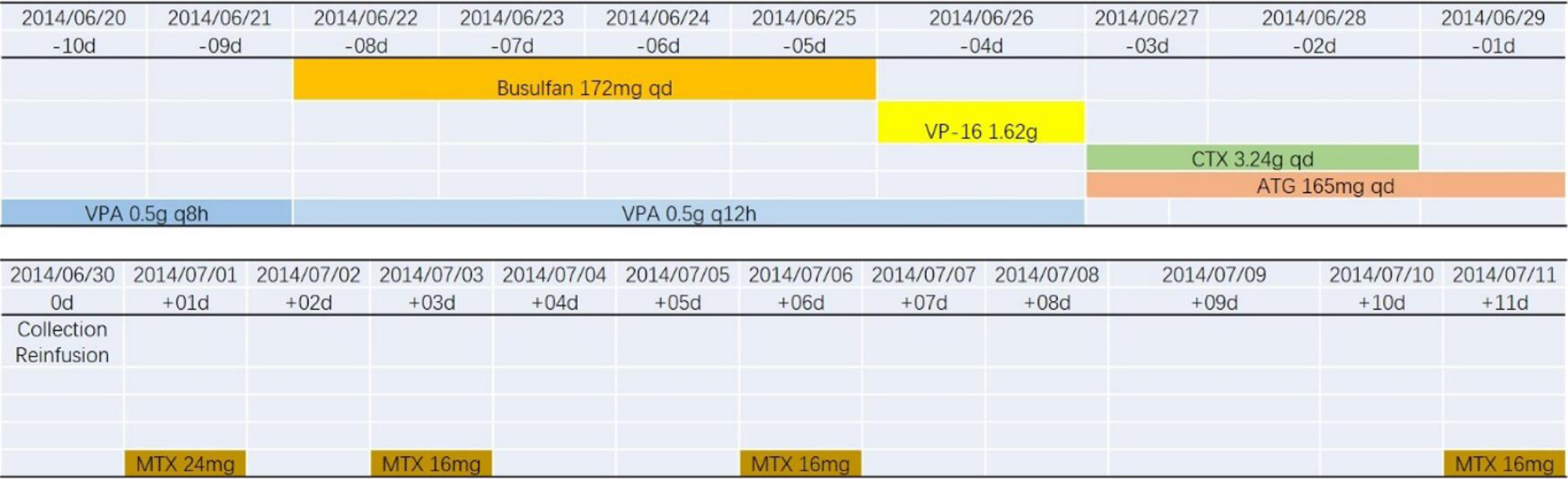
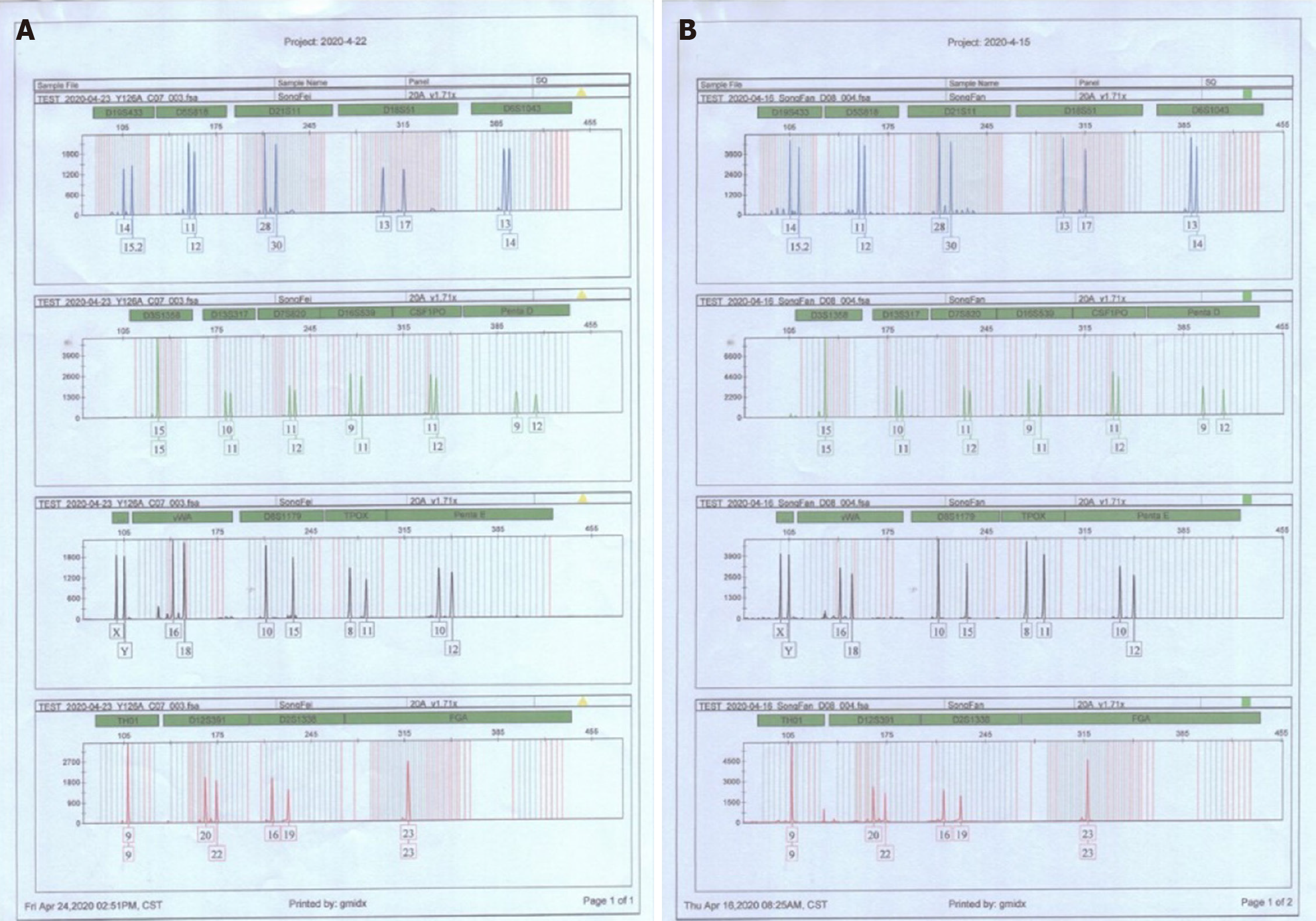
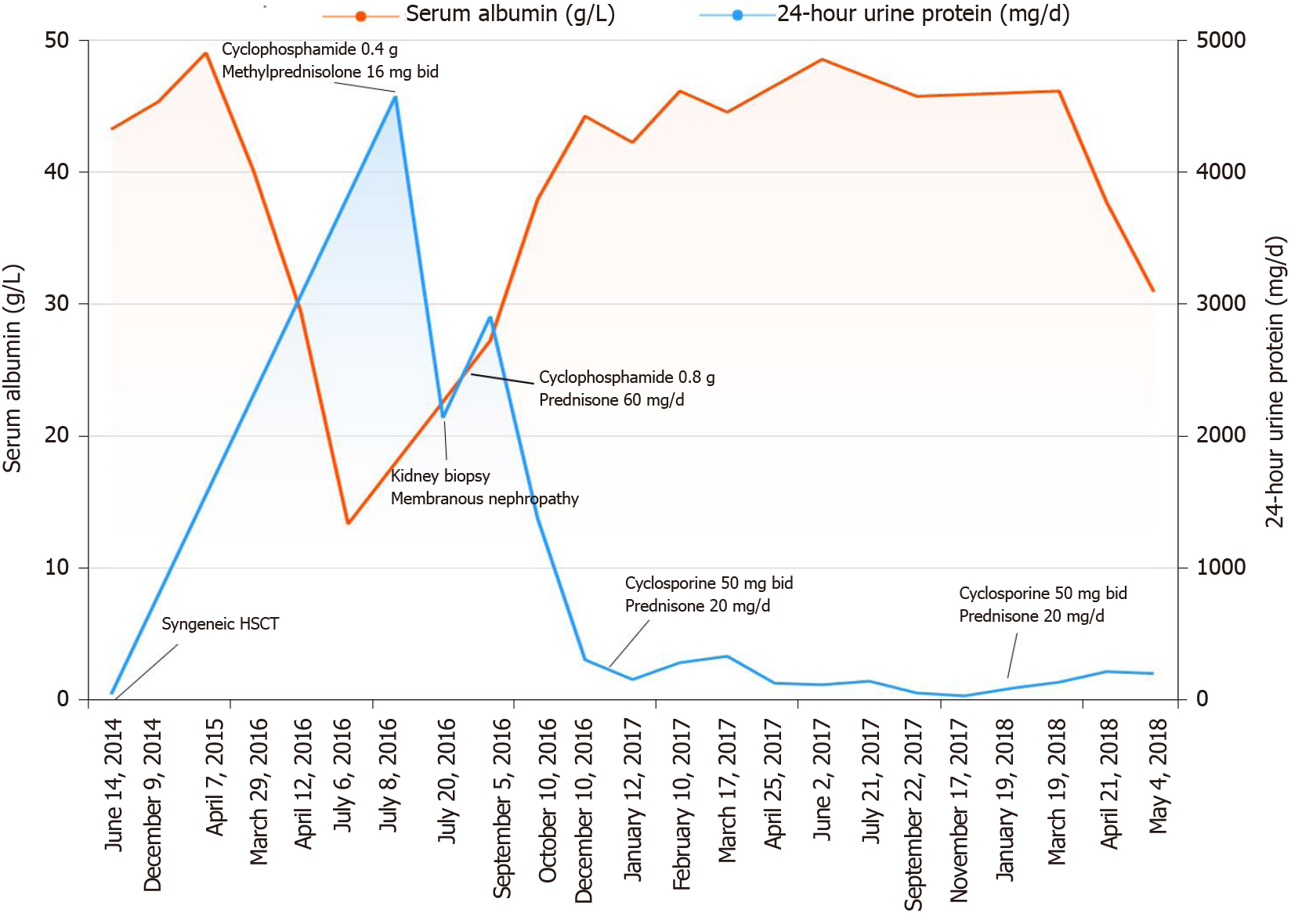
The patient diagnosed with T lymphoblastic lymphoma (stage IIIA, aaIPI 1) in September 2013 was treated with six cycles of hyper-CVAD and achieved complete remission. Then, he underwent syngeneic HSCT in June 2014 (Figure 1), and had no kidney disease history before the transplant. The donor was his twin brother, and HLA matching at high resolution for the HLA-A, B, C, DRB1 and DQB1 loci revealed that the loci were completely consistent in the donor and recipient. Additionally, short tandem repeat analysis was performed on both the donor and recipient, and the results showed that the 20 loci were completely consistent (Figure 2 and Table 1). After transplantation, the patient used 8 mg glucocorticoids daily and then tapered off gradually, and he also received 3 mg sirolimus daily for 3 mo to prevent potential GVHD, which achieved good results.
| Locus | Recipient | Locus | Donor | ||
| A1 | A2 | A1 | A2 | ||
| D19S433 | 14 | 15.2 | D19S433 | 14 | 15.2 |
| D5S818 | 11 | 12 | D5S818 | 11 | 12 |
| D21S11 | 28 | 30 | D21S11 | 28 | 30 |
| D18S51 | 13 | 17 | D18S51 | 13 | 17 |
| D6S1043 | 13 | 14 | D6S1043 | 13 | 14 |
| D3S1358 | 15 | 15 | D3S1358 | 15 | 15 |
| D13S317 | 10 | 11 | D13S317 | 10 | 11 |
| D7S820 | 11 | 12 | D7S820 | 11 | 12 |
| D16S539 | 9 | 11 | D16S539 | 9 | 11 |
| CSF1PO | 11 | 12 | CSF1PO | 11 | 12 |
| Penta D | 9 | 12 | Penta D | 9 | 12 |
| AMEL | X | Y | AMEL | X | Y |
| vWA | 16 | 18 | vWA | 16 | 18 |
| D8S1179 | 10 | 15 | D8S1179 | 10 | 15 |
| TPOX | 8 | 11 | TPOX | 8 | 11 |
| Penta E | 10 | 12 | Penta E | 10 | 12 |
| TH01 | 9 | 9 | TH01 | 9 | 9 |
| D12S391 | 20 | 22 | D12S391 | 20 | 22 |
| D2S1338 | 16 | 19 | D2S1338 | 16 | 19 |
| FGA | 23 | 23 | FGA | 23 | 23 |
The patient had a free medical history.
At admission, the patient’s temperature was 36.8 °C, the heart rate was 70 bpm, the respiratory rate was 16 breaths per minute, the blood pressure was 112/80 mmHg, and the oxygen saturation in room air was 99%. The clinical physical examination revealed moderate edema in both lower limbs without any other pathological signs.
The blood analysis results were as follows: Leukocyte count of 9.60 × 109/L, neutrophil count of 6.66 × 109/L, eosinophils at 0.20%, and hemoglobin of 132 g/L. The blood biochemistry results were as follows: Albumin of 15.7 g/L, total cholesterol of 9.45 mmol/L, calcium of 1.97 mmol/L, and alkaline phosphatase of 144.7 U/L. The thyroid function testing results were as follows: free triiodothyronine of 1.96 mmol/L, triiodothyronine of 0.61 nmol/L, thyroxine of 36.5 nmol/L, thyroglobulin of 39.30 ng/L, and free thyroxine of 9.51 pmol/L. Immunoglobulin G was 4.99 g/L, d-dimer was 1.48 mg/L, 25-hydroxyvitamin D was 7.50 nmol/L, blood kappa light chain was 0.866 g/L, and L light chain was 0.413 g/L. The patient had undergone testing for antinuclear antibody, rheumatism, hepatitis B virus antigen, hepatitis C, and syphilis, the results of which were all negative. Furthermore, his level of serum phospholipase A2 receptor (PLA2R) autoantibodies was 4.36 RU/mL (normal value < 20 RU/mL according to ELISA), which was also a negative result.
The electrocardiogram, chest X-ray, kidney, ureter and bladder ultrasonic imaging, and positron emission computed tomography/computed tomography results were all negative.
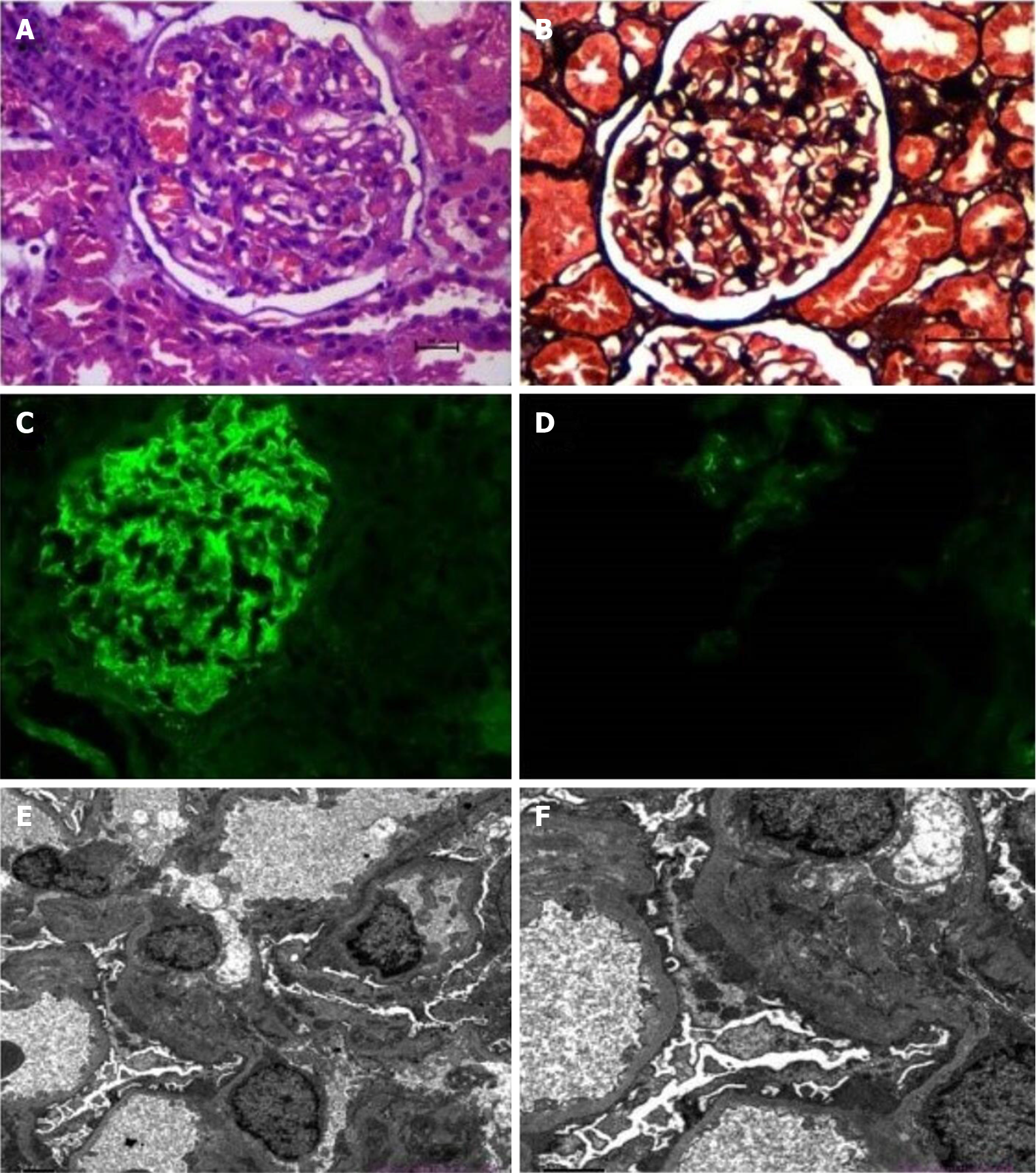
A percutaneous kidney biopsy was performed, which led to a diagnosis of glomerulonephritis with characteristics of MN (Figure 3) on the basis of light microscopy, electron microscopy and immunofluorescence findings.
Light microscopy: Thirty-two glomeruli were observed. The number of glomerular cells was not increased, and the mesangial hypercellularity was mild. The mesangial matrix expansion was slight, and few intracapillary fibrin thrombi were observed. The capillaries were open, showing few microthromboses. Basement membrane thickening was observed, with a small number of capsule adhesions but no double contours or spikes. The renal tubular epithelial cells were basically normal. The lumen showed evidence of protein casts, red blood cell casts and few cellular casts. The interstitium showed little infiltration of inflammatory cells and no obvious fibrosis, and the wall of the arteriole was not thickened (Figure 3A and B).
Electron microscopy: A large number of fibroblasts were seen in Bowman's space. There were few capillaries, and the opening was in acceptable condition. The endothelium was not hypercellular. The thickness of the basement membrane was approximately 300-500 nm. No clear mesangial area was seen, and only one podocyte showed lamellar vacuolar degeneration. There were epithelial electron dense deposits in the glomeruli (Figure 3E and F).
Immunofluorescence (IF): Findings were as follows: IgG, diffuse granular immune reactant on the basement membrane (++++); IgA, diffuse granular immune reactant on the basement membrane (+); IgM, diffuse granular immune reactant on the basement membrane (+++); C1q, diffuse granular immune reactant on the basement membrane (++); C3, diffuse granular immune reactant on the basement membrane (++); C4, diffuse granular immune reactant on the basement membrane (++); Fibrinogen, diffuse granular immune reactant on the basement membrane (+) (Figure 3C and D).
The final diagnosis of the presented case was nephrotic syndrome after syngeneic HSCT.
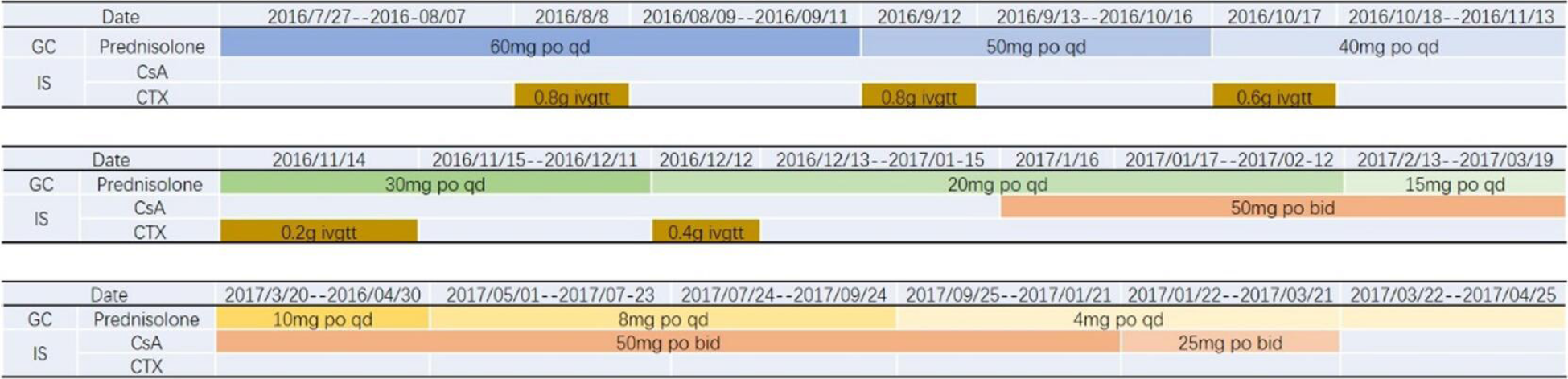
We eliminated the possibility of lymphoma recurrence, and biopsy confirmed the immune-mediated process. The patient was administered 60 mg/d methylprednisolone on 27 July 2016. On August 8th, he was treated with 0.8 g cyclophosphamide, which was also administered an additional four times (0.8 g on September 12, 0.6 g on October 17, 0.2 g on November 14, and 0.4 g on December 12).
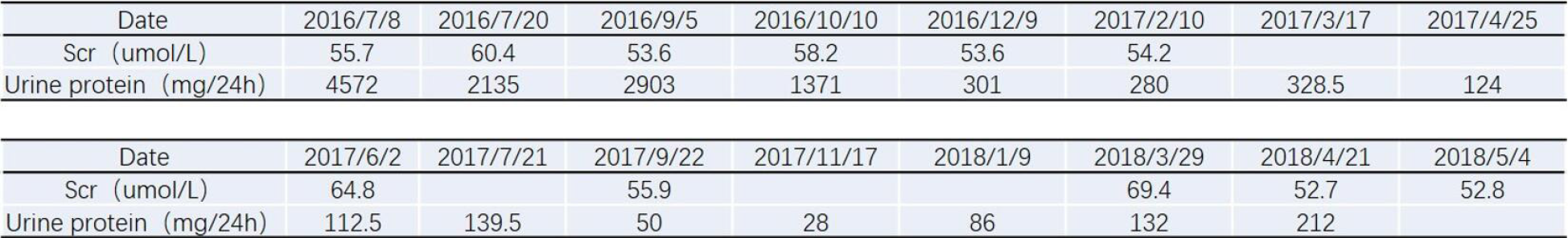
Significant improvement in the proteinuria was achieved in December 2016, with a 24-h proteinuria loss of 0.3 g (Figures 4 and 5). In January 2017, the patient was treated with cyclosporine combined with glucocorticoids, which were gradually tapered down, and his proteinuria showed long-term stability. Finally, cyclosporine (50 mg/d) was discontinued in March 2018 (Figure 6). During the follow-up period, the patient did not develop an infection.
Glomerulopathies after allogeneic HSCT most often manifest as nephrotic syndrome caused by membranous nephropathy[6,7]. Since most patients receiving allogeneic HSCT have a history of acute (30% to 68%) or chronic (74% to 86%) GVHD, GVHD is considered to cause secondary MN after HSCT[6,8,9]. Compared with that in the general population, the incidence of MN after HSCT is increased (being 0.6%-3.8% in patients 1-10 years after HSCT[5], whereas the annual incidence rate in the general population is 0.0012%[10]).
Recently, studies have shown that the pathogenesis of chronic GVHD mainly goes through three stages. First, tissue damage causes activation of antigen-presenting cells, which causes inflammation, leading to endothelial damage and reduced vascular density; second, the proliferation of allogeneic reactive T/B cells brings about thymus damage, leading to a reduction in regulatory T/B/natural killer cells, which results in the loss of peripheral tolerance. Ultimately, macrophages secrete TGF-beta and PDGF-alpha to activate fibroblasts, which leads to an increase in extracellular matrix and tissue hardening, resulting in tissue and organ lesions. However, the pathogenesis of syngeneic GVHD remains unclear.
Previous studies have shown that inducing autologous GVHD requires two major factors. The first factor is the disruption of central immune reconstruction, and the other is the failure of peripheral immune tolerance in recipient tissues after transplantation. In patients with HSCT, the thymic epithelium is destroyed by a variety of confounding factors, such as aging, conditioning regimen toxicity, and calcineurin inhibitors[11]. Alloreactive T cells contribute to the process by depleting thymic dendritic cells, medullary thymic epithelial cells (referred to as TECs) and cortical TECs[12]. Moreover, due to lymphoid clearance during pretreatment in HSCT patients, the T cell-dependent regulatory system is unable to eliminate allogeneic T cells released into the periphery, which in turn leads to the development of autoreactive inflammation[13].
In addition, B cells may be an important coparticipant, as shown by the similarity between the clinical manifestations of chronic GVHD (cGVHD) and various autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, and type 1 diabetes[11]. In a study of autoimmune diseases, more than half of developing B cells in bone marrow expressed autoreactive B cell receptors[14]. The possible role of B cells in cGVHD pathogenesis was revealed by the fact that B cells reconstituted after myeloablative conditioning exhibited B cell-receptor hyperresponsiveness, which, after activation, led to the expansion of curative B cells, which released a large number of antibodies and thereby promoted cGVHD[15].
With the discovery of anti-nuclear antibodies associated with chronic allogeneic GVHD or immune recovery, it has been further confirmed that B cells may participate in chronic allogeneic GVHD[16]. Autologous GVHD is considered to be an autoimmune syndrome and a less serve form of GVHD than the allograft response.
Nephrotic syndrome in patients with allogeneic HSCT is considered a possible manifestation of cGVHD in the kidney. A reduction in immunosuppressive medication after HSCT is related to the development of nephrotic syndrome in 63% of patients within 9 mo. MN is rarely encountered after autologous HSCT (3% autologous and 97% allogeneic HSCT)[5]. The possible reason is that changes in autoimmune lymphokines or new factors emerging after HSCT can lead to podocyte damage, which contributes to MN. Renal biopsy and treatment according to the pathological type are recommended. At present, glucocorticoids combined with calcineurin inhibitors are the main treatment. Other immunosuppressants have also been used, such as cyclophosphamide, mycophenolate, and rituximab[17]. In this case, cyclophosphamide was used for treatment, and finally, a small dose of glucocorticoid combined with cyclosporine completely relieved the condition.
In 2009, M-type PLA2R was discovered as the primary target in idiopathic MN, and great progress has been made in the diagnosis and treatment of idiopathic MN[18]. It is worth noting that a large proportion of patients with idiopathic MN have circulating antibodies against PLA2R[19].
A previous study showed that no anti-PLA2R antibody was detected in patients with allogeneic HSCT-associated MN[20]. Therefore, MN and other forms of immune-mediated glomerulonephritis in HSCT patients may represent "kidney-specific" forms of cGVHD[21], in which alloantibodies induced by donor immune cells can specifically act on podocyte antigens or proteins that bind to the receptor but that are not present in the donor. The patient was negative for PLA2R antibody, and the IF results suggested that IgG, IgA, IgM, C1q, C3, and C4 were widely deposited on basal membranes; immunosuppressive therapy was effective, perhaps as a result of this mechanism. This case suggests that GVHD may still occur in homologous transplantation, which requires close monitoring and the long-term use of small amounts of steroids and immunosuppressants.
We report a case of delayed nephrotic syndrome after syngeneic HSCT. Antibody-mediated autoimmune glomerular disease may be the underlying mechanism. After treatment with immunosuppressive agents, the nephrotic syndrome was completely relieved, but further long-term follow-up is needed.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ruiz MA S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Ando M. An Overview of Kidney Disease Following Hematopoietic Cell Transplantation. Intern Med. 2018;57:1503-1508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Jaguś D, Lis K, Niemczyk L, Basak GW. Kidney dysfunction after hematopoietic cell transplantation-Etiology, management, and perspectives. Hematol Oncol Stem Cell Ther. 2018;11:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Byrne-Dugan CJ, Collins AB, Lam AQ, Batal I. Membranous nephropathy as a manifestation of graft-vs-host disease: association with HLA antigen typing, phospholipase A2 receptor, and C4d. Am J Kidney Dis. 2014;64:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Flanagan DM, Jennings CD, Goes SW, Caywood BE, Gross R, Kaplan AM, Bryson JS. Nitric oxide participates in the intestinal pathology associated with murine syngeneic graft-versus-host disease. J Leukoc Biol. 2002;72:762-768. [PubMed] |
| 5. | Hu SL. The role of graft-versus-host disease in haematopoietic cell transplantation-associated glomerular disease. Nephrol Dial Transplant. 2011;26:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Abboud I, Peraldi MN, Hingorani S. Chronic kidney diseases in long-term survivors after allogeneic hematopoietic stem cell transplantation: monitoring and management guidelines. Semin Hematol. 2012;49:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Barbouch S, Gaied H, Abdelghani KB, Goucha R, Lakhal A, Torjemen L, Hamida FB, Abderrahim E, Maiz HB; HafedhHedri; Adel K. Chronic graft vs host disease and nephrotic syndrome. Saudi J Kidney Dis Transpl. 2014;25:1062-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Terrier B, Delmas Y, Hummel A, Presne C, Glowacki F, Knebelmann B, Combe C, Lesavre P, Maillard N, Noël LH, Patey-Mariaud de Serre N, Nusbaum S, Radford I, Buzyn A, Fakhouri F. Post-allogeneic haematopoietic stem cell transplantation membranous nephropathy: clinical presentation, outcome and pathogenic aspects. Nephrol Dial Transplant. 2007;22:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Ramachandran V, Kolli SS, Strowd LC. Review of Graft-Versus-Host Disease. Dermatol Clin. 2019;37:569-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 468] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 11. | Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, Ritz J, Antin JH, Murphy WJ, Luznik L, Shlomchik MJ, Panoskaltsis-Mortari A, Blazar BR. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, Wang M, Wang A, Todorov I, Wang J, Zeng D. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, Alyea EP, Ho VT, Soiffer RJ, Antin JH, Ritz J. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1598] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 15. | Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, Freeman GJ, Serody JS, Murphy WJ, Munn DH, Sarantopoulos S, Luznik L, Maillard I, Koreth J, Cutler C, Soiffer RJ, Antin JH, Ritz J, Dubovsky JA, Byrd JC, MacDonald KP, Hill GR, Blazar BR. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123:3988-3998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, Kikic F, Calistri E, Filì C, Geromin A, Cerno M, Fanin R. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Beyar-Katz O, Davila EK, Zuckerman T, Fineman R, Haddad N, Okasha D, Henig I, Leiba R, Rowe JM, Ofran Y. Adult Nephrotic Syndrome after Hematopoietic Stem Cell Transplantation: Renal Pathology is the Best Predictor of Response to Therapy. Biol Blood Marrow Transplant. 2016;22:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96:1292-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC. A Proposal for a Serology-Based Approach to Membranous Nephropathy. J Am Soc Nephrol. 2017;28:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 20. | Huang X, Qin W, Zhang M, Zheng C, Zeng C, Liu Z. Detection of anti-PLA2R autoantibodies and IgG subclasses in post-allogeneic hematopoietic stem cell transplantation membranous nephropathy. Am J Med Sci. 2013;346:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Chanswangphuwana C, Townamchai N, Intragumtornchai T, Bunworasate U. Glomerular diseases associated with chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation: case reports. Transplant Proc. 2014;46:3616-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |