Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.552
Peer-review started: October 3, 2020
First decision: November 23, 2020
Revised: December 2, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: January 26, 2021
Processing time: 108 Days and 21.1 Hours
High venous ammonia (VA) values have been proven to be a part of the mechanism of hepatic encephalopathy in patients with liver cirrhosis (LC) as well as acute hepatitis. Moreover, VA has been associated with poor prognosis and high mortality in these clinical settings. However, the role of ammonia in acute-on-chronic liver failure (ACLF) has not yet been clearly established.
To assess the role of VA in predicting the outcome of cirrhotic patients with ACLF in a tertiary care center.
We performed a retrospective observational study including consecutive patients with LC hospitalized for acute non-elective indications such as ascites, hepatic encephalopathy (HE), upper gastrointestinal bleeding, or bacterial infections that fulfilled the Asian Pacific Association for the Study of the Liver (APASL) criteria for ACLF. The study was conducted in “St. Spiridon” University Hospital, Iasi, Romania, a tertiary care center, between January 2017 and January 2019. The APASL ACLF Research Consortium (AARC) score was calculated and ACLF grade was established accordingly. West-haven classification was used for HE. Statistical analysis was performed using IBM SPSS version 22.0.
Four hundred and forty-six patients were included, aged 59 (50-65) years, 57.4% men. Child-Pugh, model for end-stage liver disease (MELD) and AARC scores were 11 (10-12), 19.13 ± 6.79, and 7 (6-8), respectively. 66.4% had ACLF grade I, 31.2% ACLF grade II, and 2.5% ACLF grade III. HE was diagnosed in 83.9%, 34% grade I, 37.2% grade II, 23.5% grade III, and 5.3% grade IV. Overall mortality was 7.8%. VA was 103 (78-148) μmol/L. Receiver operating characteristic analysis showed good accuracy for the prediction of in-hospital mortality for the AARC score [Area under the curve (AUC) = 0.886], MELD score (AUC = 0.816), VA (AUC = 0.812) and a fair accuracy for the Child-Pugh score (AUC = 0.799). Subsequently, a cut-off value for the prediction of mortality was identified for VA (152.5 μmol/L, sensitivity = 0.706, 1-specificity = 0.190). Univariate analysis found acute kidney injury, severe HE (grade III or IV), VA ≥ 152.5 μmol/L, MELD score ≥ 22.5, Child-Pugh score ≥ 12.5, and AARC score ≥ 8.5 to be associated with in-hospital mortality. Multivariate analysis identified AARC score ≥ 8.5 and venous ammonia ≥ 152 μmol/L to be independent predictors of in-hospital mortality.
VA could be used as an inexpensive predictor of in-hospital mortality in patients with ACLF. Patients with both ACLF and VA > 152.5 μmol/L have a high risk for a poor outcome.
Core Tip: Hyperammonemia has been associated with hepatic encephalopathy and high mortality in patients with liver cirrhosis. Acute-on-chronic liver failure is a relatively new defined syndrome presenting high 28-d mortality. The role of hyperammonemia in acute-on-chronic liver failure has not yet been clearly established. Venous ammonia presents a good predictive value for in-hospital mortality in patients with acute-on-chronic liver failure (ACLF), with a cut-off value of 152.5 μmol/L, sensitivity = 0.706, 1-specificity = 0.190 and is associated with severe hepatic encephalopathy in patients with ACLF. Thus, venous ammonia has the potential to be used as a prognostic marker in the evaluation of patients with ACLF.
- Citation: Chiriac S, Stanciu C, Cojocariu C, Singeap AM, Sfarti C, Cuciureanu T, Girleanu I, Igna RA, Trifan A. Role of ammonia in predicting the outcome of patients with acute-on-chronic liver failure. World J Clin Cases 2021; 9(3): 552-564
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/552.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.552
Hyperammonemia has been traditionally associated with hepatic encephalopathy (HE) and with liver cirrhosis (LC). Hyperammonemia has occured as a consequence of the reduction in the detoxification capabilities of the liver in the setting of liver failure as well as with increased intestinal production of ammonia[1]. However, several other conditions that determine an imbalance between the production and the clearance of ammonia such as infections with urease-producing bacteria, high-protein diet, malignancy, sarcopenia, renal failure, gastrointestinal (GI) bleeding, gastric bypass, and organ transplantation can also determine high ammonia levels[2] and sometimes lead to non-cirrhotic encephalopathy[3].
Both LC and acute liver failure are known factors for hyperammonemia, but the relation between hyperammonemia and HE is still being debated. Acute-on-chronic liver failure (ACLF) is a relatively recently recognized syndrome that is different from LC and acute liver failure in terms of outcome. Several international societies and consortia have attempted to characterize this syndrome however, a universally accepted definition has not yet been found. The latest definition was enunciated by The Asian Pacific Association for the Study of the Liver (APASL), defining ACLF as jaundice and coagulopathy complicated within four weeks by clinical ascites and/or encephalopathy in a patient with a previously diagnosed or undiagnosed chronic liver disease/cirrhosis, associated with a high 28-d mortality, following an acute hepatic insult[4]. The conditions associated with ACLF vary from hepatitis B reactivation, super infection with hepatitis E, bacterial fungal or parasitic infections, drug-induced liver injury, alcohol abuse, autoimmune hepatitis flare, acute variceal bleeding, and also relative adrenal insufficiency[4-6].
Although ammonia is widely recognized as the main factor involved in the pathogenesis of HE, the association with the severity of HE is much clearer in acute liver failure than in chronic liver disease, suggesting that in the latter there might be other factors involved, such as systemic inflammation that could lower the threshold for neurological impairment caused by ammonia[7]. Patients with acute liver failure and high ammonia levels may experience cerebral edema, followed by subsequent herniation and death[8]. Although cerebral edema has been traditionally associated with acute liver failure, several studies also reported cerebral edema in patients with LC[9,10] as well as in some cases of ACLF[11,12]. High serum ammonia levels are found in patients with chronic liver disease as well as in patients with ACLF, with higher levels being reported in the latter category[13]. However, the relation between high ammonia levels and HE in patients with ACLF has not been adequately described. Although there is data indicating hyperammonemia as a potential mechanism for HE from experimental ACLF models, there are only a few studies to demonstrate this relation in clinical settings[8]. Moreover, there is still conflicting data concerning the risk of in-hospital death for patients with ACLF and high ammonia levels. While several studies found that hyperammonemia was associated with increased mortality[14,15], others did not show worse prognosis in the cases with peak ammonia levels[16,17]. Moreover, ammonia levels are not currently influencing the clinical management of the patients with HE, and further evidence is needed to demonstrate this biomarker’s utility in predicting the mortality of patients with advanced liver disease[18].
This study aimed to assess the prognostic value of ammonia in patients with ACLF in terms of in-hospital mortality.
We conducted a retrospective study on patients hospitalized in “St. Spiridon” University Hospital, Iasi, Romania between January 2017 and January 2019. We included consecutive cirrhotic patients admitted for non-elective indications such as ascites, HE, upper GI bleeding, or bacterial infections that fulfilled the APASL diagnostic criteria of ACLF. Patients diagnosed with non-hepatic malignancy, human immunodeficiency virus infection, hematological disease as well as pregnant women were excluded from the study. LC was diagnosed based on clinical criteria, laboratory tests, typical imagistic findings, and upper digestive endoscopy consistent with portal hypertension. The end-point of the study was considered in-hospital death.
Patient electronic records were analyzed and information regarding sex, demographics, etiology of liver disease, duration of hospitalization and the in-hospital death rates was recorded. The abdominal ultrasound and upper digestive endoscopy results were obtained. Laboratory tests from fasting venous blood taken at admission were noted. Complete blood count, serum transaminase levels, total serum bilirubin, albumin, creatinine, international normalized ratio (INR), lactate, C-reactive protein (CRP), sodium, and ammonia levels were retrieved.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of “Grigore T. Popa” University of Medicine and Pharmacy of Iasi. There was no requirement for the informed written consent because of the retrospective nature of the study; all of the patients signed an informed consent upon hospitalization agreeing to receive treatment.
ACLF was defined according to APASL criteria. APASL ACLF Research Consortium (AARC) score was calculated using the online calculator available at
IBM SPSS version 22.0 was used for the statistical analysis. Continuous variables were expressed either as mean ± SD or as median (interquartile ratio), according to the parametric or non-parametric distribution. The Kolmogorov–Smirnov test was used to assess the distribution. The Student's t-test, Chi-square or Fischer’s exact tests were used for the analysis of continuous and categorical variables respectively. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used for the assessment of accuracy in predicting the outcome for Child-Pugh, model for end-stage liver disease (MELD), AARC scores and venous ammonia (VA). The cohort was then divided and further analyzed according to the cut-off value obtained for VA. Univariate and multivariate analysis was performed to assess the risk factors for in-hospital mortality. The statistical methods of the study were reviewed by a biomedical statistician.
Five hundred and twenty patients were screened. After applying the exclusion criteria 74 patients were removed from further analysis, thus 446 patients were included in the study. The median age of the participants was 59 (50-65) years and 57.4% of the patients were men. The main etiology of LC was alcohol (78.7%), followed by hepatitis C virus (HCV) infection (11.2%), hepatitis B virus (HBV) infection (6.1%), alcohol and HBV (1.8%), alcohol and HVC (1.3%), HBV and HVC (0.7%). The patients’ general characteristics are presented in Table 1.
| Variable | Value |
| Age, median (IQR) | 59 (50-65) |
| Male sex, n (%) | 256 (57.4) |
| Etiology of liver disease, n (%) | |
| Alcohol | 351 (78.7) |
| Hepatitis C virus | 50 (11.2) |
| Hepatitis B virus | 28 (6.1) |
| Alcohol + hepatitis B virus | 8 (1.8) |
| Alcohol + hepatitis C virus | 6 (1.3) |
| Hepatitis B and C virus | 3 (0.7%) |
| Albumin (g/dL), mean ± SD | 2.38 ± 0.59 |
| Bilirubin (mg/dL), median (IQR) | 2.53 (1.41-4.82) |
| INR, median (IQR) | 1.47 (1.29-1.74) |
| Platelets (× 109/L), median (IQR) | 128 (90-178) |
| WBC (× 109/L), median (IQR) | 7.41 (5.45-10.36) |
| CRP (mg/dL), median (IQR) | 1.51 (0.82-3.1) |
| Venous ammonia (μmol/L), median (IQR) | 103 (78-148) |
| Sodium (mmol/L), median (IQR) | 134 (130-137) |
| Creatinine (mg/dL), median (IQR) | 0.815 (0.63-1.27) |
| Lactate (mmol/L), median (IQR) | 1.7 (1.2-2.1) |
| Child-Pugh score, median (IQR) | 11 (10-12) |
| Child-Pugh class, n (%) | |
| A | 3 (0.7) |
| B | 84 (19.2) |
| C | 350 (80.1) |
| MELD, mean ± SD | 19.13 ± 6.79 |
| AARC score, median (IQR) | 7 (6-8) |
| ACLF, n (%) | |
| Grade I | 296 (66.4) |
| Grade II | 139 (31.2) |
| Grade III | 11(2.5) |
| Ascites, n (%) | 408 (91.5) |
| Grade I | 37(9.1) |
| Grade II | 112 (27.5) |
| Grade III | 259 (63.5) |
| HE, n (%) | 374 (83.9) |
| Grade I | 127 (34) |
| Grade II | 139 (37.2) |
| Grade III | 88(23.5) |
| Grade IV | 20(5.3) |
| Precipitating factors, n (%) | |
| Alcohol | 146 (32.7) |
| Upper GI bleeding | 89 (19.9) |
| Infections | 94 (21) |
| Hepatitis B virus activation | 7 (1.5) |
| Unknown | 110 (24.6) |
| Upper GI bleeding, n (%) | 89 (20) |
| SBP, n (%) | 24 (5.4) |
| Other infections, n (%) | 70 (15.7) |
| UTI | 28 (38.4) |
| Pneumonia | 7 (9.6) |
| CDI | 20 (27.4) |
| Sepsis | 5 (6.8) |
| Cutaneous | 11 (15.1) |
| Oral candidiasis | 2 (2.7) |
| AKI, n (%) | 52 (11.7) |
| HCC, n (%) | 11 (2.5) |
| Hospital stay (d), median (IQR) | 8 (5-11) |
| In-hospital death rate, n (%) | 35 (7.8) |
Most of the patients had advanced liver disease, as indicated by the high values of the traditional Child-Pugh and MELD prognosis scores of 11 (10-12) and 19.13 ± 6.79 respectively. The median novel AARC score was 7 (6-8), consistent with the predominance of grade I ACLF found in the majority of the patients; 66.4% of the participants had ACLF grade I, 31.2% ACLF grade II, and 2.5% ACLF grade III. Non-contrast computed tomography (CT) cranial scans were performed in 34 patients and no evidence of cerebral edema was described.
In-hospital mortality was 7.8% (Table 2). The mean survival of the deceased patients was 5 (3-10) d. The causes of death were: multi-organ failure in 19 patients, severe HE in 9 patients, and hemorrhagic shock in 7 patients. Non-survivors had higher levels of bilirubin, higher INR, lower albumin, and consequently higher Child-Pugh and MELD scores, consistent with more advanced liver disease than survivors. However, a higher inflammatory response expressed through higher CRP and higher white blood cells (WBC) count was also noted in the deceased, independently of the presence of bacterial infections, thus suggesting the important role of an exacerbated inflammatory response in the prognostic of patients with ACLF and HE. As expected, most of the non-survivors had grade II and III ACLF (62.9%, 22.9% respectively), while most of the survivors had grade I ACLF (70.8%).
| Variable | Survivors, n (%) 411 (92.2) | Non-survivors, n (%) 35 (7.8) | P value |
| Age, median (IQR) | 59 (50-65) | 60 (49-66) | 0.956 |
| Male sex, n (%) | 239 (58.2) | 17 (48.6) | 0.271 |
| Albumin (g/dL), mean ± SD | 2.4 ± 0.59 | 2.16 ± 0.5 | 0.022 |
| Bilirubin (mg/dL), median (IQR) | 2.33 (1.37-4.67) | 3.52 (1.93-6.49) | 0.013 |
| INR, median (IQR) | 1.45 (1.27-1.68) | 1.81 (1.5-2.6) | 0.005 |
| Creatinine (mg/dL), median (IQR) | 0.82 (0.63-1.26) | 0.77 (0.62-1.32) | 0.397 |
| Platelets (× 109/L), median (IQR) | 132 (92-180) | 123 (82-173) | 0.481 |
| WBC (× 109/L), median (IQR) | 7.24 (5.37-9.89) | 11.04 (6.79-13.45) | 0.014 |
| CRP (mg/dL), median (IQR) | 1.36 (0.8-2.98) | 3.15 (1.7-4.67) | 0.001 |
| Venous ammonia (μmol/L), median (IQR) | 100 (75-139) | 198 (125-345) | < 0.001 |
| Venous ammonia > 152.5 μmol/L, n (%) | 75 (19) | 24 (70.6) | < 0.001 |
| Sodium (mmol/L), median (IQR) | 134 (130-137) | 133 (130-137) | 0.215 |
| Child-Pugh score, median (IQR) | 11 (10-12) | 13 (11-15) | < 0.001 |
| Child-Pugh score ≥ 12.5 | 58 (14.4) | 19 (57.6) | < 0.001 |
| Child-Pugh class, n (%) | |||
| A | 3 (0.7) | 0 | |
| B | 84 (20.8) | 0 | |
| C | 317 (78.5) | 33 (100) | |
| MELD score, mean ± SD | 18.43 ± 6.26 | 27.31 ± 7.37 | < 0.001 |
| MELD score ≥ 22.5 | 119 (29) | 27 (77.1) | < 0.001 |
| AARC score, median (IQR) | 7 (6-8) | 9 (9-11) | < 0.001 |
| AARC score ≥ 8.5 | 42 (10.2) | 26 (74.3) | < 0.001 |
| ACLF, n (%) | |||
| Grade I | 291 (70.8) | 5(14.3) | |
| Grade II | 117 (28.5) | 22 (62.9) | |
| Grade III | 3 (0.7) | 8(22.9) | |
| Ascites, n (%) | 376 (91.5) | 32 (91.4) | 0.991 |
| HE, n (%) | 341 (83) | 33 (94.3) | 0.081 |
| Grade I | 126 (37) | 1 (3) | |
| Grade II | 135 (39.6) | 4 (12.1) | |
| Grade III | 76 (22.3) | 12 (36.4) | |
| Grade IV | 4 (1.2) | 16 (48.5) | |
| Grade I or II HE, n (%) | 261 (76.5) | 5 (15.2) | < 0.001 |
| Grade III or IV HE, n (%) | 80 (23.5) | 84.8 (28) | |
| Upper GI bleeding, n (%) | 82 (20) | 7 (21.2) | 0.862 |
| SBP, n (%) | 22 (5.4) | 2 (5.9) | 0.705 |
| Other infections, n (%) | 63 (15.3) | 7 (20) | 0.466 |
| AKI, n (%) | 39 (9.5) | 13 (38.1) | < 0.001 |
| HCC, n (%) | 9 (2.2) | 2 (5.7) | 0.211 |
| Hospital stay (d), median (IQR) | 8 (6-11) | 5 (3-10) | 0.160 |
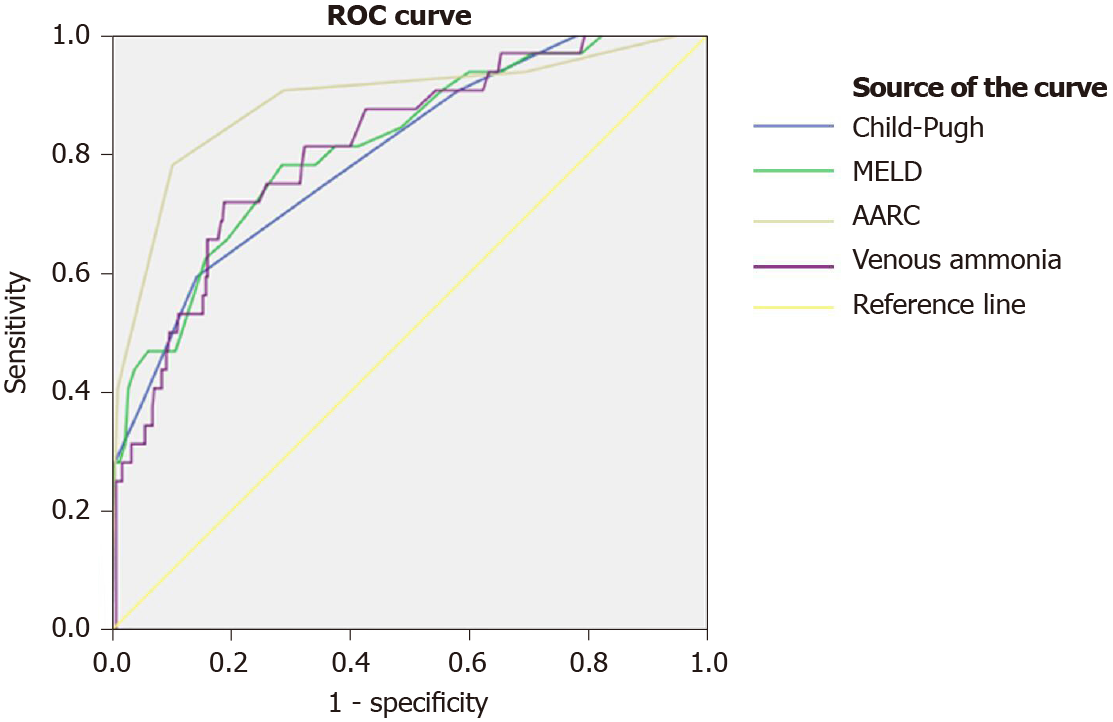
HE was diagnosed in 94.3% of the non-survivors and in 83% of the survivors (P = 0.081). However, 84% of the deceased had severe HE (grade III or IV), compared to 23.5 % of the survivors (P < 0.001). Overall VA was higher in the deceased than in the survivors' group [100 (75-139) μmol/L vs 198 (125-345) μmol/L, P < 0.001]. ROC analysis showed a slightly better accuracy for the prediction of in-hospital mortality for the AARC score (AUC = 0.886) than for the MELD score (AUC = 0.816) and the VA (AUC = 0.812). The accuracy of the Child-Pugh score in predicting mortality was fair (AUC = 0.799) (Figure 1).
Subsequently, cut-off values for the prediction of mortality were identified for VA (152.5 μmol/L, sensitivity = 0.706, 1-specificity = 0.190), AARC score (8.5, sensitivity = 0.743, 1-specificity = 0.102), MELD score (22.5, sensitivity = 0.771, 1-specificity = 0.286), and Child-Pugh score (12.5, sensitivity = 0.576, 1-specificity = 0.144).
Univariate analysis found that acute kidney injury (AKI) [odds ratio (OR) = 5.636, confidence interval (CI) (2.634-12.063)], severe HE (grade III or IV) [OR = 18.270, CI (6.830-48.870)], VA ≥ 152.5 μmol/L [OR = 7.976, CI (3.950-16.104)], MELD score ≥ 22.5 [OR = 8.282, CI (3.657-18.752)], Child-Pugh score ≥ 12.5 [OR = 8.096, CI (3.846-17.041)], and AARC score ≥ 8.5 [OR = 25.381, CI (1.151-57.770)] was associated with in-hospital mortality. However, multivariate analysis identified AARC score ≥ 8.5 and VA ≥ 152 μmol/L to be independent predictors of in-hospital mortality (Table 3).
| Variables in the equation | ||||||||
| Step 11 | B | SE | Wald | df | Sig | Exp (B) | 95%CI for EXP (B) | |
| Lower | Upper | |||||||
| AKI | 0.593 | 0.577 | 1.055 | 1 | 0.304 | 1.809 | 0.584 | 5.607 |
| MELD score ≥ 22.5 | 0.815 | 0.610 | 1.787 | 1 | 0.181 | 2.259 | 0.684 | 7.463 |
| Child-Pugh score ≥ 12.5 | 0.366 | 0.568 | 0.415 | 1 | 0.520 | 1.442 | 0.473 | 4.394 |
| AARC score ≥ 8.5 | 1.849 | 0.642 | 8.305 | 1 | 0.004 | 6.354 | 1.807 | 22.349 |
| HE grade III or IV | 1.154 | 0.628 | 3.383 | 1 | 0.066 | 3.172 | 0.927 | 10.853 |
| Venous ammonia ≥ 152 μmol/L | -1.813 | 0.508 | 12.763 | 1 | 0.000 | 0.163 | 0.060 | 0.441 |
| Constant | -2.795 | 1.368 | 4.172 | 1 | 0.041 | 0.061 | ||
HE was diagnosed in 83.9% of patients, with 34% having grade I, 37.2% grade II, 23.5% grade III, and 5.3% grade IV HE. Patients with HE had lower levels of albumin and higher levels of bilirubin, MELD, Child-Pugh and AARC scores. Moreover, Patients with HE presented more often ascites and AKI, as a consequence of the higher severity of their liver disease (Table 4).
| Variable | HE, n (%) 374 (83.9) | No. HE, n (%) 72 (16.1) | P value |
| Age, median (IQR) | 60 (50-65) | 56 (47-65) | 0.413 |
| Male sex, n (%) | 211 (56.4) | 45 (62.5) | 0.339 |
| Albumin (g/dL), mean ± SD | 2.33 ± 0.55 | 2.60 ± 0.74 | < 0.001 |
| Bilirubin (mg/dL), median (IQR) | 2.62 (1.54-5.11) | 1.72 (0.73-3.30) | 0.015 |
| INR, median (IQR) | 1.49 (1.31-1.75) | 1.34 (1.14-1.57) | 0.054 |
| Creatinine (mg/dL), median (IQR) | 0.82 (0.63-1.26) | 0.77 (0.62-1.32) | 0.520 |
| Platelets (× 109/L), median (IQR) | 127 (90-173) | 154 (93-193.5) | 0.054 |
| WBC (×109/L), median (IQR) | 7.5 (5.41-10.45) | 7.12 (5.76-9.09) | 0.898 |
| CRP (mg/dL), median (IQR) | 1.53 (0.83-3.15) | 1.29 (0.72-2.24) | 0.795 |
| Venous ammonia (μmol/L), median (IQR) | 111 (86-158) | 74 (60-93) | < 0.001 |
| Venous ammonia > 152.5 μmol/L, n (%) | 96 (26.6) | 3 (4.5) | < 0.001 |
| Sodium (mmol/L), median (IQR) | 134 (129-137) | 135 (131-138) | 0.883 |
| Child-Pugh score, median (IQR) | 11 (10-12) | 9 (8-11) | < 0.001 |
| Child-Pugh class, n (%) | |||
| A | 1 (0.3) | 2 (3) | |
| B | 47 (12.7) | 37 (55.2) | |
| C | 322 (87) | 28 (41.8) | |
| MELD, mean ± SD | 19.74 ± 6.88 | 15.97 ± 5.24 | < 0.001 |
| AARC score, median (IQR) | 7 (6-8) | 9 (9-11) | < 0.001 |
| ACLF, n (%) | |||
| Grade I | 224 (59.9) | 72(100) | |
| Grade II | 139 (37.2) | 0 | |
| Grade III | 11 (2.9) | 0 | |
| Ascites, n (%) | 337 (90.1) | 71 (98.6) | 0.018 |
| Upper GI bleeding, n (%) | 71 (19) | 18 (25) | 0.247 |
| SBP, n (%) | 23 (6.1) | 1 (1.4) | 0.150 |
| Other infections, n (%) | 64 (17.1) | 6 (8.3) | 0.061 |
| AKI, n (%) | 51 (13.6) | 1 (1.4) | 0.003 |
| HCC, n (%) | 10 (2.7) | 1 (1.4) | 0.520 |
| Hospital stay (d), median (IQR) | 8 (6-11) | 5 (3-10) | 0.061 |
| In-hospital death rate, n (%) | 33 (8.8) | 2 (2.8) | 0.081 |
VA median value was 103 (78-148) μmol/L, with higher levels in the HE than in the non-HE groups [111 (86-158) vs 74 (60-93), respectively, P < 0.001]. When further analyzing the subgroup of patients with high VA levels (≥ 152 μmol/L), significant differences were found concerning the distribution of cases with HE, severe HE, and ascites. Patients with high VA levels had a more advanced liver disease, as shown by the high MELD and AARC scores and a significantly higher in-hospital death rate (Table 5).
| Variable | Venous ammonia > 152.5 μmol/L, n (%) 99 (23.1) | Venous ammonia ≤ 152.5 μmol/L, n (%) 329 (76.9) | P value |
| Age, median (IQR) | 57 (48-65) | 60 (50-65) | 0.481 |
| Male sex, n (%) | 58 (58.6) | 190 (57.8) | 0.883 |
| Albumin (g/dL), mean ± SD | 2.33 ± 0.54 | 2.38 ± 0.59 | 0.452 |
| Bilirubin (mg/dL), median (IQR) | 2.7 (1.48-5) | 2.33 (1.37-4.7) | 0.230 |
| INR, median (IQR) | 1.5 (1.31-1.83) | 1.46 (1.27-1.72) | 0.584 |
| Creatinine (mg/dL), median (IQR) | 0.85 (0.63-1.54) | 0.81 (0.63-1.18) | 0.397 |
| Platelets (× 109/L), median (IQR) | 121 (73-173) | 134 (93-182) | 0.134 |
| WBC (× 109/L), median (IQR) | 8.39 (5.251-11.02) | 7.26 (5.47-9.92) | 0.359 |
| CRP (mg/dL), median (IQR) | 1.4 (0.84-4.16) | 1.5 (0.8-2.98) | 0.682 |
| HE, n (%) | 265 (80.5) | 96 (97) | < 0.001 |
| HE grade III or IV, n (%) | 55 (20.8) | 49 (51) | < 0.001 |
| Sodium (mmol/L), median (IQR) | 134 (131-137) | 134 (129-137) | 0.970 |
| Child-Pugh score, median (IQR) | 11 (10-12) | 11 (10-12) | 0.173 |
| Child-Pugh class, n (%) | |||
| A | 1 (1) | 2 (0.6) | |
| B | 12 (12.4) | 70 (21.4) | |
| C | 84 (86.6) | 255 (78) | |
| MELD, mean ± SD | 20.76 ± 7.72 | 18.61 ± 6.34 | 0.013 |
| AARC score, median (IQR) | 7 (6-8) | 9 (9-11) | < 0.001 |
| ACLF, n (%) | |||
| Grade I | 47 (47.5) | 238 (72.3) | |
| Grade II | 46 (46.5) | 87 (26.4) | |
| Grade III | 6 (6.1) | 4 (1.2) | |
| Ascites, n (%) | 82 (82.8) | 308 (93.6) | 0.001 |
| Upper GI bleeding, n (%) | 22 (22.4) | 57 (17.3) | 0.252 |
| SBP, n (%) | 4 (4) | 20 (6.1) | 0.440 |
| Other infections, n (%) | 16 (16.2) | 49 (14.9) | 0.758 |
| AKI, n (%) | 16 (16.2) | 32 (9.7) | 0.075 |
| HCC, n (%) | 5 (5.1) | 6 (1.8) | 0.137 |
| Hospital stay (d), median (IQR) | 8 (6-11) | 5 (3-10) | 0.969 |
| In-hospital death rate, n (%) | 24 (24.2) | 10 (3) | < 0.001 |
This study provided evidence for the utility of VA in predicting the short-term prognosis in patients with ACLF. We found a statistically significant risk for in-hospital mortality in patients with high VA that was independent of the disease severity as evaluated by the classic and novel prognostic scores.
There is still much controversy regarding the use of VA in patients with LC, concerning its value as a predictor for the development of HE, as well as its role in predicting short-term mortality. VA is considered to be unspecific for the severity of HE[21] and thus it is not being used to guide the treatment in this setting[18]. However, the utility of VA might reside in its prognostic value. For the vast majority of patients with decompensated LC, VA has not been established as an adequate indicator for poor outcome[17,21]. This is mainly due to the intricate mechanisms that lead to hyperammonemia. These mechanisms are found in numerous conditions associated with LC, such as sarcopenia, GI bleeding, infection, and AKI, all of which are associated with poor prognostic in patients with LC and ACLF[22]. There is data suggesting that sepsis and systemic inflammation exacerbate the deleterious effects that ammonia exercise on the brain[12]. Thus, patients with sepsis and LC could present HE even in the absence of high VA. By analyzing only patients with ACLF in our study, we found that elements suggestive of systemic inflammation such as WBC and CRP presented higher values in the group of non-survivors, without relation to bacterial infections. These findings are indicative of alternative pathways of developing an exacerbated inflammatory response in ACLF, other than through infection, such as endotoxemia which is secondary to bacterial translocation from the gut[23]. However, no statistically significant differences were noted in patients with and without HE or high ammonia levels regarding these inflammatory markers. These results suggest that an exacerbated inflammatory response poses a risk for in-hospital mortality in patients with ACLF, mostly determined by other physiopathological mechanisms leading to different organ dysfunctions, such as AKI. The results also indicate that the relationship between systemic inflammation and HE is complex, requiring a more in-depth analysis.
The management of patients with advanced liver disease has been greatly improved by the efforts made to identify the groups with high-risk for mortality, via numerous prognostic scores[24]. This stratification has been of paramount importance for the in-hospital management, facilitating decisions such as admission to the intensive care unit, or urgent liver transplantation[25]. The traditional prognostic scores, namely Child-Pugh and MELD scores seem to be more adequate for the prediction of outcome in the setting of decompensated LC, but not ACLF[26]. In our study, the novel AARC score presented a good accuracy for predicting mortality, better than the Child-Pugh and MELD scores. Moreover, VA showed good accuracy for predicting the outcome, similarly to the accuracy of the MELD score. We identified a cut-off value of 152.5 μmol/L for VA which accurately predicted mortality in our cohort. These observations are in accordance with recent data which supports the theory that ammonia has an independent role in the risk for short-term mortality[15]. As discussed, VA levels can be increased in the presence of several conditions frequently associated with LC, among which sarcopenia, AKI, GI bleeding, and infection, thus ammonia levels could represent an additional marker of advanced liver disease, indicative of the altered homeostasis of patients with LC and ACLF[22]. VA could therefore aid in the stratification of ACLF patients regarding in-hospital prognostic and serve as a marker of severity for this category.
In our study, comprising of patients with advanced liver disease, the presence of HE, in general, was not associated with increased mortality. However, grade III or IV HE posed a statistically significant high risk for in-hospital death and was associated with high levels of VA. These results are in accordance with the findings reported by Bajaj et al[27]. The authors analyzed 1560 patients, from which 516 presented HE, 371 grade 1-2 and 145 grade 3-4. Grade 3-4 but not grad 1-2 HE was associated with both higher in-hospital and 30-day mortality rates[27]. Ammonia levels correlate with HE grade in ACLF patients[14], but high ammonia levels have also been associated with the development of organ failure in the setting of ACLF, other than HE, thus contributing to the unfavorable prognostic of these patients[15]. Our data suggest that the main risk factor for in-hospital mortality in patients with high VA remains severe HE, thus optimal management of these patients is required.
There are several limitations to our study. As a single-center retrospective study, the possibility of selection bias could not be eliminated. Also, as per hospital protocol, arterial ammonia was not available and therefore not comparable with VA regarding the outcome. Furthermore, CT cranial scan was not routinely performed for patients with ACLF and HE, thus the frequency and the impact of cerebral edema on survival could not be optimally analyzed.
Our results suggest that VA presents a good predictive value for in-hospital mortality in patients with ACLF and that high levels of VA are associated with severe HE. VA has the potential to be used as an additional prognostic marker in the evaluation of patients with ACLF. However, prospective additional studies are required to confirm whether the use of ammonia lowering agents guided by VA levels can improve survival in these patients.
High venous ammonia (VA) values have been proven to be a part of the mechanism of hepatic encephalopathy in patients with liver cirrhosis (LC) as well as acute hepatitis. Moreover, VA has been associated with poor prognosis and high mortality in these clinical settings.
VA has been associated with hepatic encephalopathy (HE) and mortality and has been used by clinicians in acute settings. However, the role of ammonia in acute-on-chronic liver failure (ACLF) has not yet been clearly established and a cut-off value for the prediction of in-hospital mortality is not currently available.
We aimed to adequately assess the role of VA in predicting in-hospital mortality of cirrhotic patients with ACLF in a tertiary care center and to establish an indicative cut-off value for poor prognosis in these patients.
We retrospectively included consecutive cirrhotic patients fulfilling the Asian Pacific Association for the Study of the Liver (APASL) criteria for ACLF that were hospitalized for acute non-elective indications such as ascites, HE, upper gastrointestinal bleeding, or bacterial infections. The study was conducted in “St. Spiridon” University Hospital, Iasi, Romania, a tertiary care center, between January 2017 and January 2019. Patients diagnosed with non-hepatic malignancy, human immunodeficiency virus infection, hematological disease as well as pregnant women were excluded. ACLF was defined according to the APASL criteria. The APASL ACLF Research Consortium (AARC) score was calculated and ACLF grade was established accordingly. West-haven classification was used for HE. Statistical analysis was performed using IBM SPSS version 22.0.
Five hundred and twenty patients were screened and after applying the exclusion criteria 446 patients were included, aged 59 (50-65) years, 57.4% men. The main etiology of LC was alcohol (78.7%), followed by hepatitis C virus (HCV) infection (11.2%), hepatitis B virus (HBV) infection (6.1%), alcohol and HBV (1.8%), alcohol and HVC (1.3%), HBV and HVC (0.7%). 66.4% had ACLF grade I, 31.2% ACLF grade II, and 2.5% ACLF grade III. HE was diagnosed in 83.9%, 34% grade I, 37.2% grade II, 23.5% grade III, and 5.3% grade IV. Overall mortality was 7.8% and the mean survival of the deceased patients was 5 (3-10) d. ROC analysis showed good accuracy for the prediction of in-hospital mortality for the AARC score [Area under the curve (AUC) = 0.886], model for end-stage liver disease (MELD) score (AUC = 0.816), VA (AUC = 0.812) and a fair accuracy for the Child-Pugh score (AUC = 0.799). A cut-off value for the prediction of mortality was identified for VA (152.5 μmol/L, sensitivity = 0.706, 1-specificity = 0.190). We identified acute kidney injury, severe HE (grade III or IV), VA ≥ 152.5 μmol/L, MELD score ≥ 22.5, Child-Pugh score ≥ 12.5, and AARC score ≥ 8.5 to be associated with in-hospital mortality. Multivariate analysis found AARC score ≥ 8.5 and venous ammonia ≥ 152 μmol/L to be independent predictors of in-hospital mortality.
Our data indicated that VA represented a useful prognostic marker for patients with ACLF diagnosed according to the APASL definition. Moreover, the cut-off value of 152.5 μmol/L was independently associated with the risk of death with a sensitivity = 0.706 for a 1-specificity = 0.190.
Prospective large additional studies should be performed in order to confirm whether the use of ammonia lowering agents guided by VA levels could improve survival in patients with ACLF.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Meng Q S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Jaeger V, DeMorrow S, McMillin M. The Direct Contribution of Astrocytes and Microglia to the Pathogenesis of Hepatic Encephalopathy. J Clin Transl Hepatol. 2019;7:352-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Laish I, Ben Ari Z. Noncirrhotic hyperammonaemic encephalopathy. Liver Int. 2011;31:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Triplett KE, Murray R, Anstey M. Multifactorial non-cirrhotic hyperammonaemic encephalopathy. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 5. | Piano S, Favaretto E, Tonon M, Antonelli G, Brocca A, Sticca A, Mareso S, Gringeri E, Scaroni C, Plebani M, Russo FP, Burra P, Cillo U, Angeli P. Including Relative Adrenal Insufficiency in Definition and Classification of Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2020;18:1188-1196.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Trifan A, Chiriac S, Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol. 2013;19:445-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Seyan AS, Hughes RD, Shawcross DL. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress. World J Gastroenterol. 2010;16:3347-3357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 8. | Liotta EM, Kimberly WT. Cerebral edema and liver disease: Classic perspectives and contemporary hypotheses on mechanism. Neurosci Lett. 2020;721:134818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, Ranjan P, Pandey CM, Narayana PA. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Joshi D, O'Grady J, Patel A, Shawcross D, Connor S, Deasy N, Willars C, Bernal W, Wendon J, Auzinger G. Cerebral oedema is rare in acute-on-chronic liver failure patients presenting with high-grade hepatic encephalopathy. Liver Int. 2014;34:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Sawhney R, Holland-Fischer P, Rosselli M, Mookerjee RP, Agarwal B, Jalan R. Role of ammonia, inflammation, and cerebral oxygenation in brain dysfunction of acute-on-chronic liver failure patients. Liver Transpl. 2016;22:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Hu C, Huang K, Zhao L, Zhang F, Wu Z, Li L. Serum ammonia is a strong prognostic factor for patients with acute-on-chronic liver failure. Sci Rep. 2020;10:16970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Shalimar, Sheikh MF, Mookerjee RP, Agarwal B, Acharya SK, Jalan R. Prognostic Role of Ammonia in Patients With Cirrhosis. Hepatology. 2019;70:982-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Kumar R, Shalimar, Sharma H, Prakash S, Panda SK, Khanal S, Acharya SK. Persistent hyperammonemia is associated with complications and poor outcomes in patients with acute liver failure. Clin Gastroenterol Hepatol. 2012;10:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Jacoby KJ, Singh P, Prekker ME, Leatherman JW. Characteristics and outcomes of critically ill patients with severe hyperammonemia. J Crit Care. 2020;56:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Haj M, Rockey DC. Ammonia Levels Do Not Guide Clinical Management of Patients With Hepatic Encephalopathy Caused by Cirrhosis. Am J Gastroenterol. 2020;115:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Dharel N, Bajaj JS. Definition and nomenclature of hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 20. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3348] [Article Influence: 257.5] [Reference Citation Analysis (0)] |
| 21. | Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, Arroliga AC, Mullen KD. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | Patwardhan VR, Jiang ZG, Risech-Neiman Y, Piatkowski G, Afdhal NH, Mukamal K, Curry MP, Tapper EB. Serum Ammonia is Associated With Transplant-free Survival in Hospitalized Patients With Acutely Decompensated Cirrhosis [corrected]. J Clin Gastroenterol. 2016;50:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Takaya H, Namisaki T, Sato S, Kaji K, Tsuji Y, Kaya D, Fujinaga Y, Sawada Y, Shimozato N, Kawaratani H, Moriya K, Akahane T, Mitoro A, Yoshiji H. Increased Endotoxin Activity Is Associated with the Risk of Developing Acute-on-Chronic Liver Failure. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Chen BH, Tseng HJ, Chen WT, Chen PC, Ho YP, Huang CH, Lin CY. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Trebicka J, Sundaram V, Moreau R, Jalan R, Arroyo V. Liver Transplantation for Acute-on-Chronic Liver Failure: Science or Fiction? Liver Transpl. 2020;26:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 26. | Weil D, Levesque E, McPhail M, Cavallazzi R, Theocharidou E, Cholongitas E, Galbois A, Pan HC, Karvellas CJ, Sauneuf B, Robert R, Fichet J, Piton G, Thevenot T, Capellier G, Di Martino V; METAREACIR Group. Prognosis of cirrhotic patients admitted to intensive care unit: a meta-analysis. Ann Intensive Care. 2017;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Bajaj JS, O'Leary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas HE, Lai J, Thacker LR, Reddy KR. Hepatic Encephalopathy Is Associated With Mortality in Patients With Cirrhosis Independent of Other Extrahepatic Organ Failures. Clin Gastroenterol Hepatol 2017; 15: 565-574. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (1)] |