Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8609
Peer-review started: May 26, 2021
First decision: June 15, 2021
Revised: June 27, 2021
Accepted: July 21, 2021
Article in press: July 21, 2021
Published online: October 6, 2021
Processing time: 124 Days and 19.6 Hours
Percutaneous vertebroplasty (PVP) has been widely used in osteoporotic vertebral compression fracture (OVCF). Following surgery, the bone cement would be positioned permanently. However, in some cases of lumbar degenerative disease, the cemented vertebrae needs to be fixed after decompression and fusion pro
PVP involving the L3 and L4 was performed in an 82-year-old man due to OVCF. During the surgery, bone cement leakage occurred, resulting in compression of the root of the right L3 nerve. We performed a partial facetectomy to retrieve the leaked bone cement and to relieve the patient’s neurological symptoms. After 3 mo, the patient developed lumbar disc herniation in L3/4, potentially due to instability caused by the previous surgery. Therefore, it was necessary to perform intervertebral fusion and fixation. It was difficult to implant traditional trajectory pedicle screws in L3 and L4 because of the bone cement filling. Hence, we implanted CBT screws in the L3 and L4 vertebrae. As a result, the patient’s symptoms resolved and he reported satisfaction with the surgery at follow-up after 8 mo.
It is feasible to utilize CBT in cemented vertebrae for the treatment of lumbar degenerative disease.
Core Tip: It is difficult to implant traditional trajectory (TT) pedicle screws in the cemented vertebrae. Cortical bone trajectory (CBT) may be a feasible method because of short implantation depth and location at the rear of the vertebrae. We successfully implanted CBT screws in the cemented vertebrae. The application of CBT provides a new method for the fixation of the cemented vertebrae and expands the indication for CBT. Meanwhile, we used two tips to decrease the rod curvature and simplify assem
- Citation: Chen MM, Jia P, Tang H. Cortical bone trajectory fixation in cemented vertebrae in lumbar degenerative disease: A case report. World J Clin Cases 2021; 9(28): 8609-8615
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8609.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8609
Percutaneous vertebroplasty (PVP) has been widely applied for the treatment of osteoporotic vertebral compression fracture (OVCF)[1]. Polymethylmethacrylate (PMMA) is used as the bone cement, which is permanently implanted into the ver
Herein, we report the case of a patient with lumbar disc herniation who underwent PVP of two successive vertebrae. During the surgery, bone cement leakage occurred, and open decompression was performed to remove the leaked bone cement. After 3 mo, lumbar disc herniation occurred at the decompressed segment, and a cortical bone trajectory (CBT) pedicle screw was used to fix the cemented vertebrae.
An 82-year-old man complained of serious backache accompanied by radiating pain to the right lower extremity.
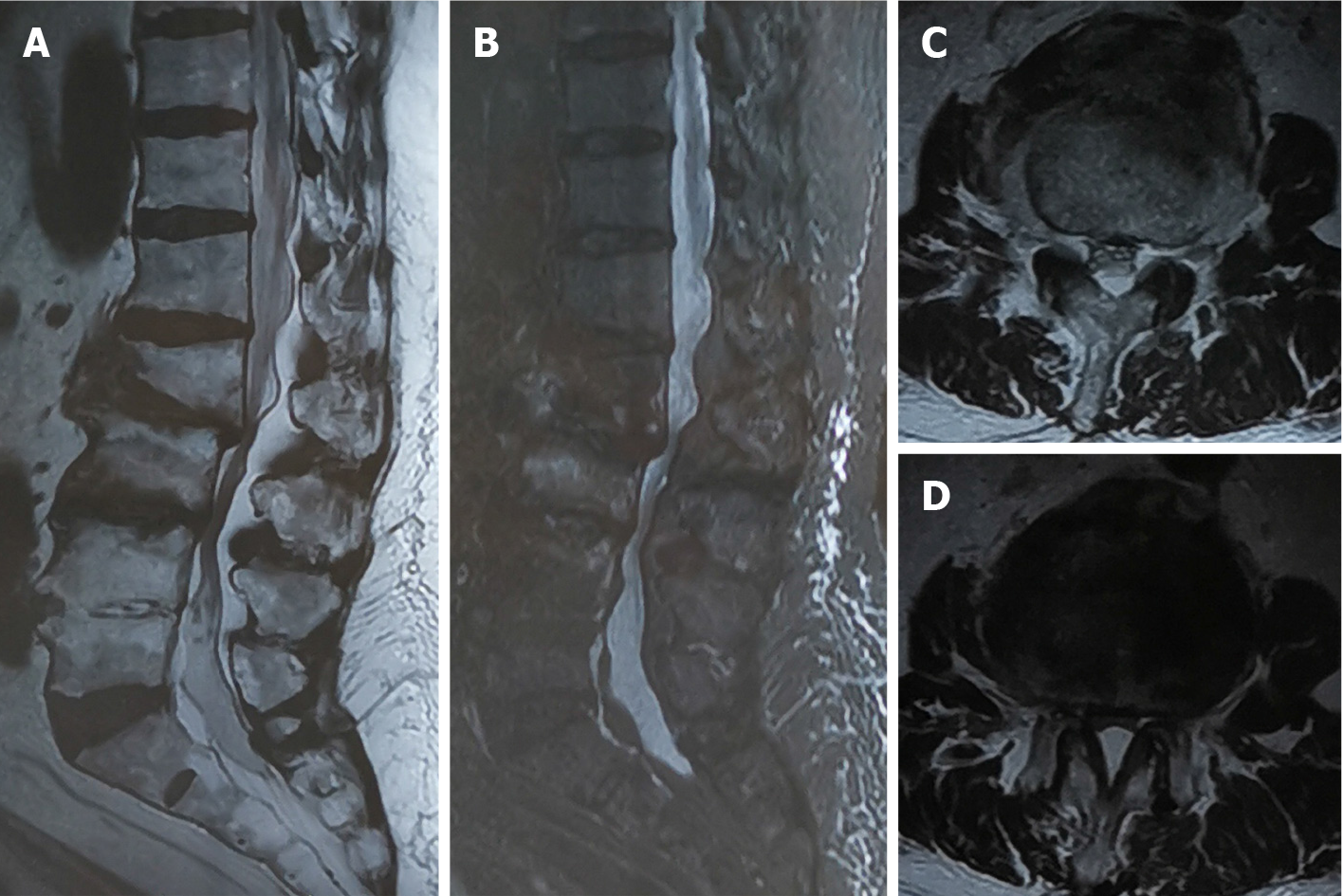
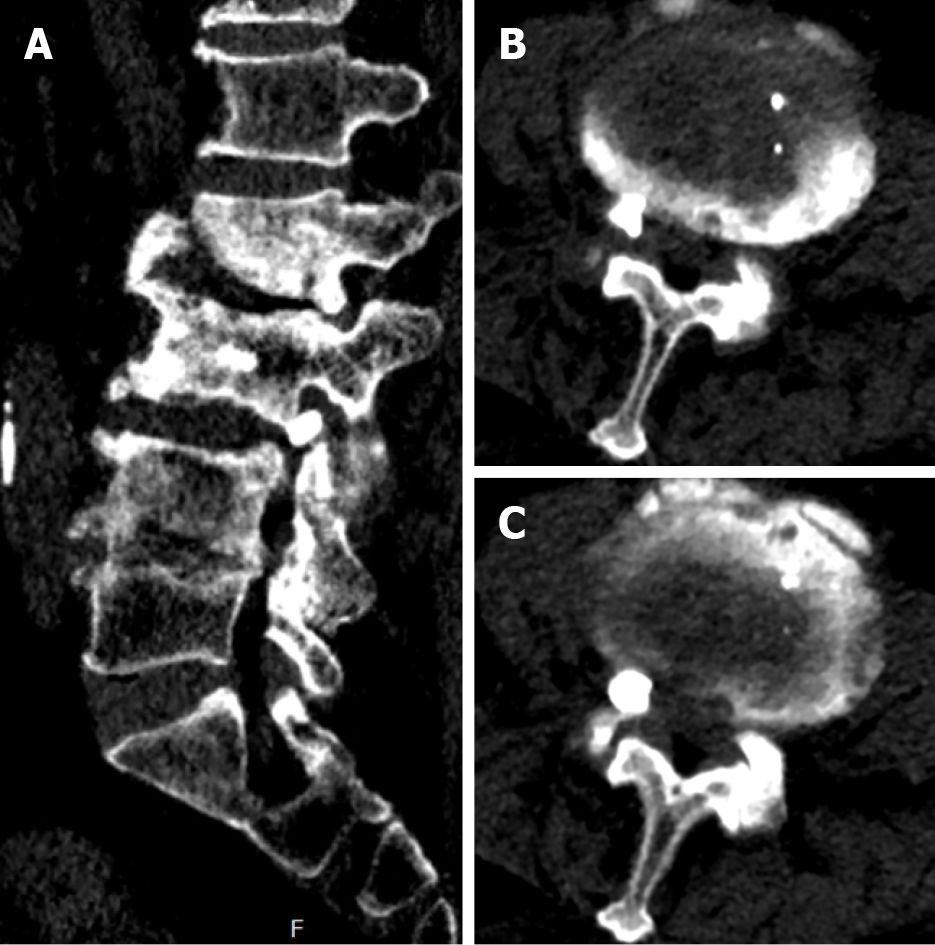
The patient presented to a local hospital for acute exacerbation of chronic lower back pain 3 mo ago. Magnetic resonance imaging (MRI) revealed a high signal area in T2-weighted images of the vertebral bodies of L3 and L4, with the L2/3 presenting corresponding segment canal stenosis (Figure 1). However, the patient did not exhibit any obvious lower extremity neurological symptoms. Therefore, PVP was performed based on the diagnosis of OVCF (L3, L4). Unfortunately, bone cement leakage occurred during the surgery, which led to severe neurological symptoms. Computed tomography (CT) revealed that the right intervertebral foramen of L3/4 had a high density shadow (Figure 2). Leaked bone cement compressed the root of the L3 nerve, which caused a stabbing pain radiating to the skin of the anterior-lateral thigh. A local surgeon administered an epidural steroid injection that can temporarily alleviate neurological symptoms. However, after 2 d, the patient’s pain recurred and was unbearable. The patient was transferred to our hospital and complained of lower back pain accompanied by radiating pain in the right thigh. The patient was maintained in bedridden status because even light activity resulted in unbearable pain. We therefore performed a partial facetectomy to retrieve the leaked bone cement using a posterior approach. Following this procedure, the neurological symptoms completely resolved, although slight backache persisted. The patient was subsequently discharged from the hospital.
After 3 mo, the patient experienced serious backache accompanied by radiating pain to the right lower extremity and revisited our hospital. The radiating pain was mainly located from the back of the thigh to the inner side of the shin. The visual analog scale score was 8.
The patient had a previous diagnosis of high blood pressure and coronary heart disease.
The patient had been drinking for about 20 years, intaking about 200 mg per day. He had no history of smoking and no significant family history.
Numbness and hypoesthesia at the medial lateral of the right shin skin, and the muscle strength of the dorsiflexion ankle decreased to level 4. A positive Lasegue’s sign on the right lower extremity was noted. Bilateral patellar and Achilles tendon reflex could not be evoked. Perianal sensation and anal sphincter muscle strength were normal. Babinski sign was negative.
Troponin-T and N-terminal pro-brain natriuretic peptide were normal. Arterial blood gas analysis revealed partial pressure of blood oxygen was 81.7 mmHg.
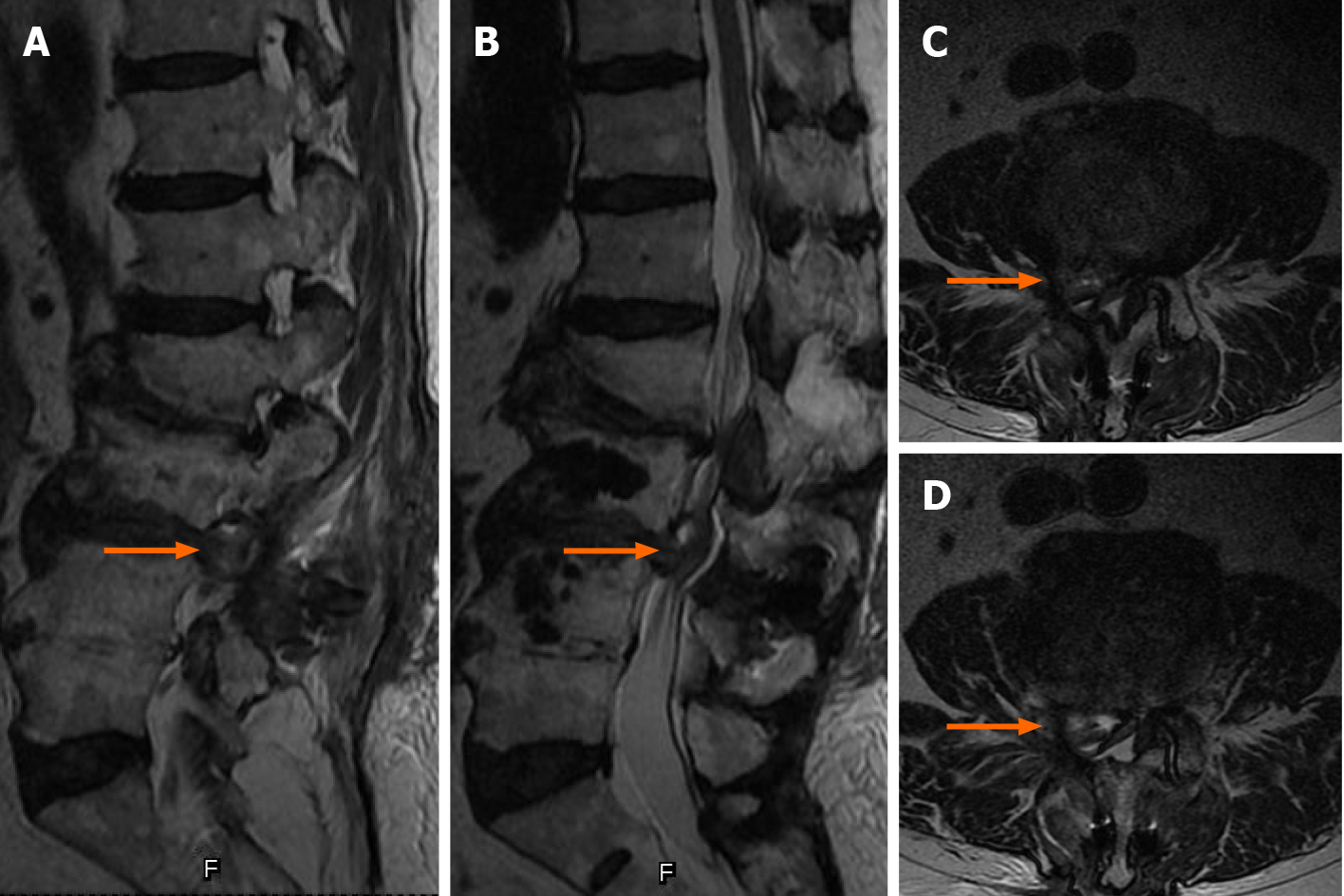
MRI revealed a right lumbar disc protrusion located at L3/4 and spinal canal stenosis at L2/3 (Figure 3).
This patient was diagnosed with lumbar disc herniation (L3/4) which compressed the root of the L4 nerve.
Since sciatica was the patient’s main symptom, discectomy and nerve root decom
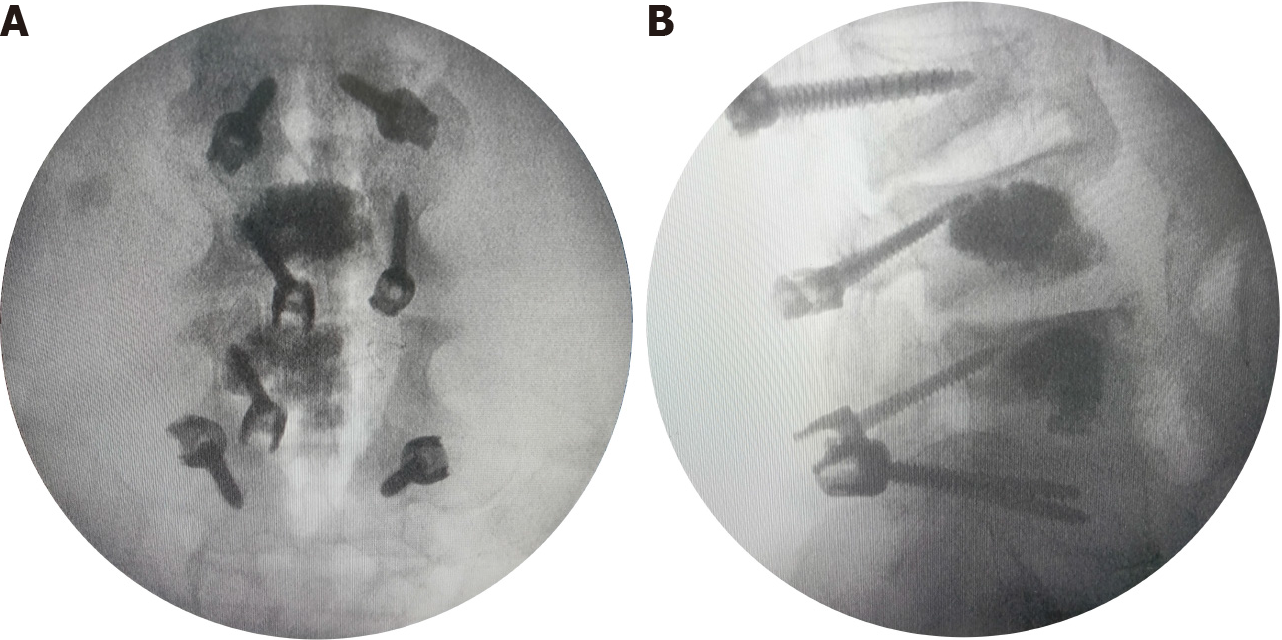
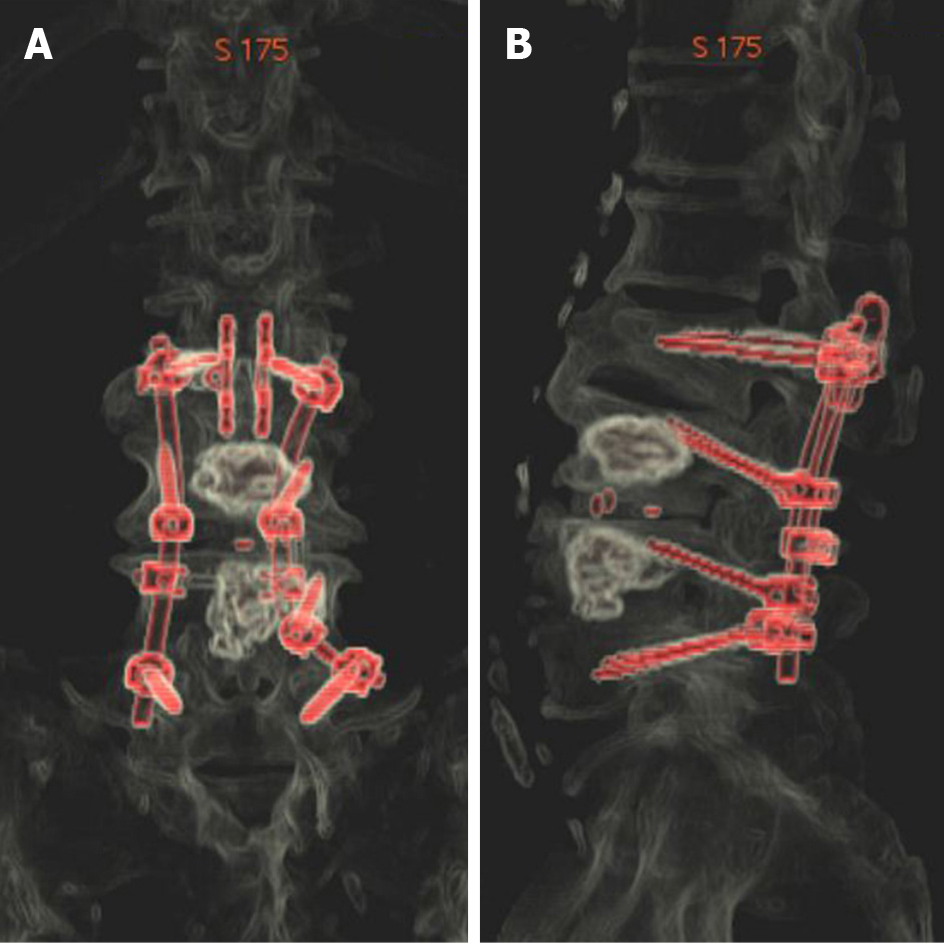
The surgery scheme was designed to include discectomy, intervertebral fusion, pedicle screw fixation, and a topping-off strategy. It was difficult to implant TT pedicle screws into L3 and L4 because of the bone cement filling. We therefore applied CBT screws to fix the cemented vertebrae. The entry point was set in the lateral point of the pars interarticularis. The angle projected from 5-o’clock to 11-o’clock in the left pedicle and from 7-o’clock to 1-o’clock in the right pedicle (Figure 4). The right isthmus of the L4 Lamina had an iatrogenic defect from the previous surgery, so it was not suitable for implantation of the pedicle screw. Subsequently, TT screws were implanted into L2 and L5. Furthermore, considering the fusion of multiple segments in the lumbar spine, an interspinous stabilization device was placed between L1 and L2 to avoid adjacent segment disease (ASD).
After surgery, the symptom of the right lower extremity radiating pain immediately resolved. On postoperative day 5, the patient left the bed and walked around the room with the use of a waist brace. At 8 mo after surgery, the patient could take care of himself without assistance, and reported satisfaction with the surgery at the outpatient clinic follow-up. The CT reconstruction images are shown in Figure 5. No related complications occurred.
PVP has been an effective measure for the treatment of OVCF[1]. PVP has been widely used in clinical practice since it immediately enhances vertebral stability and quickly relieves pain. The most common filling material is PMMA cement, which rapidly solidifies after being injected into the vertebrae and remains in the body permanently without degradation[2]. In many cases, people who experienced PVP also have concomitant lumbar degenerative disease[5]. Subsequently, several patients may need surgery for further decompression and fixation owing to severe degeneration. How
We therefore applied CBT screws to fix the cemented vertebrae. Santoni first reported the application and related biomechanical study of CBT in 2009[6]. CBT was initially designed for improving the fixation strength in patients with osteoporosis. Compared to the TT screw, the implantation of the CBT screw does not require exposure of the facet joint and is minimally invasive. Therefore, less dissection and an inferior entry point expanded the indications of CBT[7,8]. Moreover, CBT is also used in revision patients with ASD and can be considered as a rescue technique using TT screws[9,10]. Pacione reported on an 83-year-old female diagnosed with L4 OVCF and lumbar spinal stenosis (L3/4) who was treated with PVP (L4) and decompression (L3/4). CBT screws were applied in L3 and L5 for spinal stability. After 3 mo, the patient experienced an adjacent segment fracture in L3 and underwent PVP with a common procedure negating impediment of CBT[11]. Shi published the application of CBT in spinal tuberculosis in which CBT screws were implanted in diseased vertebrae to strengthen the stabilization and reduce instrumented segments. The method is suitable for diseased vertebrae fixation because of the short implantation depth and location at the rear of the vertebrae[12].
The internal fixation stabilization is essential for spine fusion in osteoporosis patients. It has been reported that CBT improved the pullout strength by 30% and the insertional torque was 1.7 times higher than that of the TT screw[6,13]. Moreover, double fixation can also increase instrumentation stability in osteoporosis patients. Ueno reported on a 64-year-old female with severe osteoporosis who underwent spinal fixation and fusion using two sets of screw systems (CBT and TT) to enhance stability; every pedicle was implanted with two different screws[14].
In our case, an interspinous stabilization device was used to prevent ASD after multiple lumbar spine fusion (topping-off). The increasing activity in the adjacent segment due to fusion of distal segments would lead to further disc degeneration. Therefore, an interspinous stabilization device, which limits the range of the adjacent segment and reduces the disc stress, can effectively decrease the incidence of ASD[15,16].
In the present case, we applied a hybrid screw technique in which CBT and TT were used in the same set. The placement of a rod could be difficult because the entry points are located in different sagittal planes. Therefore, we used two tips to decrease the rod curvature and simplify assembly. First, the CBT screws should be implanted in successive vertebrae in the hybrid screw technique. Second, the entry points in the region of the junction of the TT and CBT should be adjusted in the controllable area. In our patient, for example, the TT screw implantation in L5 can be downward and the CBT screw in L4 can be upward in the normal range. Therefore, it is feasible to reduce the curvature of the rod at the junction of the TT and CBT. However, not all cemented vertebrae can be fixed using CBT, particularly for the cement existing in the pedicle. Therefore, it is necessary to perform preoperative CT to evaluate and program the screw implantation trajectory. The limitations of this study were the limited number of cases and the short follow-up time. In the future study, more patients should be included and a longer follow-up time should be applied.
It is feasible to utilize CBT in cemented vertebrae in the treatment of lumbar dege
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bai J S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Muijs SP, van Erkel AR, Dijkstra PD. Treatment of painful osteoporotic vertebral compression fractures: a brief review of the evidence for percutaneous vertebroplasty. J Bone Joint Surg Br. 2011;93:1149-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Liu H, Liu B, Gao C, Meng B, Yang H, Yu H, Yang L. Injectable, biomechanically robust, biodegradable and osseointegrative bone cement for percutaneous kyphoplasty and vertebroplasty. Int Orthop. 2018;42:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Miller JD, Nader R. Treatment of combined osteoporotic compression fractures and spinal stenosis: use of vertebral augumentation and interspinous process spacer. Spine (Phila Pa 1976). 2008;33:E717-E720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Bonaldi G, Cianfoni A. Percutaneous treatment of lumbar compression fracture with canal stenosis and neurogenic intermittent claudication: combining kyphoplasty and interspinous spacer. J Vasc Interv Radiol. 2012;23:1437-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2930] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 6. | Santoni BG, Hynes RA, McGilvray KC, Rodriguez-Canessa G, Lyons AS, Henson MA, Womack WJ, Puttlitz CM. Cortical bone trajectory for lumbar pedicle screws. Spine J. 2009;9:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Kaye ID, Prasad SK, Vaccaro AR, Hilibrand AS. The Cortical Bone Trajectory for Pedicle Screw Insertion. JBJS Rev. 2017;5:e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Tortolani PJ, Stroh DA. Cortical Bone Trajectory Technique for Posterior Spinal Instrumentation. J Am Acad Orthop Surg. 2016;24:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Rodriguez A, Neal MT, Liu A, Somasundaram A, Hsu W, Branch CL Jr. Novel placement of cortical bone trajectory screws in previously instrumented pedicles for adjacent-segment lumbar disease using CT image-guided navigation. Neurosurg Focus. 2014;36:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Calvert GC, Lawrence BD, Abtahi AM, Bachus KN, Brodke DS. Cortical screws used to rescue failed lumbar pedicle screw construct: a biomechanical analysis. J Neurosurg Spine. 2015;22:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Pacione D, Kim I, Wilson TA, Frempong-Boadu A. Cortical screw trajectory for instrumentation and fusion in the setting of osteopathic compression fracture allows for percutaneous kyphoplasty for adjacent level compression fractures. J Clin Neurosci. 2015;22:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Shi S, Ying X, Zheng Q, Zhu B, Jin Y, Shen J, Zheng M, Hu S. Application of Cortical Bone Trajectory Screws in Elderly Patients with Lumbar Spinal Tuberculosis. World Neurosurg. 2018;117:e82-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976). 2014;39:E240-E245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Ueno M, Imura T, Inoue G, Takaso M. Posterior corrective fusion using a double-trajectory technique (cortical bone trajectory combined with traditional trajectory) for degenerative lumbar scoliosis with osteoporosis: technical note. J Neurosurg Spine. 2013;19:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Lee CH, Hyun SJ, Kim KJ, Jahng TA, Yoon SH, Kim HJ. The efficacy of lumbar hybrid stabilization using the DIAM to delay adjacent segment degeneration: an intervention comparison study with a minimum 2-year follow-up. Neurosurgery. 2013;73:ons224-231; discussion ons231-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Herren C, Beckmann A, Meyer S, Pishnamaz M, Mundt M, Sobottke R, Prescher A, Stoffel M, Markert B, Kobbe P, Pape HC, Eysel P, Siewe J. Biomechanical testing of a PEEK-based dynamic instrumentation device in a lumbar spine model. Clin Biomech (Bristol, Avon). 2017;44:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |