Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8579
Peer-review started: May 19, 2021
First decision: June 15, 2021
Revised: July 5, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: October 6, 2021
Processing time: 131 Days and 15.3 Hours
Infantile hemangiomas (IHs) are the most common childhood benign tumors, showing distinctive progression characteristics and outcomes. Due to the high demand for aesthetics among parents of IH babies, early intervention is critical in some cases. β-Adrenergic blockers and corticosteroids are first-line medications for IHs, while itraconazole, an antifungal medicine, has shown positive results in recent years.
In the present study, itraconazole was applied to treat two IH cases. The therapeutic course lasted 80-90 d, during which the visible lesion faded by more than 90%. Moreover, no obvious side effects were reported, and the compliance of the baby and parents was desirable.
Although these outcomes further support itraconazole as an effective therapeutic choice for IHs, large-scale clinical and basic studies are still warranted to improve further treatment.
Core Tip: Infantile hemangiomas (IHs) stand as the most common vascular tumors in infants, mainly due to the disorder of vascular architecture and aberrant proliferation of endothelial cells. Early intervention is critical for restraining lesion growth, reducing the risk of complications, and mitigating psychosocial stress. In the two IH cases listed in the study, oral administration of itraconazole, which was dissolved in milk for 80-90 d, yielded satisfying outcomes, including fading of lesions by more than 90%, few side effects, and desirable compliance of the baby’s parents. Overall, itraconazole has been demonstrated being an effective therapeutic choice for IHs.
- Citation: Liu Z, Lv S, Wang S, Qu SM, Zhang GY, Lin YT, Yang L, Li FQ. Itraconazole therapy for infant hemangioma: Two case reports. World J Clin Cases 2021; 9(28): 8579-8586
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8579.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8579
Infantile hemangiomas (IHs), the most common vascular tumors in infants, are caused by disorder of vascular architecture and aberrant proliferation of endothelial cells[1]. Several retrospective and prospective studies reported 1%-3% incidence of IHs among infants, 2.6%-9.9% incidence in older children, and up to 22%-30% incidence in preterm infants weighing less than 1 kg[2]. The initial onset of IHs usually appears before 4 wk of age with complete growth by 5 mo. For most IH cases, the involution begins at 12 mo and finalizes by age 4[2]. Although a large portion of IHs tend to fade spontaneously without structural or aesthetics challenges, some are accompanied by vital complications or residual disfigurement[3]. In such cases, early intervention is critical for restraining the lesion growth, reducing the risk of complications, and mitigating psychosocial stress[4].
IH intervention approaches involve medications and laser treatments, while surgery is required for patients who fail to respond to pharmacotherapy[2,3]. Orally administrated propranolol or topical applied timolol, as β-adrenergic blockers, have shown clear efficacy on the superficial IHs[5]. While systemic corticosteroids are the typical first-line therapy for IHs, their distinct adverse effects limit their application in clinic[6]. Other medications, including vincristine, interferon-α, and imiquimod, are also commonly used but result in remarkable complications[2,3].
Itraconazole is an antifungal medication to treat infections caused by fungus in the lungs, mouth, throat, toenails, and fingernails in adults[7]. However, complications associated with this medicine include heart failure, liver, and kidney disease[8]. A recent study has suggested that the treatment of IH with 5 mg/kg per day itraconazole (oral) induced a substantial alleviation of lesions[9]. The underlying reasons for the success are related to angiogenesis inhibition and depressed cellular proliferation[10]. Despite this evidence, further research is still required to confirm the contribution of itraconazole. Therefore, we describe two cases in which infants presenting IHs were treated by itraconazole for 80-90 d and exhibited satisfying results without any noticeable complications. In both cases, the infants and parents showed promising compliance to the therapeutic approaches.
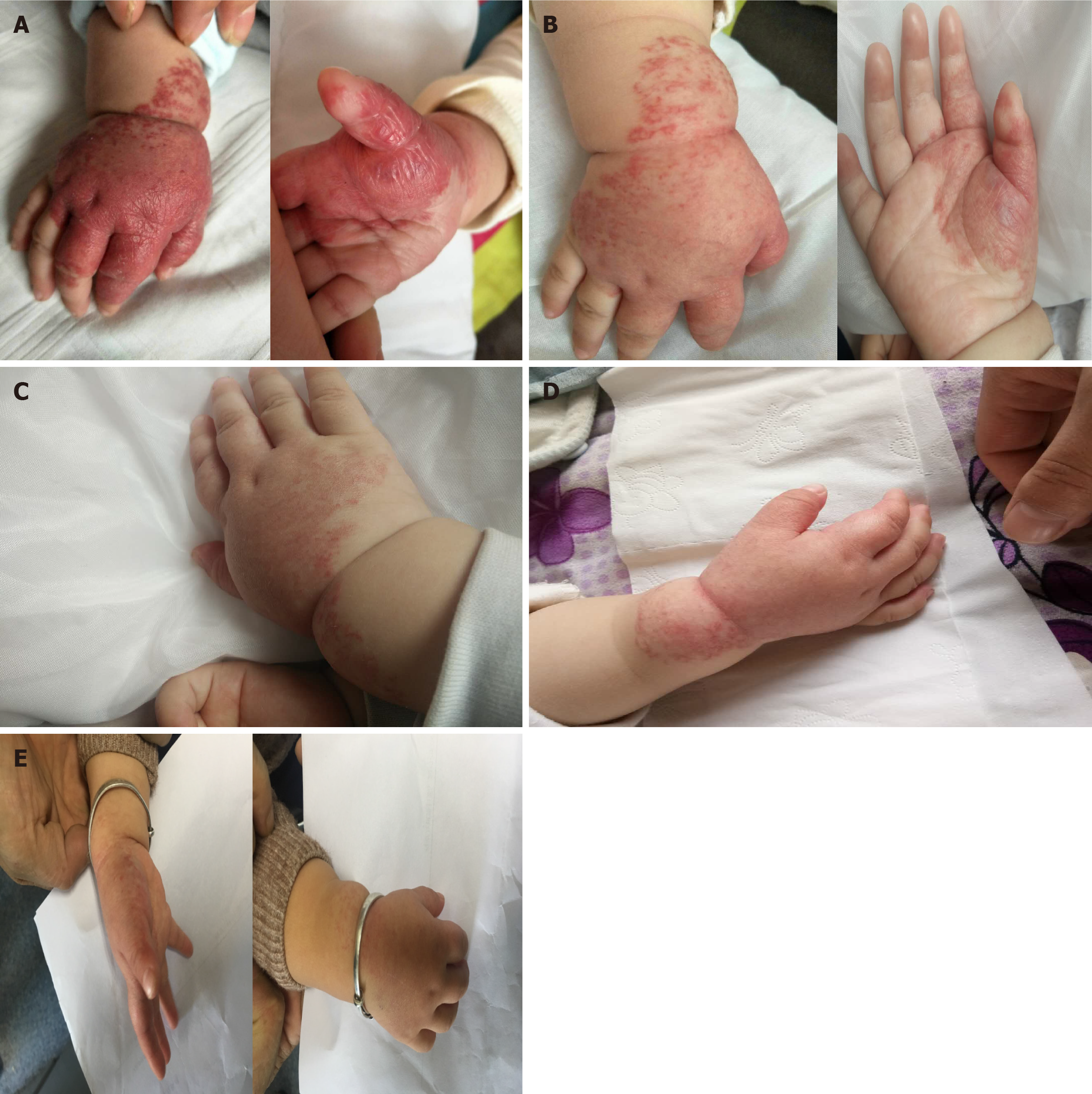
Case 1: A 4-mo-old baby presented slightly raised soft reddish-purple patches on the right hand at birth.
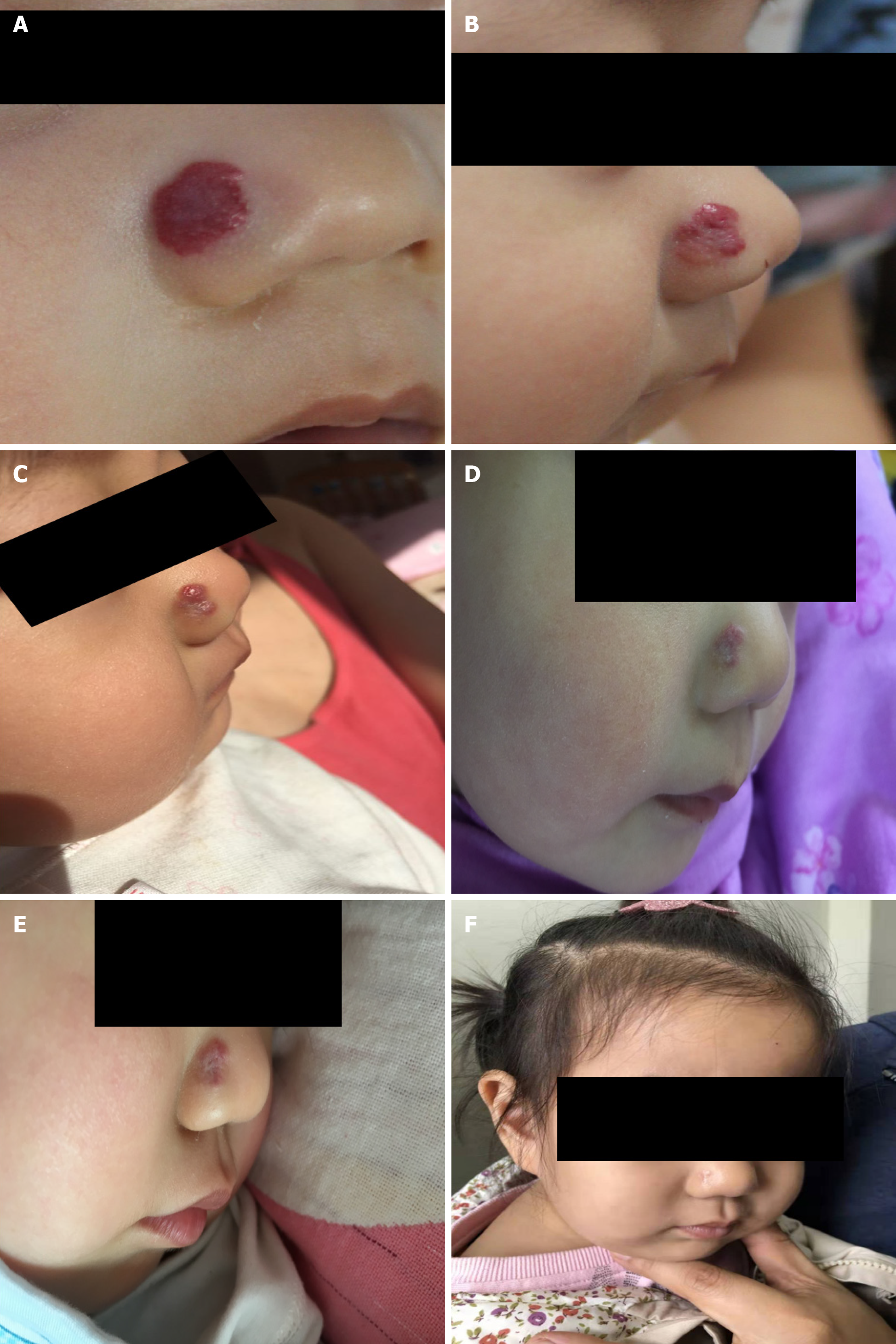
Case 2: A 6-mo-old baby visited the hospital for a bright red raised soft patch on the surface of the right ala nasi (Figure 1).
Case 1: Within 3 mo, the lesion grew rapidly in size and thickness, showing no fading tendency. The lesions were limited to the right palm and dorsum and exhibited a reddish-purple color, well-defined boundary, and nodular shape with slight lobulation (Figure 2).
Case 2: The lesion appeared 1 mo after birth and rapidly enlarged in size and thickness within 5 mo, showing no fading tendency. The patient had received radionuclide application therapy in another hospital 2 mo prior, which flattened the lesion slightly, but the response lasted only half a month. The lesion relapsed and thickened quickly, displaying a bright-red strawberry shape and clear boundary that faded upon pressing, thus was diagnosed as infantile strawberry hemangioma. The lesion extended into the nasal cavity causing vascular malformation on nasal mucosa.
Case 1: The patient showed a clear previous medical history.
Case 2: The previous medical history of the patient was clear.
Case 1: The baby’s parents reported no special personal and family medical history.
Case 2: No special personal and family medical history was reported.
Case 1: The baby weighed 5 kg, and the physical examination revealed no abnormal signs.
Case 2: The baby weighed 7.9 kg, and the physical examination found no abnormality.
Liver function was normal, and no other vital organs were involved.
Case 1: None of the imaging examinations revealed anomaly.
Case 2: All the imaging examinations were normal.
Case 1: All the clinical features supported the diagnosis of infantile cavernous hemangioma.
Case 2: The clinical features of this patient supported the diagnosis of IH.
Case 1: With the written informed consent obtained from the baby’s parents, oral itraconazole (5 mg/kg/d, Janssen Pharmaceutical Co. Ltd) was chosen as the only treatment. The medicine (2000 mg itraconazole dissolved in milk) was fed to the baby for 80 d.
Case 2: After obtaining the written informed consent from the baby’s parents, oral itraconazole (5 mg/kg/d, Janssen Pharmaceutical Co. Ltd) was chosen as the only treatment. One of two equal parts of the 100-mg itraconazole capsule was dissolved in 5 mL of milk, which was fed to the baby once a day. The treatment included 39.5 mg/d itraconazole and lasted for 90 d, thus a total of 3600 mg itraconazole was administered to the baby.
Case 1: The follow-up examinations were performed on day 43, 80, 124, and 1 year after the treatment began. No biochemical anomaly was found, and normal liver function was maintained. The patient showed fine compliance. On day 43, the lesions were less raised with a slightly faded color, narrowed size, and emergence of cracks, invagination, and ruffle on the surface (Figure 2). On day 80, the size and color of lesions were reduced further (Figure 2), therefore itraconazole administration was halted. The follow-up examination at month 4 revealed flattened lesions with light pink fibrous and adipose tissues and substantially lessened vascular structures (Figure 1). At the 1-year follow-up, only a trace of light pink fibrous and adipose tissues as well as vascular structures were left (Figure 1).
Case 2: The follow-up examinations were assigned on day 30, 60, 80, and 90. The biochemical indices and liver function remained normal, and the parents did not report any discomfort of the baby. Upon visual observation on day 30, the lesion was flattened with reduced size and color, and invagination appeared on the surface of the lesion, which indicated clear fading tendency (Figure 1). The color B type ultrasonography detected a smaller vascular malformation inside the nasal cavity. After day 90, the baby’s parents decided by themselves to stop the medication and no longer visit the hospital. One year later, a photo of the baby revealed only a small light-pink scar at the original site of the IH (Figure 1).
IH is the most common benign tumor in children with distinctive progress and outcomes. Specifically, they can grow quickly in the early infancy, then fade slowly but spontaneously grow in the following years, which is characteristic of the most uncomplicated IHs. However, if IHs are present on risky sites, such as the eye, lip, nose, cheek, or central nervous system, early intervention becomes a necessity[1]. Darrow et al[2] proved that the most remarkable growth of IHs occurs 5.5 and 6.5 wk after the onset of the disease, much sooner than previous predictions. This report also recommends early intervention before this time frame to prevent the irreversible anatomic deformation or complications[2]. While systemic corticosteroids have been the first-line option for IH therapy, propranolol was found effective in 2008[11] and demonstrated higher safety and efficacy than corticosteroids in later studies.
In 2015, Ran et al[9] reported 6 IH cases in which oral itraconazole was used as the only treatment[9]. A favorable outcome was achieved as the lesions faded by 80%-100% after 2-9 wk of itraconazole treatment (5 mg/kg/d), although minor digestive symptoms, such as diarrhea, appeared. Later, the same team reported positive results of a giant tufted angioma, which faded within 3 mo of oral itraconazole treatment[12]. The liver function and blood test were normal during the treatment, and conditions continued to improve 6 mo after withdrawal of the medicine. Moreover, Li et al[13] selected specimens of 5 IHs cases and 11 capillary malformations to perform an adenosine triphosphate sensitivity assay to detect the growth inhibition activity of propranolol, rapamycin, sildenafil, and itraconazole[13]. They found that itraconazole exhibited clear inhibition on the cellular proliferation of both IHs and capillary malformations[13]. Bessar et al[14] investigated the efficacy of propranolol and itraconazole on IHs by observing the variation of serum angiopoietin-2. They reported that oral itraconazole is a promising alternative to propranolol with shorter treatment duration and higher safety[14]. We chose itraconazole for the 2 cases considering its superiority compared to propranolol, less side effects, reliance on electrocardiogram monitoring, and therapeutic capability on cardiovascular diseases[15,16]. Both the patients were satisfied with the treatment. Nonetheless, because only a few dermatologists are aware of the benefits of itraconazole to date, in the present study, the effect of itraconazole was further observed in two babies with IHs.
Patients of the two cases exhibited lesions on exposed sites. A large and rapidly growing lesion on the right hand with no fading tendency was reported in case 1. In case 2, the lesion was presented on the ala nasi, affecting the nasal cavity and creating an apparent vascular deformation. Both cases met the need for early intervention; thus, the parents of case 1 chose itraconazole as the only treatment, and the case 2 parents selected radionuclide application therapy before oral itraconazole. Currently, the 90Sr and 32P radionuclides are the most frequently used for IHs, which emit β-rays upon decay[17]. IH tissues are more sensitive to radioactive rays than the surrounding normal tissues, whereby the ionization induces swelling and necrosis of vascular endothelial cells and the subsequent occlusion and atrophy of vascular lumen. The 90Sr applicator is used often in clinic for superficial IHs since β-rays possess a 3-mm penetrating power and, thus, cannot reach deep lesions[18]. Besides, the radionuclide application therapy brings about several complications, such as skin atrophy, pigmentation or depigmentation, and scarring[17]. In case 2, the negative response to radionuclide application suggests that the lesion is located in deeper tissues, therefore the residue scan of this case could be a complication of radioactive treatment.
As for the preparation of medicine, lipophilic itraconazole is almost completely insoluble in water[19], so its bioavailability would be reduced by 40% with fasting[20]. A previous study implied that milk as a medium can improve the dissolving velocity and solubility of itraconazole[21]. Therefore, we used milk as the delivery medium in these 2 cases, during which neither baby showed any obvious adverse effect.
For more than 30 years, itraconazole has been widely acknowledged as a safe and effective treatment in clinic to treat fungal infections of infants and children[22,23]. The United States Food and Drug Administration has approved itraconazole as an inhibitor of the hedgehog pathway to treat cancers, such as basal cell carcinoma[24]. Compared to other triazole antifungal medications, itraconazole retains the endothelium in the G1 phase of the cell cycle[25] and restrains the angiogenesis through the vascular endothelial-derived growth factor signal pathway[26]. A recent study indicated that itraconazole can reduce the platelet-derived growth factor content to suppress the activation of platelet-derived growth factor-β and downstream effectors, including phosphatidylinositol-3-kiase, Akt, 4E binding protein 1, and p70S6K[27]. This discovery partly explains the therapeutic effect of itraconazole on the growth and survival of IHs cells.
In both cases, satisfying outcomes were achieved after the patients received oral administration of itraconazole, which further demonstrated the remarkable efficacy of this medication on IHs. Since there was no need for the patients and their parents to stay in the hospital, the compliance of parents could be ensured. Considering its low price and minor side effects, itraconazole stands as a promising new therapeutic approach for IHs. Nevertheless, high-quality large-scale multicenter clinical studies and a further comparative study between itraconazole and propranolol are still needed to confirm its efficacy. In addition, considering the younger age of IH patients and the long therapeutic course of itraconazole (daily oral administration for more than 3 mo), other adverse effects, including bone growth inhibition, should be closely observed for a longer duration. Overall, more clinical and basic research regarding the effect of itraconazole on IHs are warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiba T, Kaur M, Syahputra DA S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Satterfield KR, Chambers CB. Current treatment and management of infantile hemangiomas. Surv Ophthalmol. 2019;64:608-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Darrow DH, Greene AK, Mancini AJ, Nopper AJ; Section on Dermatology, Section on Otolaryngology–Head and Neck Surgery, and Section on Plastic Surgery. Diagnosis and Management of Infantile Hemangioma. Pediatrics. 2015;136: e1060-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Fei Q, Lin Y, Chen X. Treatments for infantile Hemangioma: A systematic review and network meta-analysis. EClinicalMedicine. 2020;26:100506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Tiemann L, Hein S. Infantile Hemangioma: A Review of Current Pharmacotherapy Treatment and Practice Pearls. J Pediatr Pharmacol Ther. 2020;25:586-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Marqueling AL, Oza V, Frieden IJ, Puttgen KB. Propranolol and infantile hemangiomas four years later: a systematic review. Pediatr Dermatol. 2013;30:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Cheng CE, Friedlander SF. Infantile hemangiomas, complications and treatments. Semin Cutan Med Surg. 2016;35:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Clark NM, Grim SA, Lynch JP, 3rd. Posaconazole: Use in the Prophylaxis and Treatment of Fungal Infections. Semin Respir Crit Care Med. 2015;36:767-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Kurn H, Wadhwa R. Itraconazole. 2021 May 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–.. [PubMed] |
| 9. | Ran Y, Chen S, Dai Y, Kang D, Lama J, Ran X, Zhuang K. Successful treatment of oral itraconazole for infantile hemangiomas: a case series. J Dermatol. 2015;42:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Tsai YC, Tsai TF. Itraconazole in the Treatment of Nonfungal Cutaneous Diseases: A Review. Dermatol Ther (Heidelb). 2019;9:271-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1472] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 12. | Wu W, Shi J, Li Z, Li D, Dou S, Pradhan S, Ran X, Ran Y. Oral itraconazole for the treatment of giant tufted angioma with hair loss arising during pregnancy: A case report. J Dermatol. 2020;47:e35-e36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Li L, Yang B, Wei L, Zhang B, Han XF, Xu ZG, Ma L. Application of the adenosine triphosphate sensitivity assay in infantile vascular anomalies. BMC Pediatr. 2020;20:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Bessar H, Kandil AH, Nasr NM, Khattab F. Itraconazole Versus Propranolol: Therapeutic and Pharmacologic Effect On Serum Angiopoietin-2 In Patients With Infantile Hemangioma. J Dermatolog Treat. 2019;1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Ji Y, Chen S, Xiang B, Yang Y, Qiu L. Safety and tolerance of propranolol in neonates with severe infantile hemangiomas: a prospective study. Sci Rep. 2017;7:1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Chen S, Sun KY, Feng XW, Ran X, Lama J, Ran YP. Efficacy and safety of itraconazole use in infants. World J Pediatr. 2016;12:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Bruscino N, Bonan P, Cannarozzo G, Moretti S, Lotti T, Campolmi P. Laser use in infantile hemangiomas, when and how. Dermatol Ther. 2012;25:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Grzesik P, Wu JK. Current perspectives on the optimal management of infantile hemangioma. Pediatric Health Med Ther. 2017;8:107-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Binder B, Richtig E, Weger W, Ginter-Hanselmayer G. Tinea capitis in early infancy treated with itraconazole: a pilot study. J Eur Acad Dermatol Venereol. 2009;23:1161-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Feola D, Rapp RP. Effect of food intake on the bioavailability of itraconazole. Clin Infect Dis. 1997;25:344-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Ghazal HS, Dyas AM, Ford JL, Hutcheon GA. In vitro evaluation of the dissolution behaviour of itraconazole in bio-relevant media. Int J Pharm. 2009;366:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Cauwenbergh G, De Doncker P, Stoops K, De Dier AM, Goyvaerts H, Schuermans V. Itraconazole in the treatment of human mycoses: review of three years of clinical experience. Rev Infect Dis. 1987;9 Suppl 1:S146-S152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Grant SM, Clissold SP. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989;37:310-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 273] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Wei X, Liu W, Wang JQ, Tang Z. "Hedgehog pathway": a potential target of itraconazole in the treatment of cancer. J Cancer Res Clin Oncol. 2020;146:297-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ Jr, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Aftab BT, Dobromilskaya I, Liu JO, Rudin CM. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764-6772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Chen S, Zhuang K, Sun K, Yang Q, Ran X, Xu X, Mu C, Zheng B, Lu Y, Zeng J, Dai Y, Pradhan S, Ran Y. Itraconazole Induces Regression of Infantile Hemangioma via Downregulation of the Platelet-Derived Growth Factor-D/PI3K/Akt/mTOR Pathway. J Invest Dermatol. 2019;139:1574-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |