Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8571
Peer-review started: May 17, 2021
First decision: June 24, 2021
Revised: July 8, 2021
Accepted: July 28, 2021
Article in press: July 28, 2021
Published online: October 6, 2021
Processing time: 133 Days and 21.5 Hours
Hypereosinophilia (HE) is defined as a peripheral blood eosinophil count of > 1.5 × 109/L and may be associated with tissue damage. The clinical presentations of HE vary; however, myocardial fibrosis and thrombosis can threaten the lives of patients with sustained eosinophilia. Cerebral venous sinus thrombosis (CVST) in the setting of eosinophil-related diseases has seldom been reported. Here, we review the literature on HE with CVST to increase knowledge and encourage early diagnosis.
A previously healthy 41-year-old man was admitted to hospital with diarrhea and abdominal pain. He was treated with antibiotics for suspected acute colitis. Three days later, he experienced headache and vomiting. Brain computed tomography (CT) revealed thrombosis of the left jugular vein to the left transverse sinus vein. Platelet (PLT) count decreased to 60 × 1012/L, and absolute eosinophil count (AEC) increased to 2.41 × 109/L. He was treated with low-molecular-weight heparin. PLT count progressively decreased to 14 × 109/L, and we terminated anticoagulation and performed PLT transfusion. Six days after admission, he complained of a worsening headache. Brain CT revealed right temporal lobe and left centrum semiovale intracerebral hemorrhage, and AEC increased to 7.65 × 109/L. We used prednisolone for HE. The level of consciousness decreased, so emergency hematoma removal and decompressive craniectomy for right cerebral hemorrhage were performed. The patient was alert 2 d after surgery. He was treated with anticoagulation again 2 wk after surgery. Corticosteroids were gradually tapered without any symptomatic recurrence or abnormal laboratory findings.
HE can induce CVST, and we need to focus on eosinophil counts in patients with CVST.
Core Tip: Thromboembolism is a rare but serious complication of hypereosinophilia (HE). We report a 41-year-old man who presented with colitis, cerebral venous sinus thrombosis (CVST), and intracerebral hemorrhage caused by HE. Blood eosinophil count decreased quickly after corticosteroid therapy, and CVST caused headache, which improved after anticoagulation therapy. Good clinical outcomes were observed during a 6-mo follow-up period. We conclude that HE can induce CVST, and we need to focus on eosinophil counts in patients with CVST. Corticosteroids are a useful first-line therapy for platelet-derived growth factor receptor A/B-negative HE.
- Citation: Song XH, Xu T, Zhao GH. Hypereosinophilia with cerebral venous sinus thrombosis and intracerebral hemorrhage: A case report and review of the literature. World J Clin Cases 2021; 9(28): 8571-8578
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8571.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8571
Cerebral venous sinus thrombosis (CVST) can occur in the early stages of hypereosinophilia (HE) and can be life-threatening if not identified early. We searched the Web of Science, Scopus, Embase, and PubMed up to December 2020 using medical subject headings of “eosinophilia/HE” and “cerebral venous thrombosis/sinus thrombosis” (limits: Full text available, clinical trials, human studies, and studies in English), and identified eight publications (Table 1) related to HE with CVST in eight cases[1-8]. Herein, we report a 41-year-old man who presented with HE-associated CVST and intracerebral hemorrhage (ICH). Blood eosinophil count decreased quickly after corticosteroid therapy, and CVST caused headache, which improved after anticoagulation therapy.
| Ref. | Age/sex | Medical history | Initial symptoms | CVST | ICH | Thrombocytopenia | Treatment | Prognosis |
| Schulman et al[1], 1999 | 11/M | None | Bug bites, rash | Straight sinus; superior sagittal sinus | Brain stem | No | Prednisone, heparin | Death |
| Sakuta et al[2], 2007 | 7/M | Seizures, taking valproate | HE for 10 m | Superior sagittal sinus | Right hemisphere | 50 × 109/L | Heparin, warfarin | Cure |
| Numagam et al[3], 2008 | 76/F | None | Right mandible swelling | Left transverse sinus | Left temporal lobe | No | Prednisolone,evacuation of hematoma | Death |
| Ananth et al[4], 2016 | 17/M | Asthma | Intermittent fever, dyspnea | Transverse sinus | None | No | Prednisolone,warfarin | Death |
| Teresa et al[5], 2006 | 40/F | Rhinitis asthma, nasal polyps, taking contraceptives for 6 yr | Asthenia, myalgia, fever | Superior sagittal sinus | Bilateral parietal lobe | No | Heparin, warfarin | Cure |
| Left arm/foot paresthesia | Right lateral sinus | |||||||
| Kanno et al[6], 2005 | 34/F | None | Lump on left thigh | Left transverse sinus | Left hemisphere | 10.4 × 109/L | Antibiotics, corticosteroid, decompression surgery | Death |
| Chan et al[7], 2004 | 49/F | None | Headache, diplopia | Cavernous sinus, transverse sigmoid sinuses | None | No | Bilateral endoscopic sphenoidectomy, antihistamines, steroids, itraconazole | Cure |
| Sano et al[8], 2014 | 67/M | Prostatic hypertrophy | Slight fever | Superior sagittal sinus | Bilateral parietal lobes, rightoccipital lobe | No | Evacuation of hematoma | Cure |
| Heparin, warfarin,anticoagulation |
A 41-year-old man was admitted to the Department of Neurology of our hospital after experiencing headache and vomiting for 1 d on September 9, 2020.
Six days before admission, the patient presented with diarrhea (2–3 times per day) and abdominal pain with a slight fever. Self-medication with trimebutine maleate and compound Lactobacillus acidophilus showed no improvement. Three days before admission, the patient visited our hospital on emergency and was treated with antibiotics (levofloxacin) for suspected colitis. The patient achieved remission from diarrhea, but still demonstrated abdominal distension. The patient did not experience chest tightness or heart palpitations. Six days after admission, he complained of a worsening headache and developed left hemiplegia. His consciousness rapidly deteriorated with signs of brain herniation. Thirteen days after admission (2 d after surgery), he had no symptoms, but he did demonstrate acne on his skin.
The patient had a history of tonsillectomy, appendectomy, and kidney stones.
The patient denied a past history of drug or alcohol abuse, smoking, promiscuous sexual behavior, raw food consumption, and travel. There was no family history of neurological or blood system diseases.
After admission, the patient’s body temperature was 36.7 ℃, respiratory rate was 16 bpm, heart rate was 42 bpm, and blood pressure was 135/72 mmHg. There was no detectable rash, bradycardia, arrhythmia, or murmur, and both lungs sounded clear with no rales. Abdominal distension without tenderness was noted, and the patient’s neurological examination was normal.
On September 6, 2020, white blood cell count was elevated (12.6 × 109/L) with an absolute eosinophil count (AEC) of 0.97 × 109/L, hemoglobin concentration of 155 g/L, platelet (PLT) count of 238 × 1012/L, and C-reactive protein concentration of 110 mg/L. On September 9, 2020, PLT count decreased to 60 × 1012/L, AEC increased to 2.41 × 109/L, D-dimer increased to 50.22 mg/L, prothrombin time (PT) was 12.8 s, and activated partial PT was 36.2 s. Total immunoglobulin E was 389.4 IU/mL, erythrocyte sedimentation rate was 21 mm/h, and glycan antigen was 125 U/mL, but a serological allergen screen was negative. Tests for parasites in the stool were negative. Anti-neutrophil cytoplasmic, antinuclear, and cardiolipin antibodies were negative. Procalcitonin, troponin, cardiac enzymes, liver and kidney function, folic acid, vitamin B12, and thyroid function were all normal.
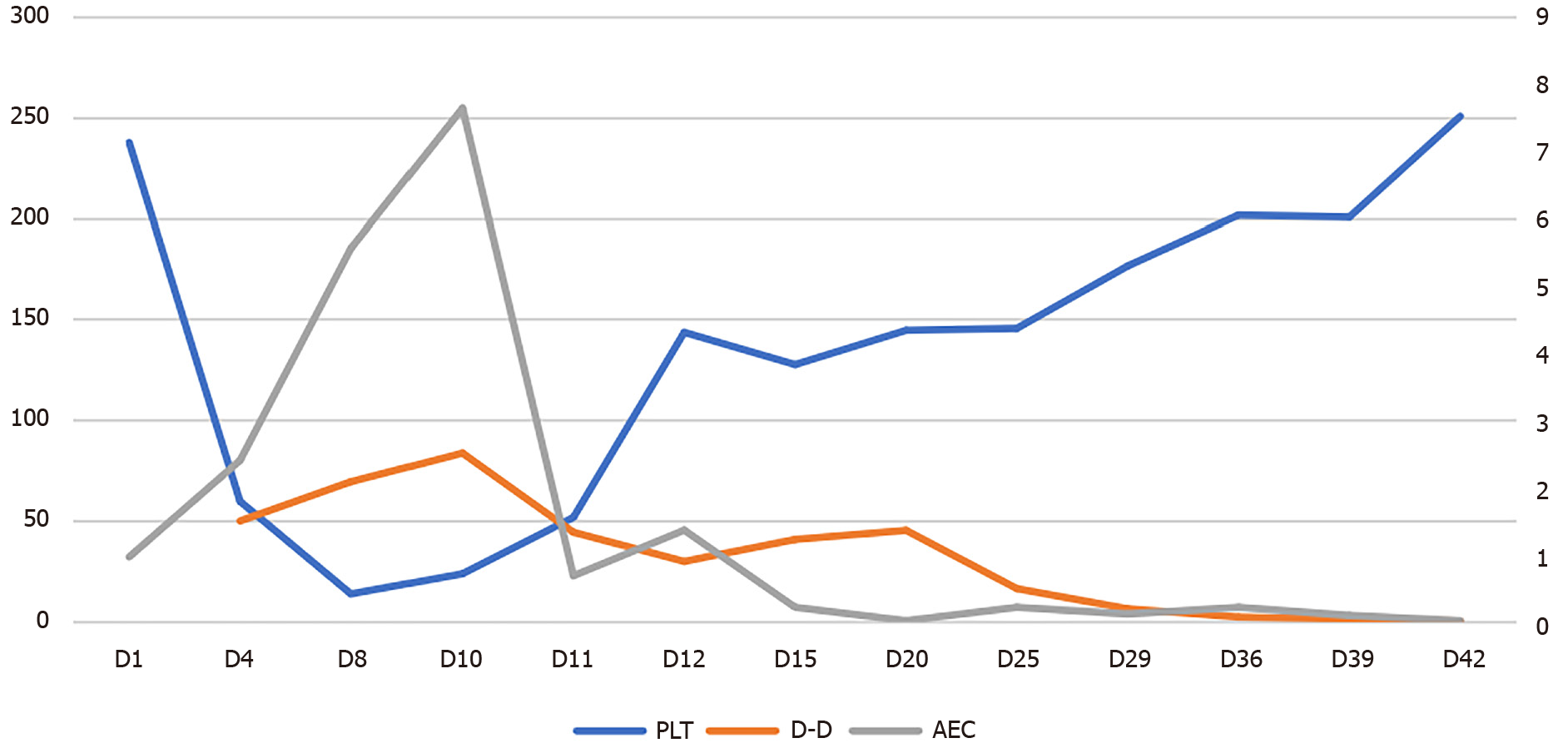
Serology for human immunodeficiency virus, hepatitis viruses, and Treponemapallidum was normal. Bone marrow biopsy showed normal cellularity with increased eosinophils (35%). FIPL1 and platelet-derived growth factor receptor (PDGFR) A/B gene fusion and chromosomal analysis were normal. However, AEC progressively increased up to 7.65 × 109/L, PLT count progressively decreased to a minimum of 14 × 109/L, and D-dimer continuously increased to 69.76 mg/L (Figure 1).
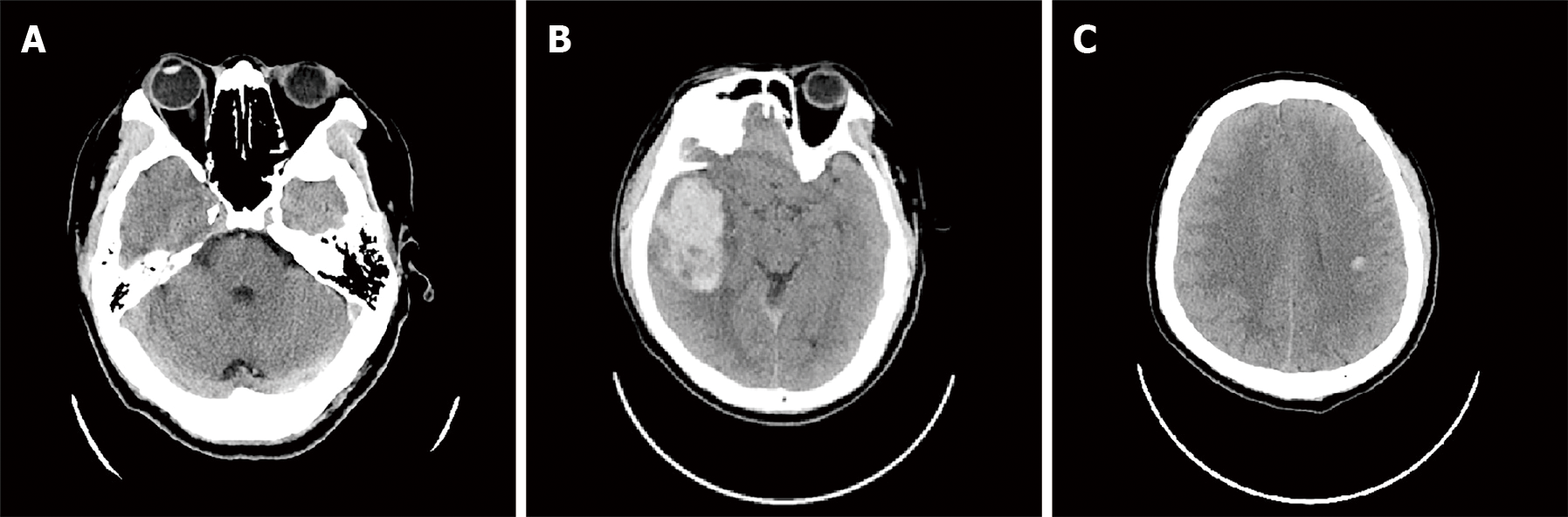
On September 6, 2020, abdominal computed tomography (CT) revealed swelling with peripheral exudation changes in the wall of the transverse and sigmoid colon. On September 9, 2020, brain CT revealed thrombosis of the left jugular vein to the left transverse sinus vein (Figure 2A). Chest CT demonstrated inflammation in both lungs.
Electrocardiography showed significant sinus bradycardia with irregularity, sinus arrest, and frequent borderline escape, with normal myocardial enzymes and troponin. Echocardiography showed no abnormal findings. On September 15, 2020, brain CT revealed right temporal lobe and left centrum semiovale ICH (Figure 2B and C). Chest CT showed bilateral pleural effusion. Duplex ultrasonography showed bilateral intermuscular venous thrombosis of the calf.
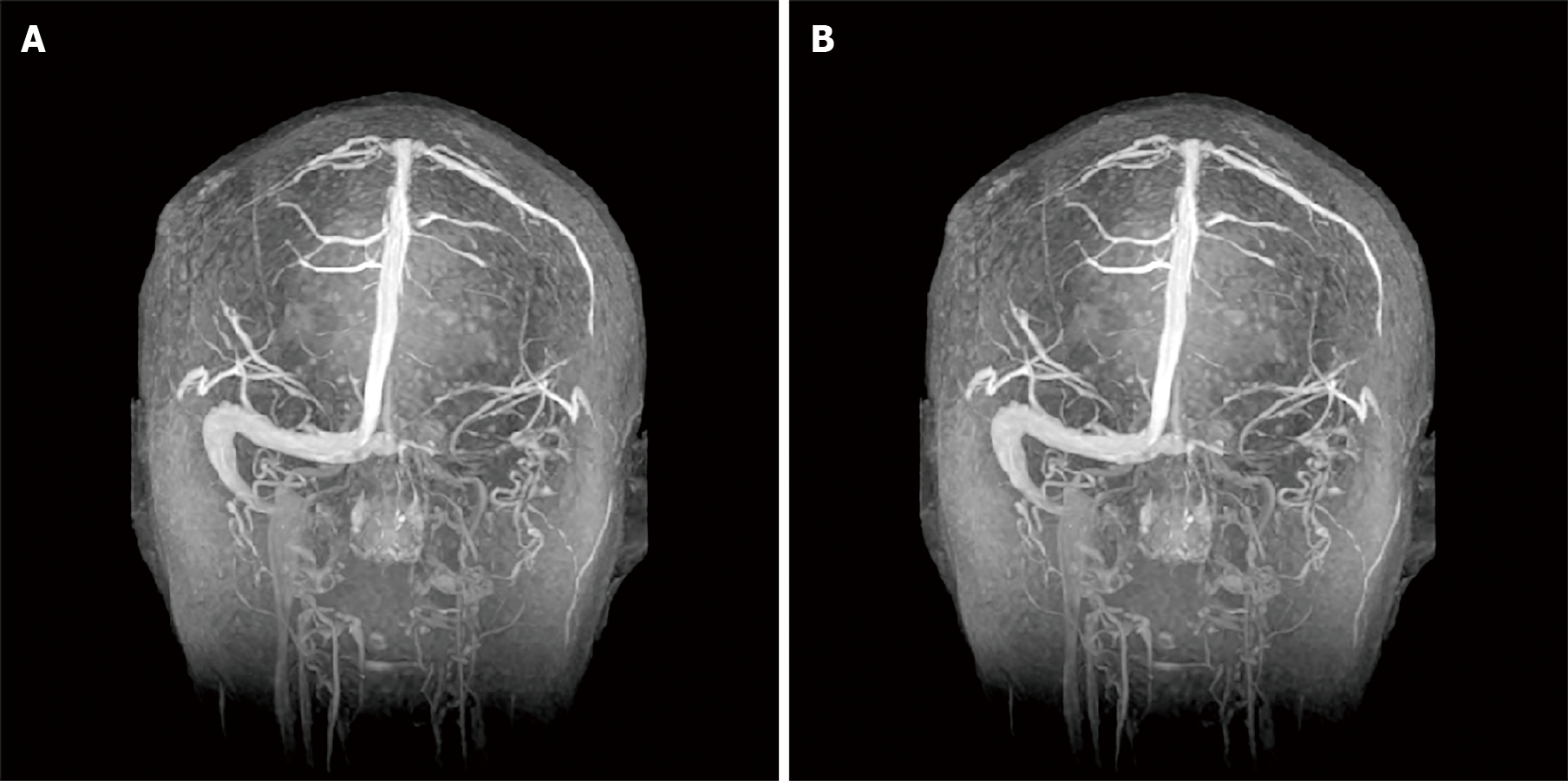
On September 30, 2020, enhanced cranial magnetic resonance venogram showed stenosis of the left internal jugular vein, transverse sinus, sigmoid sinus, confluent sinus, straight sinus, and inferior sagittal sinus (Figure 3A).
HE induced CVST, and the cause of HE was unknown, so the diagnosis changed from HE to idiopathic HE syndrome (HES). Thrombocytopenia may have been related to consumptive reduction by thromboembolism, and ICH occurred secondarily to thrombocytopenia.
The patient was treated with antibiotic agents due to suspected colitis, and low-molecular-weight heparin was initiated after the diagnosis of CVST. When PLT count decreased to 14 × 109/L, we terminated anticoagulation and antibiotic agents and performed PLT transfusion. We used intravenous immunoglobulin (32 g/d for 5 d) for suspected heparin-induced thrombocytopenia (HIT) and prednisolone 80 mg/day due to HE after ICH. Hematoma removal and decompressive craniectomy for right ICH were performed after PLT count increased to 80 × 109/L after PLT transfusion. The patient was treated with anti-coagulation again (heparin and warfarin) 2 wk after surgery. Corticosteroids were gradually tapered, and the total treatment course was 3 mo. Warfarin was continued, and the patient still uses it today.
A 6-mo follow-up showed that the patient did not experience any further symptoms. However, thrombosis in the left internal jugular vein, transverse sinus, sigmoid sinus, and confluent sinus, as well as partial venous thrombosis in the straight sinus and inferior sagittal sinus, was unchanged (Figure 3B).
Eosinophils are multifunctional granular leukocytes that represent approximately 3%–5% of circulating blood leukocytes with an AEC in healthy adults of 0.35–0.5 × 109/L. Eosinophils are normally present in gastrointestinal tract, except in the squamous esophagus, and are important in homeostasis and reconstitution of tissue[9]. Eosinophilia encompasses a broad range of non-hematologic (secondary or reactive) and hematologic (primary or clonal) disorders with potential for end-organ damage[10]. HE is usually linked to allergies, infections (parasitic or fungal), drugs, neoplastic disorders, autoimmune diseases, and atopy[9,10]. When blood HE induced organ damage, the diagnosis changes from HE to HES[9].
Of the cases reported in the literature, three occurred secondarily to eosinophilic granulomatosis, one was secondary to an allergic reaction to a fungus, and three was idiopathic HES. Our case was also idiopathic HES.
Peripheral blood eosinophilia can occur in patients with inflammatory bowel disease[11-13], and peripheral blood eosinophilia may be a biomarker of disease severity. Eosinophilic colitis (EC) should be suspected in any patient with intestinal symptoms with peripheral blood eosinophilia, but EC is a rare condition[14].When accompanied by peripheral blood HE, colitis can occur as an isolated gastrointestinal disorder or as part of HES[15]. Our patient’s colitis may be a part of HES.
Thrombosis is one of the most serious HE-related organ damage. It has been suggested that approximately one-quarter of patients with HES develop thrombo
Thrombocytopenia is more common than thrombocytosis in patients with HE (31% vs 16%, respectively)[24]. The mechanisms of thrombocytopenia in HE are not fully understood, but may be related to consumptive thrombocytopenia caused by thromboembolism. In a previous study, thrombocytopenia was present in five of ten patients with central nervous system involvement but no thrombosis of HE[25]. In our review, two of eight cases reported in the literature developed thrombocytopenia, which may have been underpinned by an immunological mechanism. One case of thrombotic thrombocytopenic purpura with HE was caused by an ADAMTS13 inhibitor[26]. Other cases of HE with an initial presentation of idiopathic thrombocytopenia have been reported[27]. Spontaneous HIT is rare and may be associated with antibodies against platelet factor-4, but it may occur without previous heparin exposure[28,29].
HE with ICH was accompanied by CVST in five of eight cases reported in the literature. Of these, three deaths were observed due to cerebral herniation. Multiple mechanisms may account for ICH. ICH may occur secondarily to CVST or thrombocytopenia, as a side effect of anticoagulant drugs, or due to direct endothelial injury or vasculitis caused by eosinophilic infiltration.
Corticosteroids are a first-line therapy for PDGFRA/B-negative HE[10]. HE can induce CVST, and we need to focus on eosinophil counts in patients with CVST. Early initiation of steroid therapy can potentially prevent disease progression. Persistent eosinophilia is associated with a shorter time to thromboembolism relapse[30], so the goal of therapy is to maintain the eosinophil count below 1500/μL.
HE can induce CVST, and we need to focus on eosinophil counts in patients with CVST. Corticosteroids are a useful first-line therapy for PDGFRA/B-negative HE and HES.
We thank the patient and his family who participated in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A(Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Le PH, Maglangit SACA S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Guo X
| 1. | Schulman H, Hertzog L, Zirkin H, Hertzanu Y. Cerebral sinovenous thrombosis in the idiopathic hypereosinophilic syndrome in childhood. Pediatr Radiol. 1999;29:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Sakuta R, Tomita Y, Ohashi M, Nagai T, Murakami N. Idiopathic hypereosinophilic syndrome complicated by central sinovenous thrombosis. Brain Dev. 2007;29:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Numagami Y, Tomita T, Murakami K, Masaki I, Kubo K, Michiharu N. Sinus thrombosis in idiopathic hypereosinophilic syndrome causing fatal cerebral haemorrhage. J Clin Neurosci. 2008;15:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Ananth S, Sankaralingam R, Manoj M. Aggressive eosinophilic granulomatosis with polyangiitis and transverse sinus thrombosis. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Teresa Sartori M, Briani C, Munari M, Amistà P, Pagnan A, Zampieri P. Cerebral venous thrombosis as a rare onset of Churg-Strauss syndrome. Thromb Haemost. 2006;96:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Kanno H, Ouchi N, Sato M, Wada T, Sawai T. Hypereosinophilia with systemic thrombophlebitis. Hum Pathol. 2005;36:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Chan YC, Ho KH, Chuah YS, Lau CC, Thomas A, Tambyah PA. Eosinophilic meningitis secondary to allergic Aspergillus sinusitis. J Allergy Clin Immunol. 2004;114:194-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Sano H, Fukuoka T, Maruyama H, Hayashi T, Tanahashi N. Cerebral sinovenous thrombosis in a patient with transient eosinophilia. Intern Med. 2014;53:2139-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94:1149-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, Hellmann A, Metzgeroth G, Leiferman KM, Arock M, Butterfield JH, Sperr WR, Sotlar K, Vandenberghe P, Haferlach T, Simon HU, Reiter A, Gleich GJ. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 507] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 11. | Barrie A, Mourabet ME, Weyant K, Clarke K, Gajendran M, Rivers C, Park SY, Hartman D, Saul M, Regueiro M, Yadav D, Binion DG. Recurrent blood eosinophilia in ulcerative colitis is associated with severe disease and primary sclerosing cholangitis. Dig Dis Sci. 2013;58:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Sadi G, Yang Q, Dufault B, Stefanovici C, Stoffman J, El-Matary W. Prevalence of Peripheral Eosinophilia at Diagnosis in Children With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2016;62:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Prathapan KM, Ramos Rivers C, Anderson A, Koutroumpakis F, Koutroubakis IE, Babichenko D, Tan X, Tang G, Schwartz M, Proksell S, Johnston E, Hashash JG, Dunn M, Wilson A, Barrie A, Harrison J, Hartman D, Kim SC, Binion DG. Peripheral Blood Eosinophilia and Long-term Severity in Pediatric-Onset Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Mansoor E, Saleh MA, Cooper GS. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15:1733-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Kuang FL, Curtin BF, Alao H, Piligian B, Berry A, Holland-Thomas N, Powers A, Quezado M, Lumbard K, Fay MP, Klion AD, Kumar S, Khoury P. Single-Organ and Multisystem Hypereosinophilic Syndrome Patients with Gastrointestinal Manifestations Share Common Characteristics. J Allergy Clin Immunol Pract. 2020;8:2718-2726.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Ogbogu PU, Rosing DR, Horne MK 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, Lohse P, Patel KD, Engelmann B. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Uderhardt S, Ackermann JA, Fillep T, Hammond VJ, Willeit J, Santer P, Mayr M, Biburger M, Miller M, Zellner KR, Stark K, Zarbock A, Rossaint J, Schubert I, Mielenz D, Dietel B, Raaz-Schrauder D, Ay C, Gremmel T, Thaler J, Heim C, Herrmann M, Collins PW, Schabbauer G, Mackman N, Voehringer D, Nadler JL, Lee JJ, Massberg S, Rauh M, Kiechl S, Schett G, O'Donnell VB, Krönke G. Enzymatic lipid oxidation by eosinophils propagates coagulation, hemostasis, and thrombotic disease. J Exp Med. 2017;214:2121-2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Venge P, Dahl R, Hällgren R. Enhancement of factor XII dependent reactions by eosinophil cationic protein. Thromb Res. 1979;14:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Rohrbach MS, Wheatley CL, Slifman NR, Gleich GJ. Activation of platelets by eosinophil granule proteins. J Exp Med. 1990;172:1271-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Ojima-Uchiyama A, Masuzawa Y, Sugiura T, Waku K, Fukuda T, Makino S. Production of platelet-activating factor by human normodense and hypodense eosinophils. Lipids. 1991;26:1200-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Slungaard A, Vercellotti GM, Tran T, Gleich GJ, Key NS. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J Clin Invest. 1993;91:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Mukai HY, Ninomiya H, Ohtani K, Nagasawa T, Abe T. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br J Haematol. 1995;90:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Schooley RT, Flaum MA, Gralnick HR, Fauci AS. A clinicopathologic correlation of the idiopathic hypereosinophilic syndrome. II. Clinical manifestations. Blood. 1981;58:1021-1026. [PubMed] |
| 25. | Lee D, Ahn TB. Central nervous system involvement of hypereosinophilic syndrome: a report of 10 cases and a literature review. J Neurol Sci. 2014;347:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Ohguchi H, Sugawara T, Harigae H. Thrombotic thrombocytopenic purpura complicated with hypereosinophilic syndrome. Intern Med. 2009;48:1687-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | van Grotel M, de Hoog M, de Krijger RR, Beverloo HB, van den Heuvel-Eibrink MM. Hypereosinophilic syndrome in children. Leuk Res. 2012;36:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Hwang SR, Wang Y, Weil EL, Padmanabhan A, Warkentin TE, Pruthi RK. Cerebral venous sinus thrombosis associated with spontaneous heparin-induced thrombocytopenia syndrome after total knee arthroplasty. Platelets. 2021;32: 936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, Sztriha LK. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - A report of two UK cases. Brain Behav Immun. 2021;95:514-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Réau V, Vallée A, Terrier B, Plessier A, Abisror N, Ackermann F, Benainous R, Bohelay G, Chabi-Charvillat ML, Cornec D, Desbois AC, Faguer S, Freymond N, Gaillet A, Hamidou M, Killian M, Le Jeune S, Marchetti A, Meyer G, Osorio-Perez F, Panel K, Rautou PE, Rohmer J, Simon N, Tcherakian C, Vasse M, Zuelgaray E, Lefevre G, Kahn JE, Groh M. Venous thrombosis and predictors of relapse in eosinophil-related diseases. Sci Rep. 2021;11:6388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |