Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.581
Peer-review started: July 15, 2020
First decision: September 29, 2020
Revised: October 17, 2020
Accepted: November 9, 2020
Article in press: November 9, 2020
Published online: January 26, 2021
Processing time: 189 Days and 0.8 Hours
Lung cancer is a major cause of death among patients, and non-small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancers in many countries.
To evaluate the clinical benefit (CB) of COX-2 inhibitors in patients with advanced NSCLC using systematic review.
We searched the six electronic databases up until December 9, 2019 for studies that examined the efficacy and safety of the addition of COX-2 inhibitors to chemotherapy for NSCLC. Overall survival (OS), progression free survival (PFS), 1-year survival rate (SR), overall response rate (ORR), CB, complete response (CR), partial response (PR), stable disease (SD), and toxicities were measured with more than one outcome as their endpoints. Fixed and random effects models were used to calculate risk estimates in a meta-analysis. Potential publication bias was calculated using Egger’s linear regression test. Data analysis was performed using R software.
The COX-2 inhibitors combined with chemotherapy were not found to be more effective than chemotherapy alone in OS, progression free survival, 1-year SR, CB, CR, and SD. However, there was a difference in overall response rate for patients with advanced NSCLC. In a subgroup analysis, significantly increased ORR results were found for celecoxib, rofecoxib, first-line treatment, and PR. For adverse events, the increase in COX-2 inhibitor was positively correlated with the increase in grade 3 and 4 toxicity of leukopenia, thrombocytopenia, and cardiovascular events.
COX-2 inhibitor combined with chemotherapy increased the total effective rate of advanced NSCLC with the possible increased risk of blood toxicity and cardiovascular events and had no effect on survival index.
Core Tip: This study demonstrated that in patients who received adjuvant chemotherapy for advanced non-small cell lung cancer, COX-2 inhibitors improved the overall response rate and had no improvement on prolonged mortality. However, COX-2 enhanced both the overall response rate and the 1-year survival rate. Concerning toxicity, celecoxib plus chemotherapy resulted in a higher incidence of hematologic toxicities. Meanwhile, rofecoxib may augment the risk of cardiovascular events.
- Citation: Xu YQ, Long X, Han M, Huang MQ, Lu JF, Sun XD, Han W. Clinical benefit of COX-2 inhibitors in the adjuvant chemotherapy of advanced non-small cell lung cancer: A systematic review and meta-analysis. World J Clin Cases 2021; 9(3): 581-601
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/581.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.581
The proportion of non-small cell lung cancer (NSCLC) is more than 80% of all lung tumors. Most patients have advanced NSCLC at stage ШB or IV when diagnosed and confirmed and have to receive alleviative treatment in order to maintain their lives[1,2]. The median survival time is 6-10 mo for patients who are diagnosed with advanced NSCLC in performance status 0-2 while adopting palliative first-line chemotherapy[3-5]. For decades, chemotherapy has been the cornerstone of standard cancer treatment[6]. At present, the efficacy of various chemotherapy regimens has reached its peak[7]. New treatment strategies are hypothesized to improve the clinical benefit (CB) of advanced NSCLC.
Increased COX-2 expression was reported in close to 70% of NSCLC adeno-carcinomas[8,9]. COX-2 expression is upregulated in the early stage of tumori-genesis, and it can lead to poor prognosis by promoting tumor cell proliferation, angiogenesis, invasion, and metastasis[10-12]. By any reasonable assessment, this targeted treatment initially achieved great success but also produced unpredictable and occasionally serious side effects. Comparison between nonselective nonsteroidal anti-inflammatory drugs and rofecoxib has shown that rofecoxib contributes to a decrease of gastrointestinal hemorrhage but not a decrease of thrombosis[13]. However, with respect to adverse events, celecoxib has no significant improvement on decreasing gastrointestinal events. The meta-analysis by Chen et al[14] reported that celecoxib has a positive influence on the treatment of advanced cancers but increased the risk of cardiovascular events by using celecoxib, which cannot be ignored. Other studies[15-17] indicated that celecoxib increased the overall response rate (ORR) of advanced NSCLC with no significant difference in cardiovascular events. The study related to COX-2 for intervention of NSCLC is mired in controversy in the medical field. Therefore, this systematic review based on randomized controlled trials (RCTs) was conducted to appraise the benefit of chemotherapy-assisted addition of COX-2 for advanced NSCLC.
Six electronic databases, including the MEDLINE and EMBASE from Ovid, the Cochrane Library, CNKI, Wanfang Date, and CBD, were searched through December 9, 2019 using “cyclooxygenase-2 inhibitor,” “COX-2,” “apricoxib,” “celecoxib,” “rofecoxib,” “non-small cell lung cancer,” “NSCLC,” and “randomized controlled trial.”
The following inclusion criteria for clinical trials: (1) The language was limited to Chinese and English; (2) The benefit of the addition of COX-2 to chemotherapy (the principle of quantitative simplicity) were compared; (3) The NSCLC stage IIIB or IV patients used were defined and confirmed; (4) Outcomes such as overall survival (OS), progression free survival (PFS), 1-year survival rate (SR), ORR, CB, complete response (CR), partial response (PR), stable disease (SD), and toxicities were measured with more than one outcome as their endpoints. The primary outcomes were the OS, PFS, 1-year SR, ORR, and CB. The rate of CR and PR and the rate of grade 3 and 4 toxicity are regarded as the secondary endpoints; and (5) The study type was RCT.
Studies with criteria were excluded: (1) Patients experienced chemotherapy, immunotherapy, or any systemic therapy for NSCLC before; (2) The study was a duplicate; and (3) The data could not be extracted or obtained through contact with the author.
The data extracted were study design, patient characteristics, interventions, controls, and outcomes. The data acquisition was done independently by two authors.
The methodological quality was mainly focused on five aspects, including randomization methods, stratification factors, double blind, follow-up, and intent to treat, which were independently evaluated by two commentators. If there was a dispute, a third reviewer was consulted.
The hazard ratio (HR) was considered a reasonable effect size for OS and PFS outcomes after careful consideration. The existing HR with 95% confidence interval (CI) values provided from the original research, and then HR data was obtained. If the HR and 95%CI values were not provided, the Kaplan-Meier survival curve[18] was adopted. The relative risk (RR) with 95%CI was employed for other dichotomous outcomes[19,20]. The statistical test was performed for heterogeneity, and I2 > 40% and P < 0.1 were considered as evidence for heterogeneity as well[20]. There is a theory that if the condition of the data were homogeneous under a fixed-effects model, then the heterogeneity of the results was derived from the type of Cox inhibitor and the difference in treatment line. Based on these modifiers, subgroups were conducted to address and analyze the heterogeneity. Ideally, the data are still heterogeneous, for which we introduce a stochastic effect model. The fixed-effects model was used instead when I2 ≤ 40%[21]. Besides treatment line (first-line and second-line) and phase (II and III), COX-2 inhibitor types (celecoxib, rofecoxib, and apricoxib) were also identified as significant source of heterogeneity. Egger’s test was a methodological tool to solve quantitative detection publication bias[20]. All data analyses were performed by R 5.3.1 software.
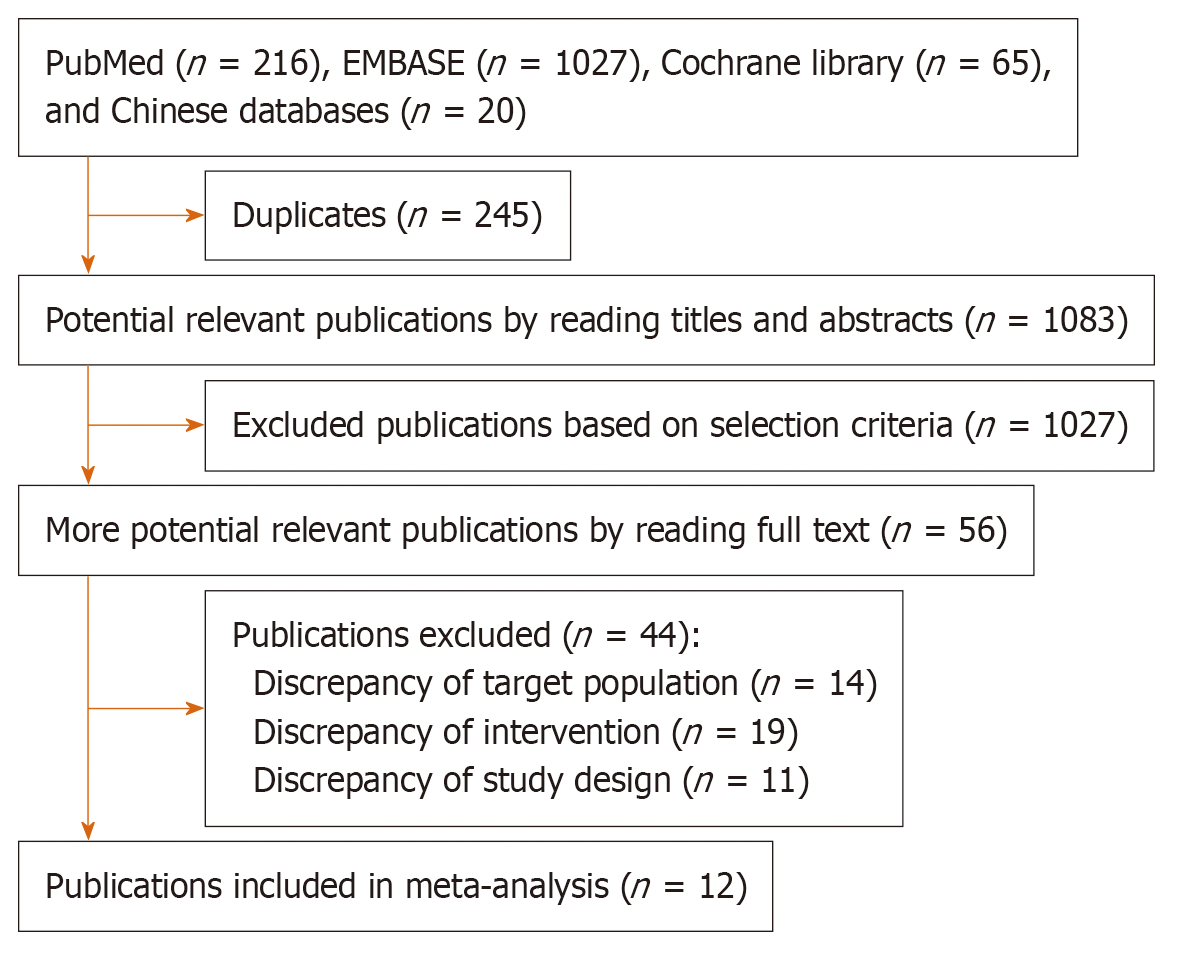
There are 1328 publications picked from the six databases (Figure 1). Ultimately, 12 studies[22-33] involving 2273 patients were screened and included in this meta-analysis. The COX-2 inhibitors, including celecoxib, apricoxib, and rofecoxib, were adopted in these studies and with most of the trials opting for celecoxib. Only three studies chose rofecoxib or apricoxib. Table 1 showed the characteristics of the 12 studies.
| Trials or Ref. | Year | Phase | Study period | Country | Sample (I/C) | Age (I/C) | Male (female) (I/C) | Histology (I/C) (AC, SCC, Other) | Extent of disease, Stage | ECOG PS or Karnofsky score | Treatment Line | Interventions | Control | Follow-up in mo |
| Lilenbaum et al[22] | 2006 | II | Feb 2002 to Sept 2003 | United States | 133 (67/66) | 62.7 (37-84)/63.5 (41-78) | 40 (27)/40 (26) | NA, NA, NA | ШB, IV | ECOG 0-1 | Second | Celecoxib 400 mg po bid + DTX 35 mg/m2 or GEM 1000 mg/m2 + CPT-11 60-100 mg/m2 ivgtt day 1 and day 8, q3w | DTX 35 mg/m2 or GEM 1000 mg/m2 + CPT-11 60-100 mg/m2 ivgtt day 1 and day 8, q3w | NA |
| GECO[23] | 2007 | Ш | Jan 2003 to May 2005 | Italy | 400 (149/251) | 61.5 (29-71)/59.0 (37-70) | 120 (29)/202 (49) | 68/134, 47/53, 34/64 | ШB, IV | ECOG 0-1 | First | Rofecoxib 50 mg po qd + GEM 1200 mg/m2 in 30-min or PCI GEM 1200 mg/m2 over 120-min iv infusions days 1 and 8 + DDP 80 mg/m2 ivgtt qd day 1, q3w | GEM 1200 mg/m2 in 30-min or PCI GEM 1200 mg/m2 over 120-min iv infusions days 1 and 8 + DDP 80 mg/m2 ivgtt qd day 1, q3w | 22 |
| Zhou et al[29] | 2007 | II | June 2004 to June 2005 | China | 65 (32/33) | 57.0 (45-77)/55.5 (40-76) | 24 (8)/24 (9) | 17/19, 9/8, 5/3 | ШB, IV | ECOG 0-2 | First | Celecoxib 400 mg po bid days 1-12 + NVB 25 mg/m2 iv qd day 1 and 8 + DDP 75 mg/m2 ivgtt qd days 1 and 2, q3w | NVB 25 mg/m2 iv qd days 1 and 8 + DDP 75 mg/m2 ivgtt qd days 1 and 2, q3w | NA |
| Xiong et al[28] | 2008 | II | Jan 2003 to Jan 2006 | China | 60 (30/30) | 56.4/58.3 | 16 (14)/17 (13) | 16/17, 10/10, 4/3 | ШB, IV | ECOG 0-2 | First | Celecoxib 400 mg po bid + NVB 25 mg/m2 iv qd days 1 and 8 + DDP 70 mg/m2 ivgtt qd days 1-3, q3w | NVB 25 mg/m2 iv qd days 1 and 8 + DDP 70 mg/m2 ivgtt qd days 1-3, q3w | NA |
| CYCLUS[24] | 2011 | Ш | May 2003 to May 2006 | Sweden | 316 (158/158) | 66 (38-85)/65 (37-85) | 73 (85)/87 (71) | 77/94, 38/27, 43/36 | ШB, IV | ECOG 0-2 | First | Celecoxib 400 mg po bid + GEM or NVB + CBP or DDP, ivgtt q3w1 | Placebo + GEM or NVB + CBP or DDP, ivgtt q3w | 36 |
| NVALT-4[25] | 2011 | Ш | July 2003 to Dec 2007 | Netherlands | 561 (281/280) | 62 (40-84)/61 (33-84) | 184 (97)/171 (109) | 138/132, 44/57, 99/91 | ШB, IV | ECOG 0-2 | First | Celecoxib 400 mg po bid + DTX 75 mg/m2 ivgtt qd day 1 + CBP ivgtt qd day 1, q3w2 | Placebo + DTX 75 mg/m2 ivgtt qd day 1 + CBP ivgtt qd day 1, q3w | NA |
| Liu et al[30] | 2012 | NA | Jan 2006 to May 2011 | China | 46 (24/22) | 62 (49-75)/64 (52-76) | 14 (10)/15 (7) | 15/14, 9/8, 0/0 | ШB, IV | Karnofsky ≥ 70 | First | Celecoxib 400 mg po bid days 1-5 + DTX 75 mg/m2 ivgtt qd day 1 + DDP 100 mg/m2 ivgtt qd day 1, q3w | DTX 75 mg/m2 ivgtt qd day 1 + DDP 100 mg/m2 ivgtt qd day 1, q3w | NA |
| Sörenson et al[32] | 2013 | Ш | May 2006 to May 2009 | Sweden | 107 (52/55) | 65 (37-84) | 50/57 | 65, 16, 26 | ШB, IV | NA | First | Celecoxib at a dose of 400 mg bid + carboplatin plus gemcitabine/vinorelbine | Carboplatin + gemcitabine/ vinorelbine | 5 |
| Gitlitz et al[33] | 2014 | II | NA | United States | 120 (78/42) | 63 (35-81)/65 (36-84) | 78 (42)/42 (25) | 45/24, 21/11, 12/7 | ШB, IV | ECOG 0-2 | Second | Apricoxib (400 mg/d) + erlotinib (150 mg/d) on 21-d cycles | Placebo + erlotinib (150 mg/d) on 21-d cycles | NA |
| 0822-GCC[26] | 2015 | II | NA | United States | 72 (36/36) | 62/66 | 20 (16)/20 (16) | 24/25, 8/6, 4/5 | ШB, IV | ECOG 0-2 | Second | Apricoxib 400 mg po qd + DTX 75 mg/m2 or PET 500 mg/m2, q3w | Placebo 400 mg po qd DTX 75 mg/m2 or PET 500 mg/m2, q3w | NA |
| Teng et al[31] | 2015 | II | Aug 2009 to May 2012 | China | 81 (41/40) | 57.7 (28-72)/57.3 (33-76) | 30 (11)/26 (14) | 28/26, 13/14, 0/0 | ШB, IV | ECOG 0-1 | First | Celecoxib 200 mg po bid + NVB 25 mg/m2 ivgtt days 1 and 8 + DDP 70 mg/m2 ivgtt qd day 1, q4w | NVB 25 mg/m2 ivgtt days 1 and 8 + DDP 70 mg/m2 ivgtt qd day 1, q4w | NA |
| CALGB-30801[27] | 2017 | Ш | Nov 2013 to Jan 2016 | United States | 312 (154/158) | 64 (38-83)/64 (36-89) | 82 (72)/87 (71) | NA, 44/43, NA | ШB, IV | ECOG 0-2 | First | Celecoxib 400 mg po bid + CBP + PET 500 mg/m2 day 1, q3w for nonsquamous or Celecoxib 400 mg po bid + CBP day 1 + GEM 1000 mg/m2 day 1 and day 8, q3w for squamous | Placebo + CBP + PET 500 mg/m2 day 1, q3w for nonsquamous or placebo + CBP day 1 + GEM 1000 mg/m2 day 1 and day 8, q3w for squamous | 31 |
Of these 12 studies, only two trials[31,33] have not reported a random component in their sequence-generation process. Five studies[24,26,27,32,33] were designed with a double-blind trial. Although only five studies[9,12,23,24,27] described specific follow-up times, and all studies used intention-to-treat strategy in the evaluation of outcome measures with the exception of one study[29]. The result of methodological quality is shown in Table 2.
| Trial or Ref. | Year | Randomization methods | Stratification factors | Double blind | Follow-up | Intent to treat |
| Lilenbaum et al[26] | 2006 | Centralized | ECOG PS, age, sex, disease stage, response to treatment | No | NA | Yes |
| GECO[23] | 2007 | Centralized | Treatment, gender, PS, disease stage, tumor histology, center (three categories according to size) | No | Median follow-up of 22 mo of alive patients (range 0-40) | Yes |
| Zhou et al[29] | 2007 | Envelopes | Types | No | NA | No: 4 of 65 excluded from analysis |
| Xiong et al[28] | 2008 | Random number table | Disease stage, COX-2 expression | No | NA | Yes |
| CYCLUS[24] | 2011 | Minimization | ECOG PS, sex, stage, smoking status | Yes | After randomization, the follow-up time ranged from 0 to 36 mo | Yes |
| NVALT-4[25] | 2011 | Centralized | PS, extent of disease, use of salicylic acid, histology, COX-2 expression, treatment | No | NA | Yes |
| Liu et al[30] | 2012 | Mechanical sampling method | Stage | No | NA | Yes |
| Sörenson et al[32] | 2013 | Minimization | ECOG PS, sex, stage, smoking status | Yes | After randomization, the follow-up time ranged from 0 to 36 mo | Yes |
| Gitlitz et al[33] | 2014 | NA | ECOG PS, sex, age | Yes | The median follow-up time was 30 mo | Yes |
| 0822-GCC[26] | 2015 | Centralized | ECOG PS, sex, stage, race | Yes | NA | Yes |
| Teng et al[31] | 2015 | NA | Serum DKK-1 levels | No | NA | Yes |
| CALGB-30801[27] | 2017 | Stratified random permuted-blocks procedure | Sex, histology and chemotherapy, smoking status, stage, age group, PS | Yes | The median follow-up time was 31 mo | Yes |
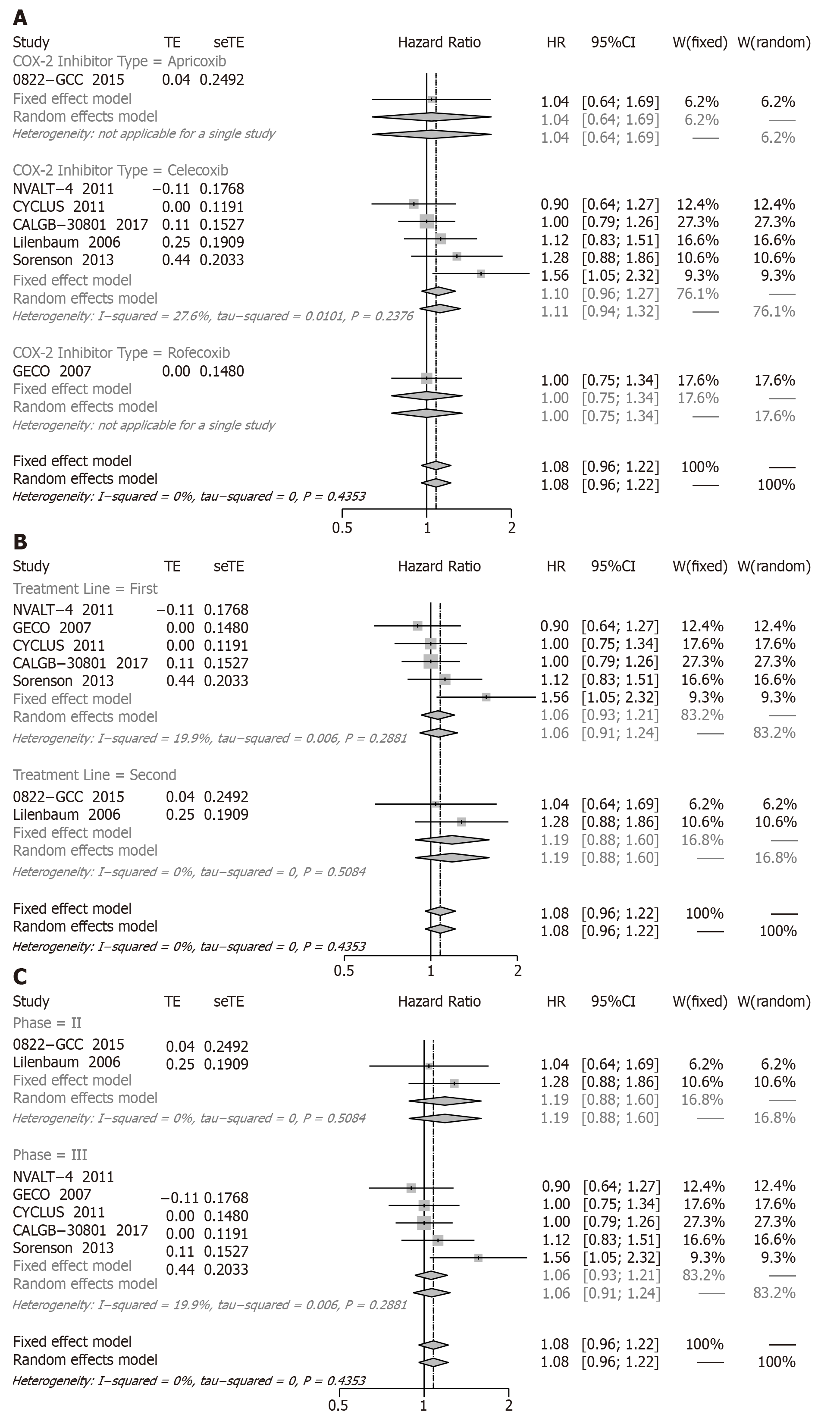
OS: A total of seven studies showed that compared with chemotherapy alone the result of combinations of treatments revealed that there was no statistically significant difference in OS (HR = 1.08, 95%CI: 0.96 to 1.22; I2: 0%) (Figure 2).
In order to evaluate the CB of COX-2 inhibitors, the subgroup analyses were conducted according to the type of COX-2 inhibitor and treatment line. No CB in OS was observed among the groups: apricoxib (HR = 1.04, 95%CI: 0.64 to 1.69), celecoxib (HR = 1.10, 95%CI: 0.96 to 1.27), and rofecoxib (HR = 1.00, 95%CI: 0.75 to 1.34) (Figure 2A). Conducting subgroups by the type of treatment line compared with chemotherapy alone, the COX-2 inhibitors plus chemotherapy of first-line treatment (HR = 1.06, 95%CI: 0.93 to 1.21) and second-line treatment (HR = 1.19, 95%CI: 0.88 to 1.60) were not statistically different (Figure 2B). In subgroup analyses of phase, phase II (HR = 1.19, 95%CI: 0.88 to 1.60) and phase III (HR = 1.06, 95%CI: 0.93 to 1.21) were not remarkably different (Figure 2C).
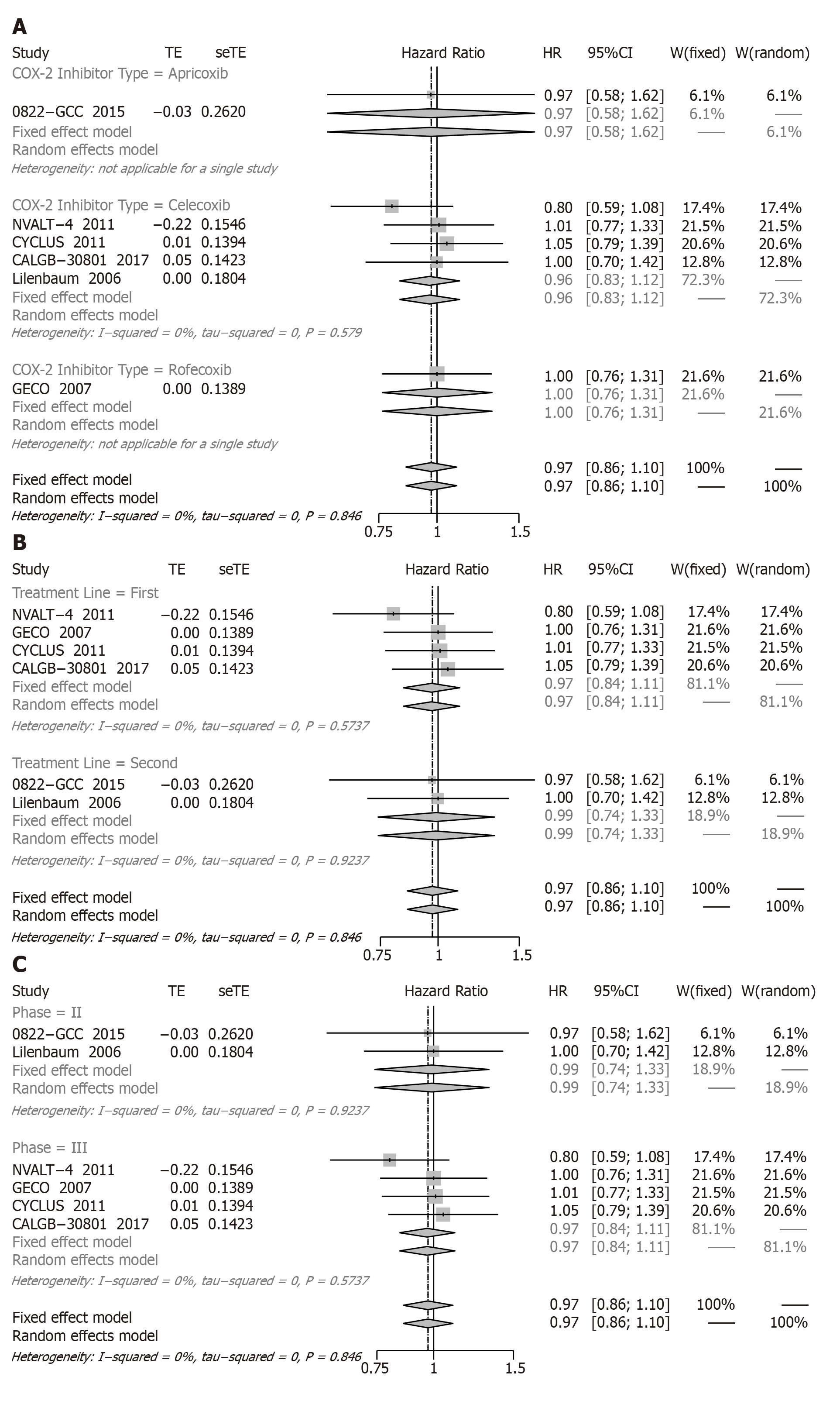
PFS: Six RCTs involving 1794 patients presented the relative data for PFS. Compared with chemotherapy alone, the COX-2 inhibitors plus chemotherapy (Figure 3) also did not represent a significant difference in PFS (HR = 0.97, 95%CI: 0.86 to 1.10).
Due to its lack of efficacy on PFS, we also performed further subgroup analysis, and all subgroup results were not significantly different (Figure 3A-C).
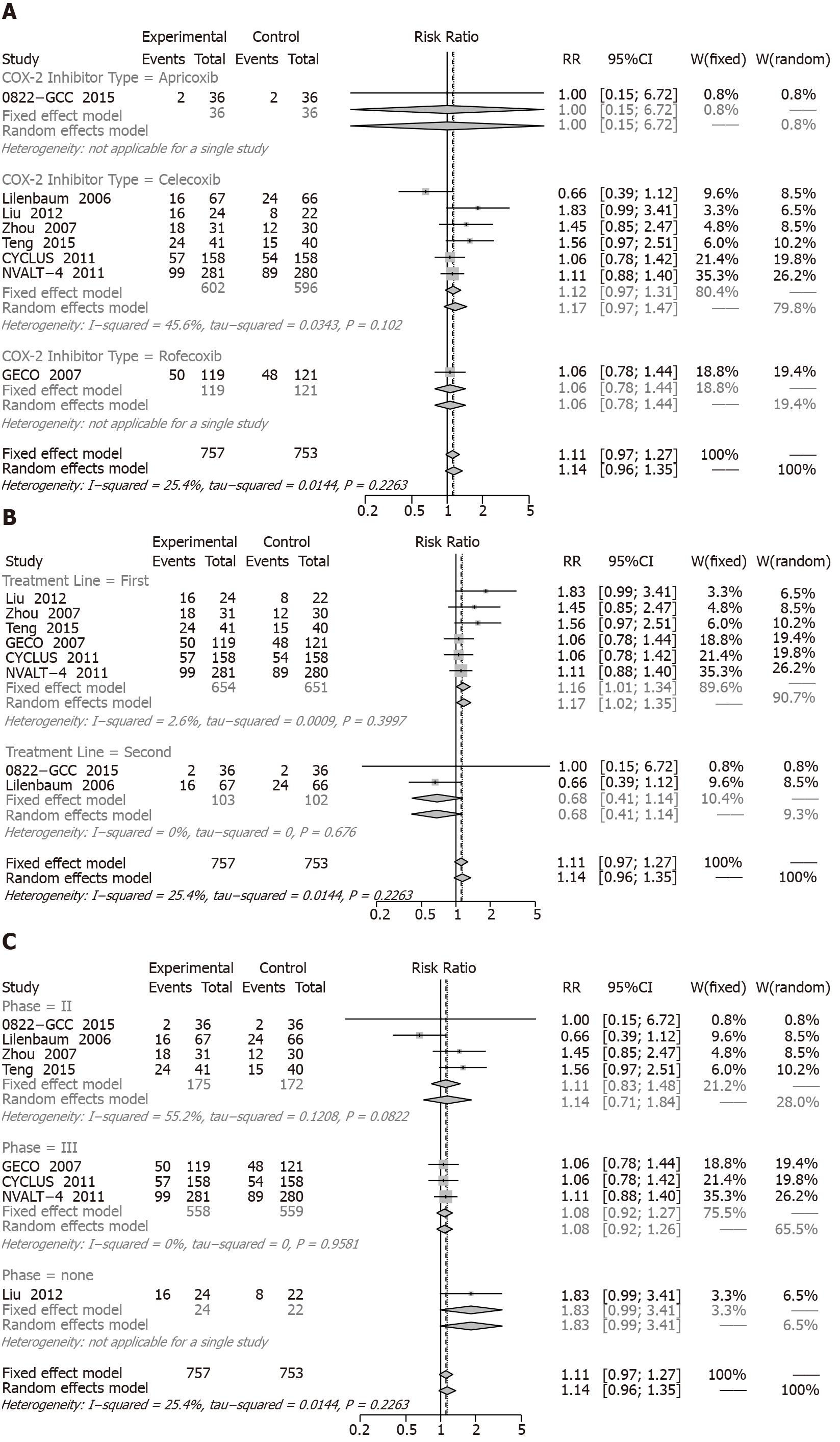
One-year SR: Eight RCTs including 1674 patients reported 1-year mortality rates (Figure 4). Compared with chemotherapy alone, the COX-2 inhibitors plus chemotherapy were not significantly different (RR = 1.11, 95%CI: 0.97 to 1.27).
Additionally, the results of the subgroup analysis were not significantly different among the types of COX-2 inhibitors: apricoxib (RR = 1.00, 95%CI: 0.15 to 6.72), celecoxib (RR = 1.12, 95%CI: 0.97 to 1.31), and rofecoxib (RR = 1.06, 95%CI: 0.78 to 1.44) (Figure 4A). However, when grouped by type of treatment line, the significant increase of 1-year SR (RR = 1.16; 95%CI: 1.01 to 1.34) was observed in first-line treatment, but there was no change in the second-line treatment (RR = 0.68; 95%CI: 0.41 to 1.14) (Figure 4B). In subgroup analyses of phase, phase II (HR = 1.14, 95%CI: 0.71 to 1.84) and phase III (HR = 1.08, 95%CI: 0.92 to 1.27) were not significantly different (Figure 4C).
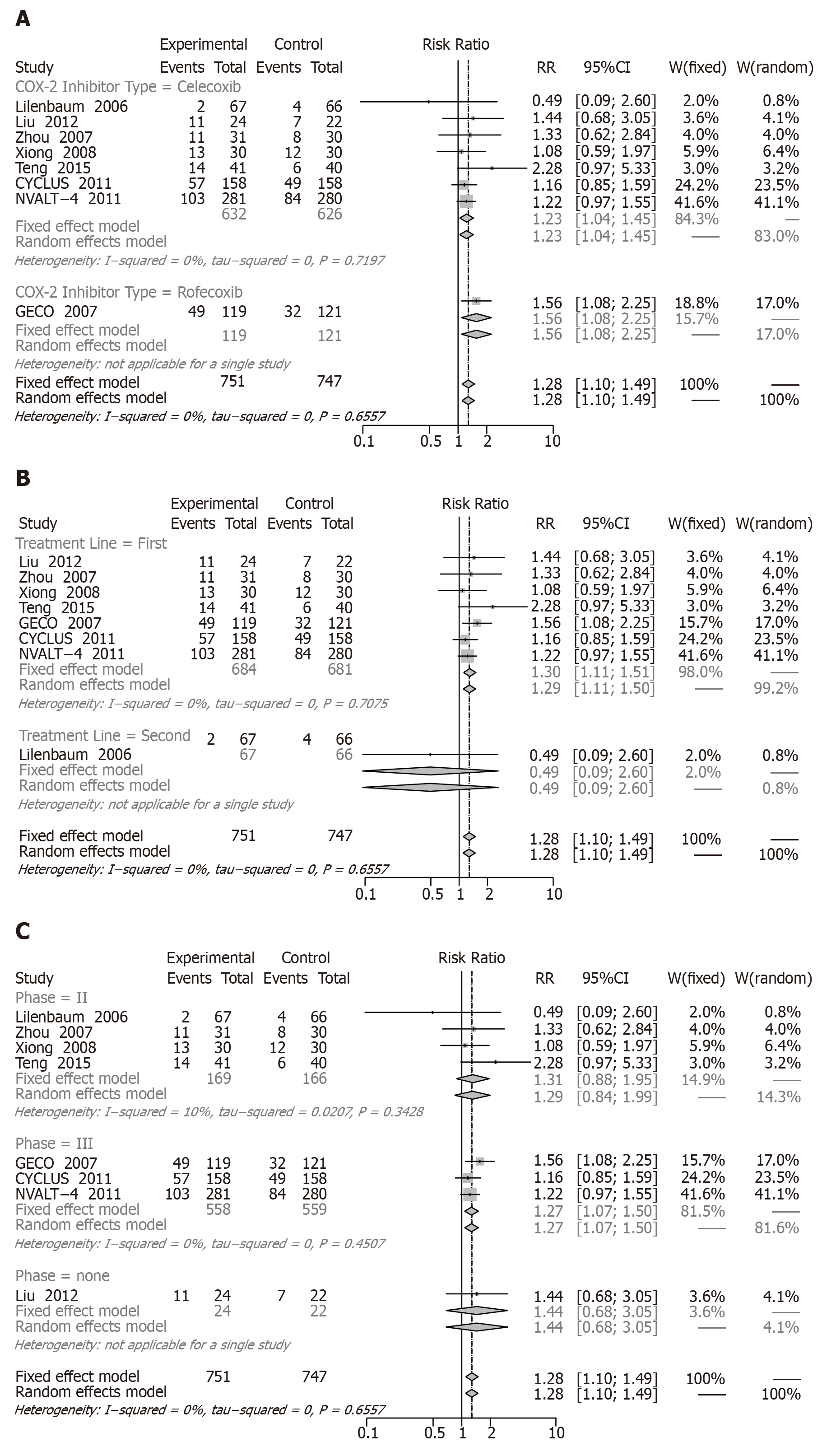
ORR: Eight RCTs including 1662 patients reported ORRs. Comparison of two groups as shown in Figure 5 resulted in an increase in the ORR (RR = 1.28, 95%CI: 1.10 to 1.49).
In the subgroup analysis, significantly increased ORRs were observed in celecoxib (RR = 1.23, 95%CI: 1.04 to 1.45), rofecoxib (RR = 1.56, 95%CI: 1.08 to 2.25), first-line treatment (RR = 1.30, 95%CI: 1.11 to 1.51), and phase III (RR = 1.27, 95%CI: 1.07 to 1.50). Second-line treatment (RR = 0.49, 95%CI: 0.09 to 2.60) and phase II (RR = 1.31, 95%CI: 0.88 to 1.95) with COX-2 inhibitors reported no significant differences (Figure 5A-C).
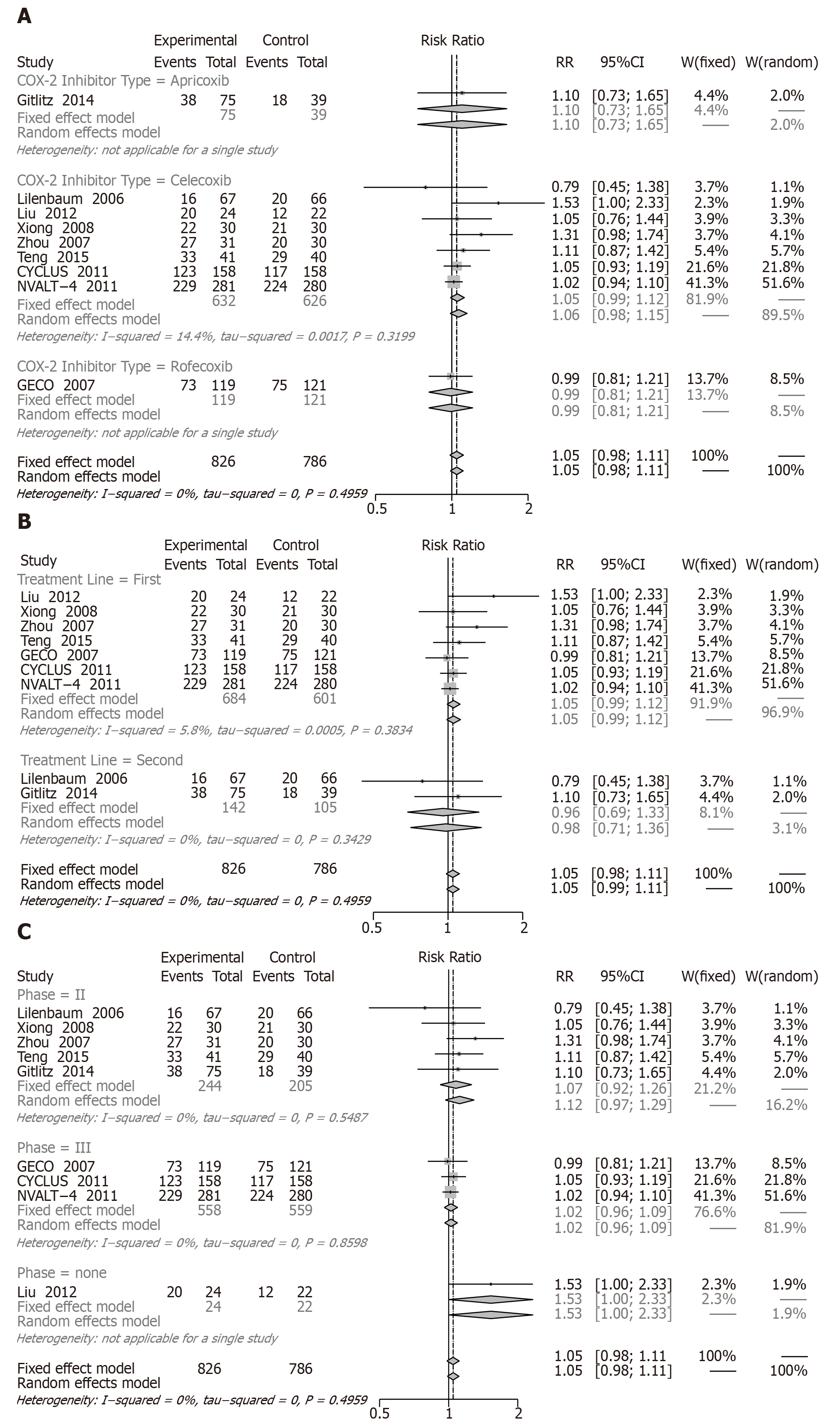
CB: Nine RCTs including 1776 patients reported a CB (Figure 6). Compared with chemotherapy alone, the COX-2 inhibitors plus chemotherapy did not represent a significant difference in CB (RR = 1.05, 95%CI: 0.98 to 1.11; I2: 0%).
As mentioned above, no significantly different results were found in the three subgroup analyses: apricoxib (RR = 1.10, 95%CI: 0.73 to 1.65; I2: NA), celecoxib (RR = 1.05, 95%CI: 0.99 to 1.12; I2: 14.4%), rofecoxib (RR = 0.99, 95%CI: 0.81 to 1.21; I2: NA), first-line treatment (RR = 1.05, 95%CI: 0.99 to 1.12; I2: 5.8%), second-line treatment (RR = 0.96, 95%CI: 0.69 to 1.33; I2: 0.0%), phase II (RR = 1.07, 95%CI: 0.92 to 1.26; I2: 0%), and phase III (RR = 1.53, 95%CI: 1.00 to 2.33; I2: NA) (Figure 6A-C).
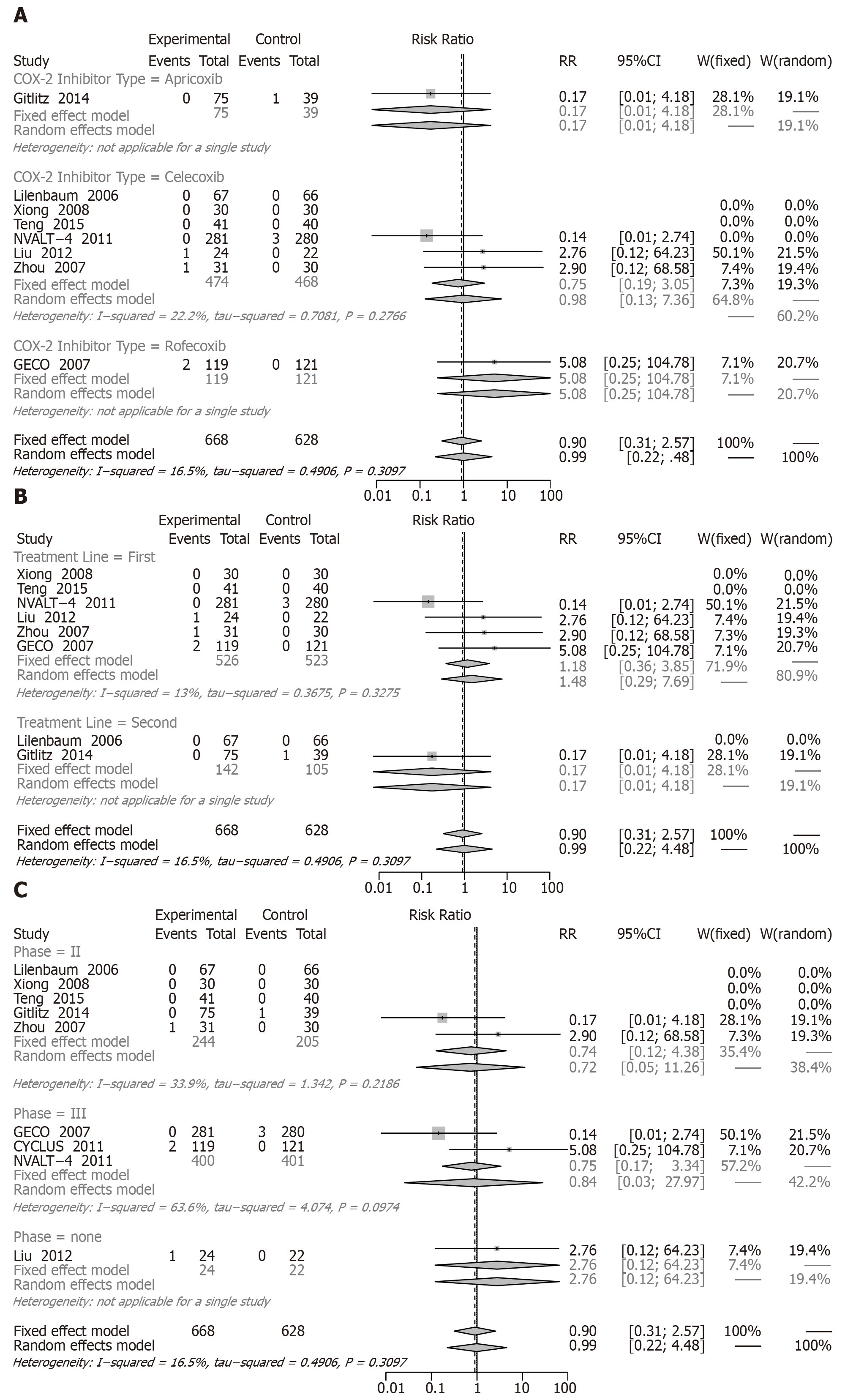
CR: When we assessed the effect on CR involving eight RCTs (1460 patients, there were no differences between combined treatment and chemotherapy alone (RR = 0.90, 95%CI: 0.31-2.57) (Figure 7).
The results of two subgroup analyses showed no significant difference: apricoxib (RR = 0.17, 95%CI: 0.01 to 4.18), celecoxib (RR = 0.75, 95%CI: 0.19 to 3.05), rofecoxib (RR = 5.08, 95%CI: 0.25 to 104.78), first-line treatment (RR = 1.18, 95%CI: 0.36 to 3.85), second-line treatment (RR = 0.17, 95%CI: 0.01 to 4.18), phase II (RR = 0.74, 95%CI: 0.12 to 4.38), and phase III (RR = 0.84, 95%CI: 0.03 to 28.0) (Figure 7A-C).
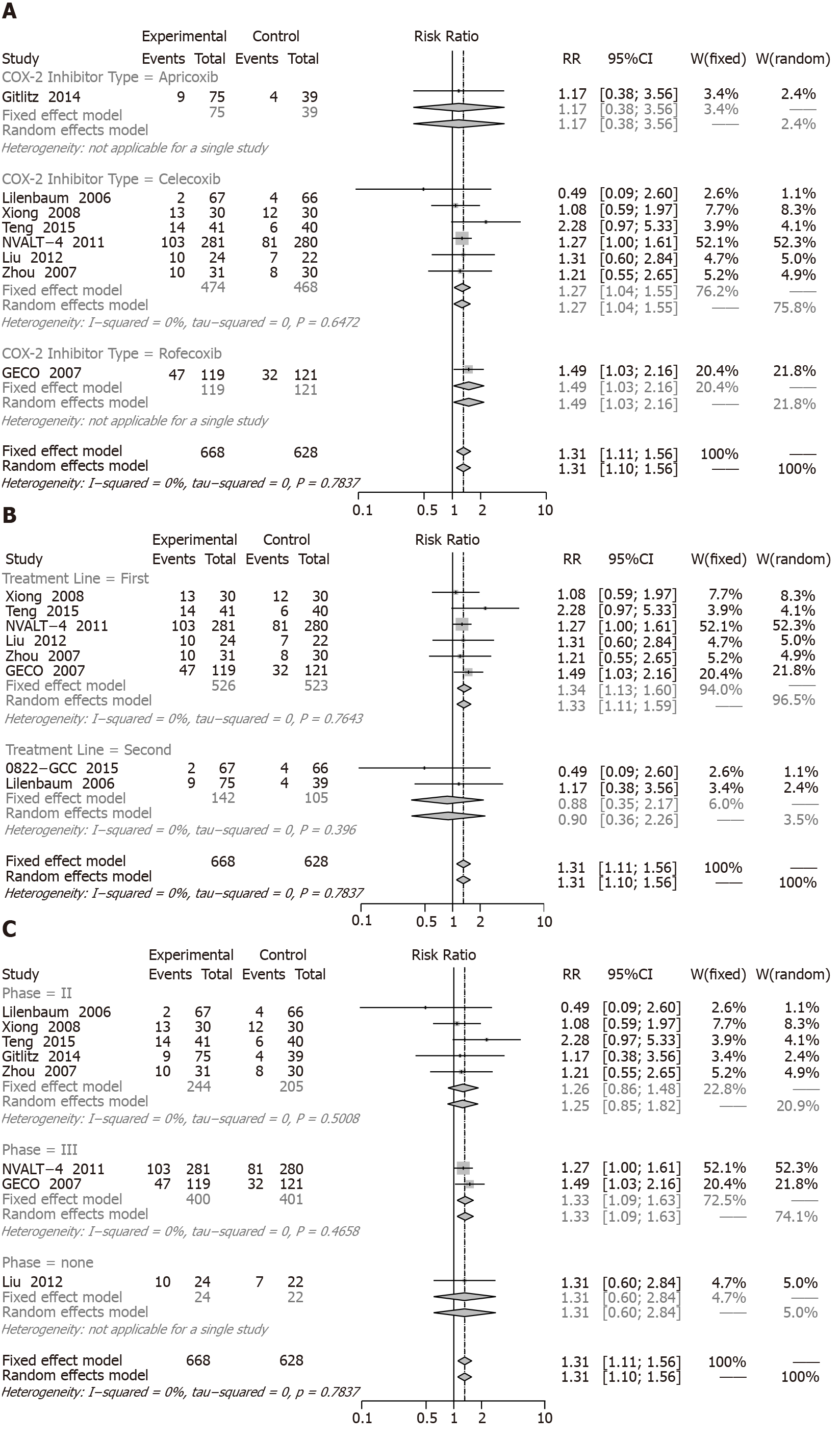
PR: When we assessed the effect on PR involving eight RCTs (1460 patients, COX-2 inhibitors combined with chemotherapy had a significant increase (RR = 1.31, 95%CI: 1.11 to 1.56) compared with chemotherapy alone (Figure 8).
The following details of subgroup analysis were represented, and the significantly increased ORRs were observed for celecoxib (RR = 1.27, 95%CI: 1.04 to 1.55), rofecoxib (RR = 1.49, 95%CI: 1.03 to 2.16), first-line treatment (RR = 1.34, 95%CI: 1.13 to 1.60), and phase III (RR = 1.33, 95%CI: 1.09 to 1.63). Apricoxib (RR = 1.17, 95%CI: 0.38 to 3.56), second-line treatment (RR = 0.88, 95%CI: 0.35 to 2.17), and phase II (RR = 1.26, 95%CI: 0.86 to 1.84) with COX-2 inhibitors showed no remarkably differences (Figure 8A-C).
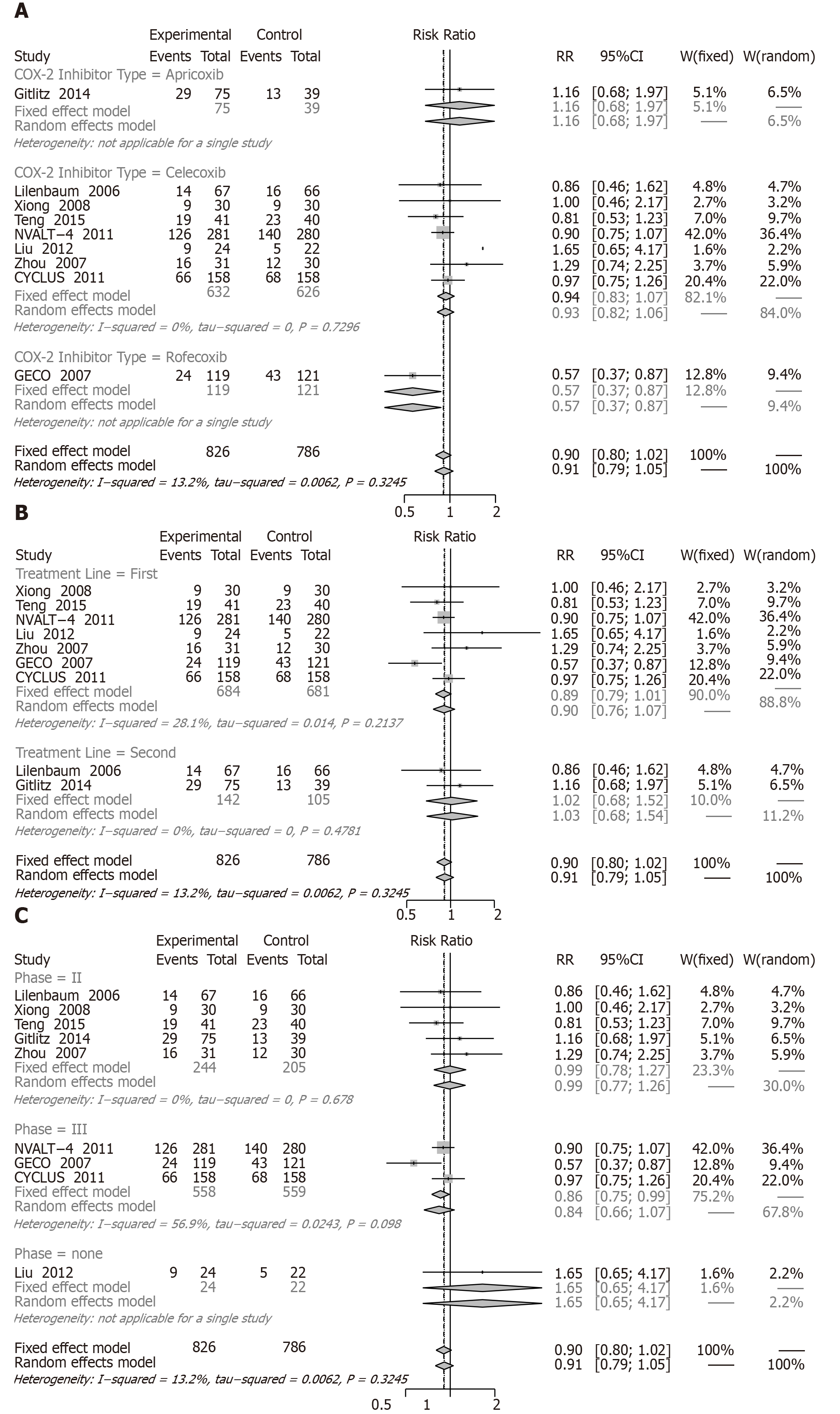
SD: When we assessed the effect on SD involving nine RCTs with 1776 patients, COX-2 inhibitors plus chemotherapy resulted in a significant increase in SD (RR = 0.90, 95%CI: 0.80 to 1.02) compared with chemotherapy alone (Figure 9).
Subgroup analysis showed an insignificant increase in SD for apricoxib (RR = 1.16, 95%CI: 0.68 to 1.97), celecoxib (RR = 0.94, 95%CI: 0.83 to 1.07), first-line treatment (RR = 0.89, 95%CI: 0.79 to 1.01), second-line treatment (RR = 1.02, 95%CI: 0.68 to 1.52), phase II (RR = 0.99, 95%CI: 0.78 to 1.27), and phase III (RR = 0.84, 95%CI: 0.66 to 1.07). However, a change was noted for rofecoxib (RR = 0.57, 95%CI: 0.37 to 0.87).
Toxicity: The increase in COX-2 inhibitor was positively correlated with the increase in grade 3 and 4 toxicity of leukopenia (RR = 1.20, 95%CI: 1.03 to 1.40), thrombocytopenia (RR = 1.33, 95%CI: 1.05 to 1.68), and cardiovascular events (RR = 2.39, 95%CI: 1.06 to 5.42) (Table 3).
| Toxicity | RCT, n | RR (95%CI) | P value for between groups | Toxicity | RCT, n | RR (95%CI) | P value for between groups |
| Leucopenia | 8 | 1.20 (1.03, 1.40) | 0.020 | Diarrhea | 3 | 1.31 (0.64, 2.71) | 0.460 |
| COX-2 inhibitor type | COX-2 inhibitor type | ||||||
| Celecoxib | 6 | 1.26 (1.07, 1.49) | 0.280 | Celecoxib | 2 | 1.24 (0.59, 2.62) | 0.940 |
| Rofecoxib | 1 | 0.80 (0.43, 1.50) | Rofecoxib | 1 | 3.05 (0.13, 74.1) | ||
| Apricoxib | 1 | 0.92 (0.47, 1.80) | Apricoxib | 1 | 2.69 (0.33, 22.3) | ||
| Treatment line | Treatment line | ||||||
| First-line | 6 | 1.20 (1.02, 1.42) | 0.900 | First-line | 2 | 0.91 (0.40, 2.07) | 0.080 |
| Second-line | 2 | 1.19 (0.76, 1.87) | Second-line | 2 | 4.10 (0.95, 17.60) | ||
| Phase | Phase | ||||||
| II | 4 | 1.14 (0.77, 1.69) | 0.720 | II | 2 | 4.10 (0.95, 17.60) | 0.080 |
| III | 4 | 1.21 (1.03, 1.44) | III | 2 | 0.91 (0.40, 2.07) | ||
| Thrombocytopenia | 8 | 1.33 (1.05, 1.68) | 0.017 | Gastric ulcer | 2 | 1.00 (0.25, 3.97) | 0.997 |
| COX-2 inhibitor type | COX-2 inhibitor type | ||||||
| Celecoxib | 6 | 1.40 (1.08, 1.81) | 0.560 | Celecoxib | 2 | 1.00 (0.25, 3.97) | NA |
| Rofecoxib | 1 | 1.02 (0.59, 1.76) | Rofecoxib | NA | NA | ||
| Apricoxib | 1 | 3.00 (0.13, 71.30) | Apricoxib | NA | NA | ||
| Treatment line | Treatment line | ||||||
| First-line | 6 | 1.24 (0.97, 1.58) | 0.090 | First-line | 2 | 1.00 (0.25, 3.97) | NA |
| Second-line | 2 | 2.66 (1.14, 6.17) | Second-line | NA | NA | ||
| Phase | Phase | ||||||
| II | 4 | 2.69 (1.19, 6.07) | 0.070 | II | 2 | 1.00 (0.25, 3.97) | NA |
| III | 4 | 1.23 (0.96, 1.56) | III | NA | NA | ||
| Anemia | 5 | 1.32 (0.75, 2.33) | 0.343 | Asthenia | 7 | 0.84 (0.56, 1.28) | 0.426 |
| COX-2 inhibitor type | COX-2 inhibitor type | ||||||
| Celecoxib | 3 | 2.76 (0.96, 7.97) | 0.110 | Celecoxib | 5 | 0.94 (0.60, 1.48) | 0.590 |
| Rofecoxib | 1 | 0.80 (0.38, 1.69) | Rofecoxib | 1 | 0.51 (0.16, 1.64) | ||
| Apricoxib | 2 | 3.14 (0.51, 19.50) | Apricoxib | 2 | 0.94 (0.20, 4.44) | ||
| Treatment line | Treatment line | ||||||
| First-line | 3 | 1.07 (0.56, 2.05) | 0.140 | First-line | 5 | 0.92 (0.60, 1.42) | 0.560 |
| Second-line | 3 | 2.91 (0.89, 9.98) | Second-line | 3 | 0.53 (0.15, 1.88) | ||
| Phase | Phase | ||||||
| II | 4 | 3.03 (1.00, 9.24) | 0.100 | II | 4 | 0.75 (0.28, 2.02) | 0.900 |
| III | 2 | 1.01 (0.52, 1.97) | III | 3 | 0.86 (0.54, 1.39) | ||
| Nausea | 7 | 0.85 (0.53, 1.36) | 0.507 | Cardiotoxicity | 5 | 2.39 (1.06, 5.42) | 0.037 |
| COX-2 inhibitor type | COX-2 inhibitor type | ||||||
| Celecoxib | 5 | 0.87 (0.50, 1.51) | 0.960 | Celecoxib | 3 | 1.55 (0.53, 4.50) | 0.540 |
| Rofecoxib | 1 | 0.76 (0.27, 2.13) | Rofecoxib | 1 | 4.58 (1.01, 20.70) | ||
| Apricoxib | 2 | 1.00 (0.15, 6.72) | Apricoxib | 1 | 3.00 (0.13, 71.30) | ||
| Treatment line | Treatment line | ||||||
| First-line | 6 | 0.84 (0.52, 1.37) | 0.860 | First-line | 4 | 2.35 (1.01, 5.49) | 0.880 |
| Second-line | 2 | 1.00 (0.15, 6.72) | Second-line | 1 | 3.00 (0.13, 71.30) | ||
| Phase | Phase | ||||||
| II | 4 | 1.44 (0.58, 3.59) | 0.400 | II | 1 | 3.00 (0.13, 71.30) | 0.880 |
| III | 3 | 0.67 (0.36, 1.25) | III | 4 | 2.35 (1.01, 5.49) | ||
| Neurotoxicity | 4 | 1.02 (0.23, 4.45) | 0.977 | ||||
| COX-2 inhibitor type | |||||||
| Celecoxib | 3 | 1.02 (0.18, 5.83) | 0.100 | ||||
| Rofecoxib | 1 | 1.02 (0.06, 16.07) | |||||
| Apricoxib | NA | NA | |||||
| Treatment line | |||||||
| First-line | 4 | 1.02 (0.23, 4.45) | 1.000 | ||||
| Second-line | NA | NA | |||||
| Phase | |||||||
| II | 2 | 3.09 (0.13, 73.20) | 0.420 | ||||
| III | 2 | 0.68 (0.11, 4.04) | |||||
Subgroup analysis of leukopenia in Table 3 showed that all of celecoxib (RR = 1.26, 95%CI: 1.07 to 1.49), first-line treatment (RR = 1.20, 95%CI: 1.02 to 1.42), and phase III (RR = 1.21, 95%CI: 1.03 to 1.44) increased the risk of leukopenia. Subgroup analysis of thrombocytopenia showed that celecoxib (RR = 1.40, 95%CI: 1.08 to 1.81), second-line treatment (RR = 2.66, 95%CI: 1.14 to 6.17), and phase II (RR = 2.69, 95%CI: 1.19 to 6.07) significantly increased the incidence of thrombocytopenia. Subgroup analysis of cardiovascular events showed that rofecoxib (RR = 4.58, 95%CI: 1.01 to 20.7), first-line treatment (RR = 2.35, 95%CI: 1.01 to 5.49), and phase III (RR = 2.35, 95%CI: 1.01 to 5.49) increased the risk of cardiovascular events. However, the risks of other toxicities were not found to be increased significantly (Table 3).
Publication bias: In the results of publication bias using Egger’s test, all primary outcomes (POS: 0.314, PPFS: 0.807, PORR: 0.883, P1-year SR: 0.624, and PCB: 0.220) were not significantly different. With respect to secondary outcomes, we did not obtain significant difference (data not shown).
Based on extensive preclinical and clinical studies, COX-2 inhibitors have shown significant CBs in both therapy and the chemoprevention of lung cancer. In this study, COX-2 inhibitors can increase the efficacy of chemotherapy regarding ORR. In a subgroup analysis, we found that celecoxib and rofecoxib might improve the ORR of patients with advanced NSCLC. Based on the treatment line, an increased ORR was found in first-line treatment with COX-2 inhibitors for advanced NSCLC patients. However, the second-line treatment with COX-2 inhibitors did not yield a significant effect in the ORR, possibly due to the inclusion of only one article. Teng et al[31] reported a higher ORR with celecoxib added to chemotherapy, whereas a study by Schneider et al[34] showed that celecoxib did not seem to improve the response rate. The most plausible explanation may be that different chemotherapy regimens were used. Teng et al[31] used gemcitabine/cisplatin, whereas Schneider et al[34] used docetaxel. However, for the CB, a significant difference was not discovered. The findings of the subgroup analysis were consistent with those of previous studies[22,28,30]. Although no evidence showed that COX-2 inhibition could improve the CB for advanced NSCLC patients, Edelman et al[35] highlighted the importance of seeking molecular oriented therapy using COX-2 inhibitors. COX-2 inhibitors plus chemotherapy has no improvement on the 1-year SR for advanced NSCLC patients. In the subgroup analysis of the treatment line, COX-2 inhibitors in first-line treatment revealed a significant increase of the 1-year SR. Accordingly, the conclusion was made that COX-2 inhibitors more effectively improved both ORR and the 1-year SR for people suffering with advanced NSCLC using first-line chemotherapy. The meta-analysis by Zhou et al[16] stated that the COX-2 inhibitors may increase the ORR with advanced NSCLC.
Toxicity exists differently for individuals in incidence and severity[36]. Compared to chemotherapy alone, COX-2 inhibitors associated with chemotherapy might have a higher incidence of hematological toxicity, except for anemia. In addition, it was confirmed by subgroup analysis that combined treatment (celecoxib plus chemotherapy) could increase the risk of hematological toxicity, particularly for two periods (in that first-line treatment with leukopenia and second-line treatment with thrombocytopenia). This is consistent with previous meta-analyses[15,16]. Nevertheless, it is likely that COX-2 is necessary for marrow recovery after cytotoxic chemo-therapy[37]. A study[38] suggested that the directed differentiation of erythroid, myeloid, and megakaryocytic progenitors is related to the level of COX-2. Therefore, COX-2 inhibitors may also result in higher risk of hematological toxicity while increasing the ORR by using COX-2 inhibitors.
This study illustrated that COX-2 augmented the risk of cardiovascular events as well. Cardiovascular events with higher incidences happened when using rofecoxib. The influence of rofecoxib on cardiovascular events still needed to be investigated for a few studies, whereas celecoxib had no effect on cardiovascular events. On the basis of classifying the treatment line, it slightly increased the risk of cardiovascular events of advanced NSCLC through first-line treatment associated with COX-2, but no obvious differences were observed for second-line treatment. Prostacyclin[39], a substance that associates with the expression of COX-2, existed in rofecoxib. Therefore, rofecoxib might participate in the process of formation of thrombosis. In vitro experiments have proven that celecoxib has a lower specific effect on COX-2 than rofecoxib and is less likely to cause thrombosis, which indicates the rationality of our hypothesis. Given that patients may not benefit from COX-2 selective nonsteroidal anti-inflammatory drugs[40], it makes sense to use aspirin to prevent vascular events again.
There are several meta-analyses concerning published research on the CB profile of COX-2[14-17]. The superiority of the ORR alone made it difficult to adequately demonstrate that the inhibition of the COX-2 inhibitors could improve the efficacy. A relevant study[15] analyzed six studies, setting forth all endpoints that did not conduct subgroup analysis. In addition, no subgroup analyses were performed when toxicities were assessed by Zhou et al[16]. Dai et al[17] study describing all efficacy endpoints with subgroup analysis, but other efficacy outcomes (CB, CR, PR and SD) were lacking, and toxicity was not performed by subgroup analysis to explore the difference in different types of COX-2 inhibitors and the treatment line. In this meta-analysis, 12 studies were included, and five main outcomes (ORR, CB, 1-year SR, OS and PFS) and four secondary outcomes (CR, PR, SD and toxicity) were defined above. Moreover, considering the potential clinical heterogeneity, subgroup analyses were employed based on the different types of COX-2 inhibitors and treatment line.
This study has some limitations. First, there are not many clinical trials that met the study design of this systematic review, especially in subgroup analysis, the small number of trials for rofecoxib, apricoxib, or second-line treatment limited the analytical power. Hence, more clinical studies are needed to further confirm our results about combined treatment and chemotherapy alone for advanced NSCLC. Second, due to the lack of data on the response rate and survival outcomes in the included RCTs, this may result in too small a result sample and the accuracy of the results.
This meta-analysis demonstrated that, in terms of ORR for patients who received adjuvant chemotherapy of advanced NSCLC, COX-2 inhibitors improved the ORR and have no improvement on prolonged mortality. However, the COX-2 inhibitors could enhance both the ORR and improve the 1-year SR, particularly with first-line chemotherapy. Concerning toxicity, celecoxib plus chemotherapy resulted in a higher incidence of hematologic toxicities. Meanwhile, rofecoxib may augment the risk of cardiovascular events.
The proportion of non-small cell lung cancer (NSCLC) is more than 80% of all lung tumors. Most patients have advanced NSCLC at stage ШB or IV when diagnosed and have to receive alleviative treatment in order to maintain their lives. The median survival time is 6-10 mo for patients who are diagnosed with advanced NSCLC in performance status 0-2 when adopting palliative first-line chemotherapy.
The motivation of this study is to investigate COX-2 for intervention of NSCLC, which is mired in controversy in the medical field.
This systematic review based on randomized controlled trials was conducted to appraise the benefit of chemotherapy-assisted addition of COX-2 for advanced NSCLC.
We searched the six electronic databases up until December 9, 2019 for studies that examined the efficacy and safety of the addition of COX-2 inhibitors to chemotherapy for NSCLC. Overall survival(OS), progression free survival (PFS), 1-year survival rate (SR), overall response rate (ORR), clinical benefit (CB), complete response (CR), partial response (PR), stable disease (SD), and toxicities were measured with more than one outcome as their endpoints. Fixed and random effects models were used to calculate risk estimates in a meta-analysis. Potential publication bias was calculated using Egger’s linear regression test. Data analysis was performed using R software.
The COX-2 inhibitors combined with chemotherapy were not found to be more effective than chemotherapy alone in OS, PFS, 1-year SR, CB, CR, and SD. However, there was a difference in ORR for patients with advanced NSCLC. In a subgroup analysis, significantly increased ORR results were found for celecoxib, rofecoxib, first-line treatment, and PR. For adverse events, the increase in COX-2 inhibitor was positively correlated with the increase in grade 3 and 4 toxicity of leukopenia, thrombocytopenia and cardiovascular events.
COX-2 inhibitor combined with chemotherapy increased total effective rate of advanced NSCLC with the possible increased risk of blood toxicity and cardiovascular events and had no effect on survival index.
This study can provide reference value for the application of COX-2 in the treatment of lung cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ampollini L S-Editor: Huang P L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Grønberg BH, Bremnes RM, Fløtten O, Amundsen T, Brunsvig PF, Hjelde HH, Kaasa S, von Plessen C, Stornes F, Tollåli T, Wammer F, Aasebø U, Sundstrøm S. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 6478] [Article Influence: 404.9] [Reference Citation Analysis (0)] |
| 3. | Helbekkmo N, Sundstrøm SH, Aasebø U, Brunsvig PF, von Plessen C, Hjelde HH, Garpestad OK, Bailey A, Bremnes RM; Norwegian Lung Cancer Study Group. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer. 2007;97:283-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | von Plessen C, Bergman B, Andresen O, Bremnes RM, Sundstrom S, Gilleryd M, Stephens R, Vilsvik J, Aasebo U, Sorenson S. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer. 2006;95:966-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Sederholm C, Hillerdal G, Lamberg K, Kölbeck K, Dufmats M, Westberg R, Gawande SR. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol. 2005;23:8380-8388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Lasalvia-Prisco E, Goldschmidt P, Galmarini F, Cucchi S, Vázquez J, Aghazarian M, Lasalvia-Galante E, Golomar W, Gordon W. Addition of an induction regimen of antiangiogenesis and antitumor immunity to standard chemotherapy improves survival in advanced malignancies. Med Oncol. 2012;29:3626-3633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 8. | Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001;107:1491-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Achiwa H, Yatabe Y, Hida T, Kuroishi T, Kozaki K, Nakamura S, Ogawa M, Sugiura T, Mitsudomi T, Takahashi T. Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin Cancer Res. 1999;5:1001-1005. [PubMed] |
| 10. | Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-1311. [PubMed] |
| 11. | Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761-3764. [PubMed] |
| 12. | Li Y, Li S, Sun D, Song L, Liu X. Expression of 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase-2 in non-small cell lung cancer: Correlations with angiogenesis and prognosis. Oncol Lett. 2014;8:1589-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563-7568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1109] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 14. | Chen J, Shen P, Zhang XC, Zhao MD, Zhang XG, Yang L. Efficacy and safety profile of celecoxib for treating advanced cancers: a meta-analysis of 11 randomized clinical trials. Clin Ther. 2014;36:1253-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Hou LC, Huang F, Xu HB. Does celecoxib improve the efficacy of chemotherapy for advanced non-small cell lung cancer? Br J Clin Pharmacol. 2016;81:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Zhou YY, Hu ZG, Zeng FJ, Han J. Clinical Profile of Cyclooxygenase-2 Inhibitors in Treating Non-Small Cell Lung Cancer: A Meta-Analysis of Nine Randomized Clinical Trials. PLoS One. 2016;11:e0151939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Dai P, Li J, Ma XP, Huang J, Meng JJ, Gong P. Efficacy and safety of COX-2 inhibitors for advanced non-small-cell lung cancer with chemotherapy: a meta-analysis. Onco Targets Ther. 2018;11:721-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4954] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 19. | Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 475] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 20. | Higgins JP. Cochrane handbook for systematic reviews of interventions, v.5.1. 2011 Available from: https://training.cochrane.org/handbook/archive/v5.1/. |
| 21. | Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 22. | Lilenbaum R, Socinski MA, Altorki NK, Hart LL, Keresztes RS, Hariharan S, Morrison ME, Fayyad R, Bonomi P. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non-small-cell lung cancer. J Clin Oncol. 2006;24:4825-4832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Gridelli C, Gallo C, Ceribelli A, Gebbia V, Gamucci T, Ciardiello F, Carozza F, Favaretto A, Daniele B, Galetta D, Barbera S, Rosetti F, Rossi A, Maione P, Cognetti F, Testa A, Di Maio M, Morabito A, Perrone F; GECO investigators. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol. 2007;8:500-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Koch A, Bergman B, Holmberg E, Sederholm C, Ek L, Kosieradzki J, Lamberg K, Thaning L, Ydreborg SO, Sörenson S; Swedish Lung Cancer Study Group. Effect of celecoxib on survival in patients with advanced non-small cell lung cancer: a double blind randomised clinical phase III trial (CYCLUS study) by the Swedish Lung Cancer Study Group. Eur J Cancer. 2011;47:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Groen HJ, Sietsma H, Vincent A, Hochstenbag MM, van Putten JW, van den Berg A, Dalesio O, Biesma B, Smit HJ, Termeer A, Hiltermann TJ, van den Borne BE, Schramel FM. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: the NVALT-4 study. J Clin Oncol. 2011;29:4320-4326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Edelman MJ, Tan MT, Fidler MJ, Sanborn RE, Otterson G, Sequist LV, Evans TL, Schneider BJ, Keresztes R, Rogers JS, de Mayolo JA, Feliciano J, Yang Y, Medeiros M, Zaknoen SL. Randomized, double-blind, placebo-controlled, multicenter phase II study of the efficacy and safety of apricoxib in combination with either docetaxel or pemetrexed in patients with biomarker-selected non-small-cell lung cancer. J Clin Oncol. 2015;33:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Edelman MJ, Wang X, Hodgson L, Cheney RT, Baggstrom MQ, Thomas SP, Gajra A, Bertino E, Reckamp KL, Molina J, Schiller JH, Mitchell-Richards K, Friedman PN, Ritter J, Milne G, Hahn OM, Stinchcombe TE, Vokes EE; Alliance for Clinical Trials in Oncology. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance). J Clin Oncol. 2017;35:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Xiong J, Xiang X, Zhang L, Zhong L, Chen W, Yu F. A Phase ІІ Study of Vinorelbine/Cisplatin with or without Cox-2 Inhibitor in First-Line Treatment of Non-small Cell Lung Cancer. Zhongliu Fangzhi Yanjiu. 2008;35:201-203. |
| 29. | Zhou SW, Zhou CC, Xu JF, Lv MJ. First-line regimen of vinorelbine and cisplatin (NP) combined with cyclooxygenase-2 inhibitor celecoxib in advanced nonsmall-cell lung cancer. Tongji Daxue Xuebao. 2007;28:87-91. |
| 30. | Liu GH, Huang JA. Clinical study of celecoxib combined with chemotherapy in the treatment of patients with advanced lung cancer. Zhonghua Zhongliu Fangzhi Zazhi. 2012;19:1661-1663. |
| 31. | Teng JJ, Pei J, Han BH, Jiang LY, Zhong H, Gu AQ, Chu TQ. Serum DKK-1 Levels in the advanced non-small cell lung caner patients: a randomized clinical study on combination of celecoxib with cisplatin-based chemotherapy. Shijie Linchuang Yaowu. 2015;36:388-394. |
| 32. | Sörenson S, Fohlin H, Lindgren A, Lindskog M, Bergman B, Sederholm C, Ek L, Lamberg K, Clinchy B. Predictive role of plasma vascular endothelial growth factor for the effect of celecoxib in advanced non-small cell lung cancer treated with chemotherapy. Eur J Cancer. 2013;49:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Gitlitz BJ, Bernstein E, Santos ES, Otterson GA, Milne G, Syto M, Burrows F, Zaknoen S. A randomized, placebo-controlled, multicenter, biomarker-selected, phase 2 study of apricoxib in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Schneider BJ, Kalemkerian GP, Kraut MJ, Wozniak AJ, Worden FP, Smith DW, Chen W, Gadgeel SM. Phase II study of celecoxib and docetaxel in non-small cell lung cancer (NSCLC) patients with progression after platinum-based therapy. J Thorac Oncol. 2008;3:1454-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Edelman MJ, Watson D, Wang X, Morrison C, Kratzke RA, Jewell S, Hodgson L, Mauer AM, Gajra A, Masters GA, Bedor M, Vokes EE, Green MJ. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1205] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 37. | Lorenz M, Slaughter HS, Wescott DM, Carter SI, Schnyder B, Dinchuk JE, Car BD. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp Hematol. 1999;27:1494-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Soza-Ried C, Hess I, Netuschil N, Schorpp M, Boehm T. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proc Natl Acad Sci USA. 2010;107:17304-17308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 938] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 40. | Belknap S. Review: studies on the cardiovascular effects of selective COX-2 inhibitors show mixed results. ACP J Club. 2002;136:53. [PubMed] |