Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8498
Peer-review started: May 19, 2021
First decision: June 15, 2021
Revised: June 27, 2021
Accepted: July 30, 2021
Article in press: July 30, 2021
Published online: October 6, 2021
Processing time: 132 Days and 7.3 Hours
Uveal melanoma is the most common primary intraocular malignant tumor affecting the eyes in adults. Nearly half of all primary uveal melanoma tumors metastasize; yet, there are currently no effective treatments for metastatic uveal melanoma. At the time of diagnosis, less than 4% of patients with uveal melanoma have detectable metastatic disease. Uveal melanoma disseminates hematogenously, with the most common site of metastasis being liver (93%), followed by lung (24%) and bone (16%).
A 57-year-old woman was diagnosed with a dysplastic nevus on her eyelid, which was histologically confirmed as malignant melanoma after resection. The patient had no evidence of metastasis to other organs and received both radiation therapy and chemotherapy. After systemic treatment, a metastatic left neck lymph node was found and another round of chemotherapy was performed after resection. Positron emission tomography-Computed Tomography tracking after completion of chemotherapy revealed two metastatic liver nodules. The patient underwent partial liver resection and showed no signs of recurrence at 1 year after surgery.
Surgery is an effective treatment for metastatic uveal melanoma. In patients with liver metastatic lesions, hepatectomy improves outcome.
Core Tip: Uveal melanoma is the most common primary intraocular malignant tumor affecting the eyes in adults. Nearly half of all primary uveal melanoma tumors metastasize; yet, there are currently no effective treatments for metastatic uveal melanoma. We reported a case of malignant melanoma. We found that the surgical approach to treatment of uveal melanoma with liver metastases improves prognosis.
- Citation: Kim YH, Choi NK. Surgical treatment of liver metastasis with uveal melanoma: A case report. World J Clin Cases 2021; 9(28): 8498-8503
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8498
Uveal melanoma is the most common primary intraocular malignant tumor affecting the eyes in adults[1]. While this type of melanoma can affect any part of the uveal tract, the choroid is predominant (86.3%), whereas iris and ciliary body are far less frequently involved[2]. Most patients with uveal melanoma are between 50 and 80 years of age, with the peak occurrence in the 70s[1] and a mean age of 58 years at the time of diagnosis[3]. Exposure to solar ultraviolet radiation is a well-known risk factor for the development of cutaneous melanoma, but evidence of its role in the development of uveal melanoma is inconclusive[4]. Other possible etiological factors include fair skin and oculodermal melanocytosis[5,6].
Because of the lack of lymphatics in the eye itself, uveal melanoma disseminates hematogenously, showing a high propensity for the liver, which accounts for 93% of cases of metastases, followed by the lungs (24%) and bones (16%). It can also metastasize to the brain, skin, and other sites. Indeed, most patients with metastatic disease have metastases at multiple sites[7]. Recent efforts to reduce mortality caused by metastatic uveal melanoma have not been successful. Survival in the presence of metastatic disease is 2–7 mo, depending on the treatment method, and the 1-year overall survival is low, ranging from 13%-29%. The most common contributors to negative prognosis are an age of more than 60 years, male sex, a short interval between primary diagnosis and manifestation of first metastases, and multiple liver metastases[8,9].
Metastatic uveal melanoma requires complex therapy. Treatment most often includes systemic chemotherapy (e.g., dacarbazine or fotemustine) and immunotherapy[9]. However, treatment is complicated by the presence of isolated liver metastases, which necessitate surgery and chemoembolization[8,10].
A 57-year-old woman was admitted for treatment of liver nodules. Three years prior, she had been diagnosed with histologically-confirmed uveal melanoma in the left eye and had been treated with chemotherapy after undergoing resection of the affected eye and upper and lower eyelids. At her 3-year follow-up, metastatic lesions were found on the liver via positron emission tomography (PET) and computed tomography (CT).
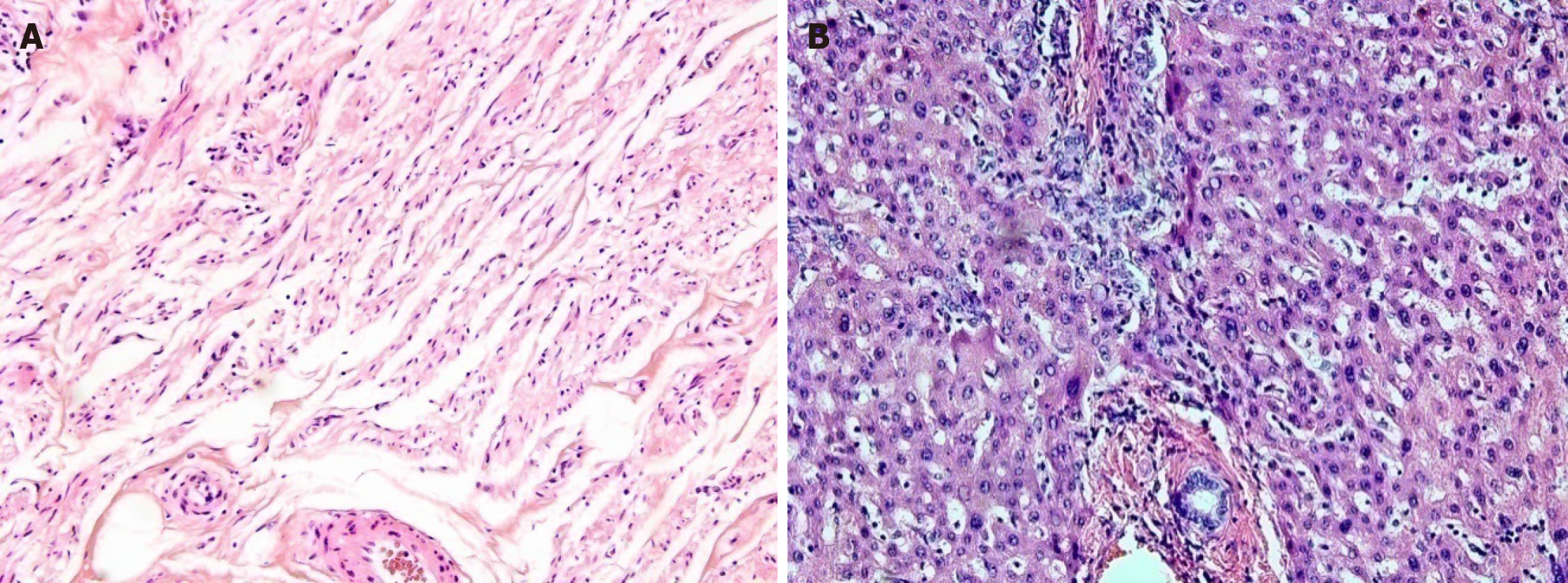
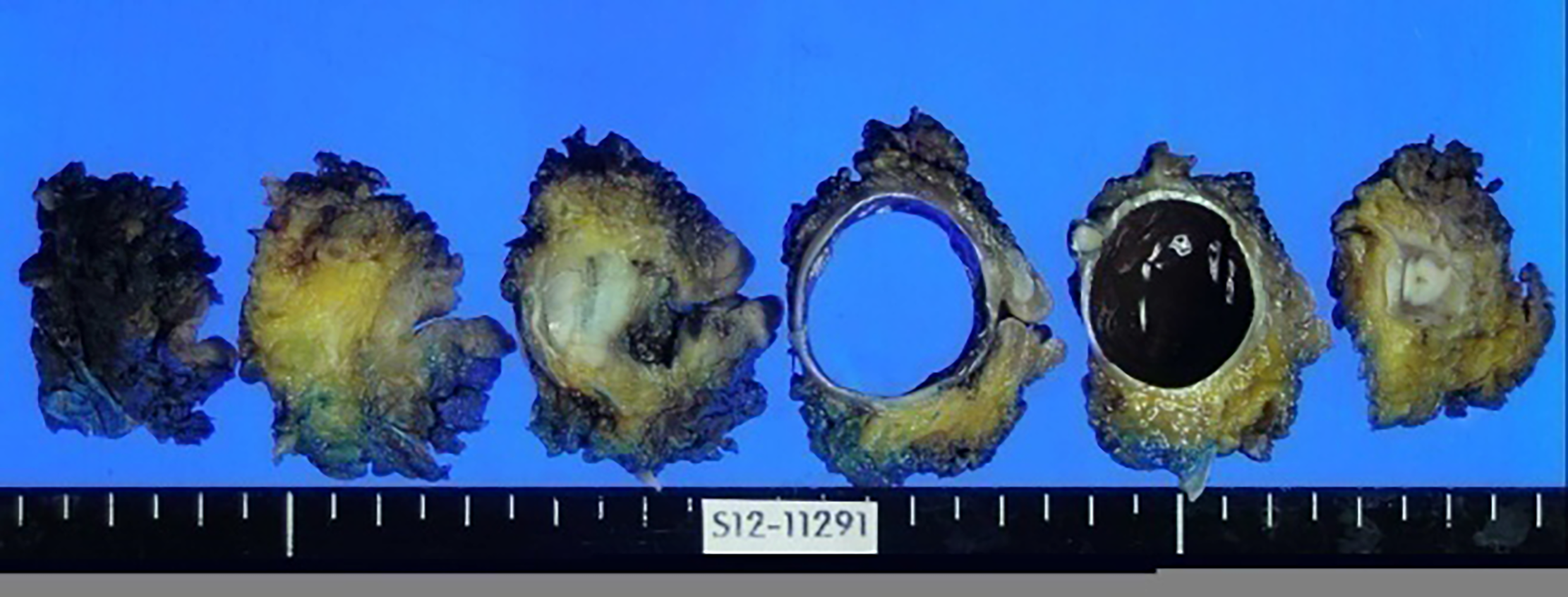
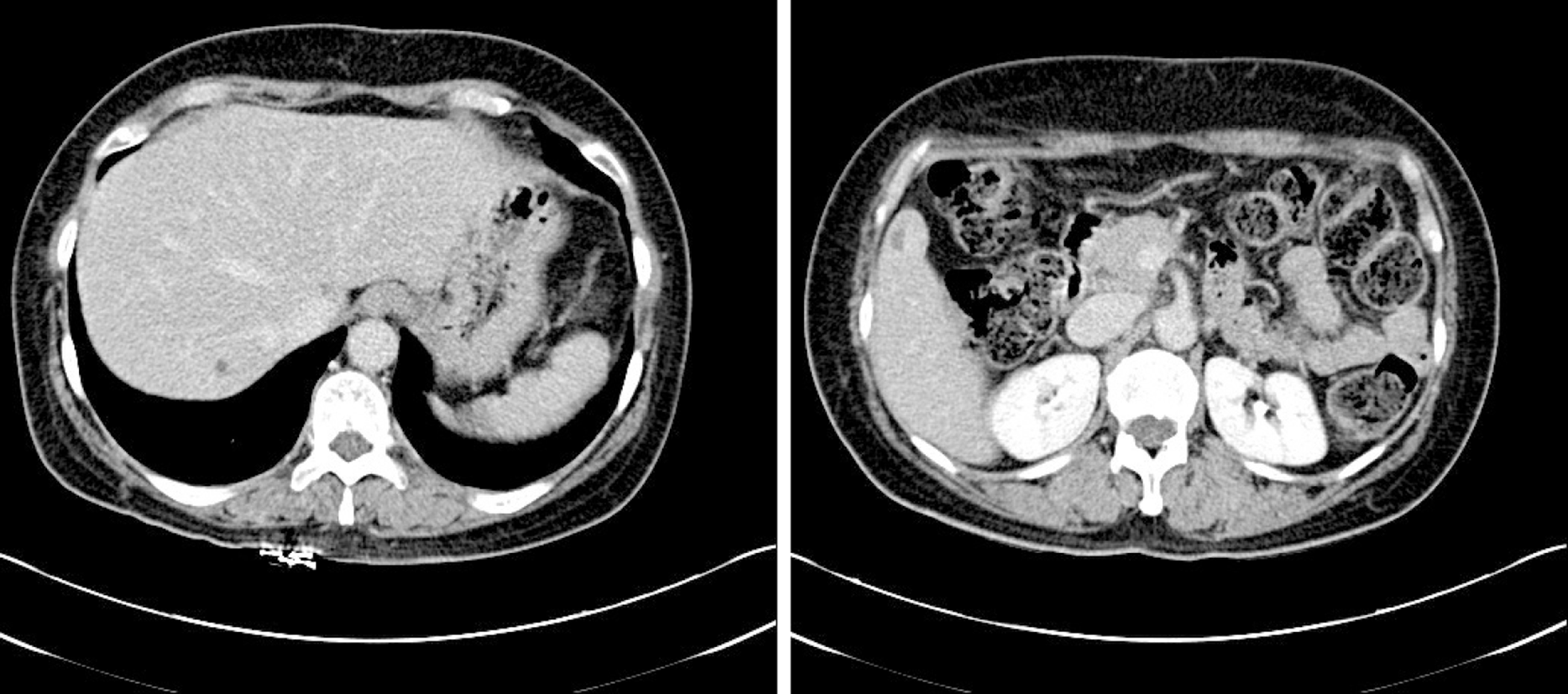
Originally, the patient had visited the outpatient ophthalmology clinic with complaint of left eyeball discomfort. In May 2011, the patient had been diagnosed with dysplastic nevus. Accordingly, a mass was removed from the left eye and confirmed by histology as malignant melanoma (Figure 1A); the patient underwent subsequent treatment by radiotherapy (total 6400 cGY, 32 wk) and chemotherapy (single-agent dacabazine 150 mg/m2 daily for 5 d every 4 wk, four rounds). In December 2012, she underwent total exenteration of the left eye for local invasion of the melanoma (Figure 2), and a follow-up PET-CT revealed metastases in the left jugular lymph nodes. In March 2013, a left radical neck lymph node dissection was performed, after which the patient underwent chemotherapy with high-dose interferon (IFN)-alpha administered on a 2-d interval schedule for 48 wk. Neither abdominal nor thoracic CT showed metastatic lesions in the solid organs or ascites. In June 2014, the patient completed IFN-alpha chemotherapy, but follow-up PET-CT in July 2014 revealed two metastatic nodules in the right lobe of the liver, being 2.5 cm (segment #7) and 2.3 cm (segment #5) in size (Figure 3).
The patient did not have any underlying disease.
The patient had no notable family or personal history.
The patient had no unusual abdominal symptoms.
The whole blood count, serum electrolyte levels, liver function test results, urea nitrogen level, creatinine level, and C-reactive protein level were normal.
Serial PET-CT found hypermetabolic sites. In December 2011, a PET-CT had revealed the metastatic lesion in the left eyelid, and metastases had been detected in the left jugular lymph nodes in December 2012. In June 2014, the follow-up PET-CT revealed two metastatic nodules in the right lobe of the liver (2.5 cm in segment #7 and 2.3 cm in segment #5) (Figure 3).
The clinical features and radiological findings supported a diagnosis of liver metastasis from uveal melanoma.
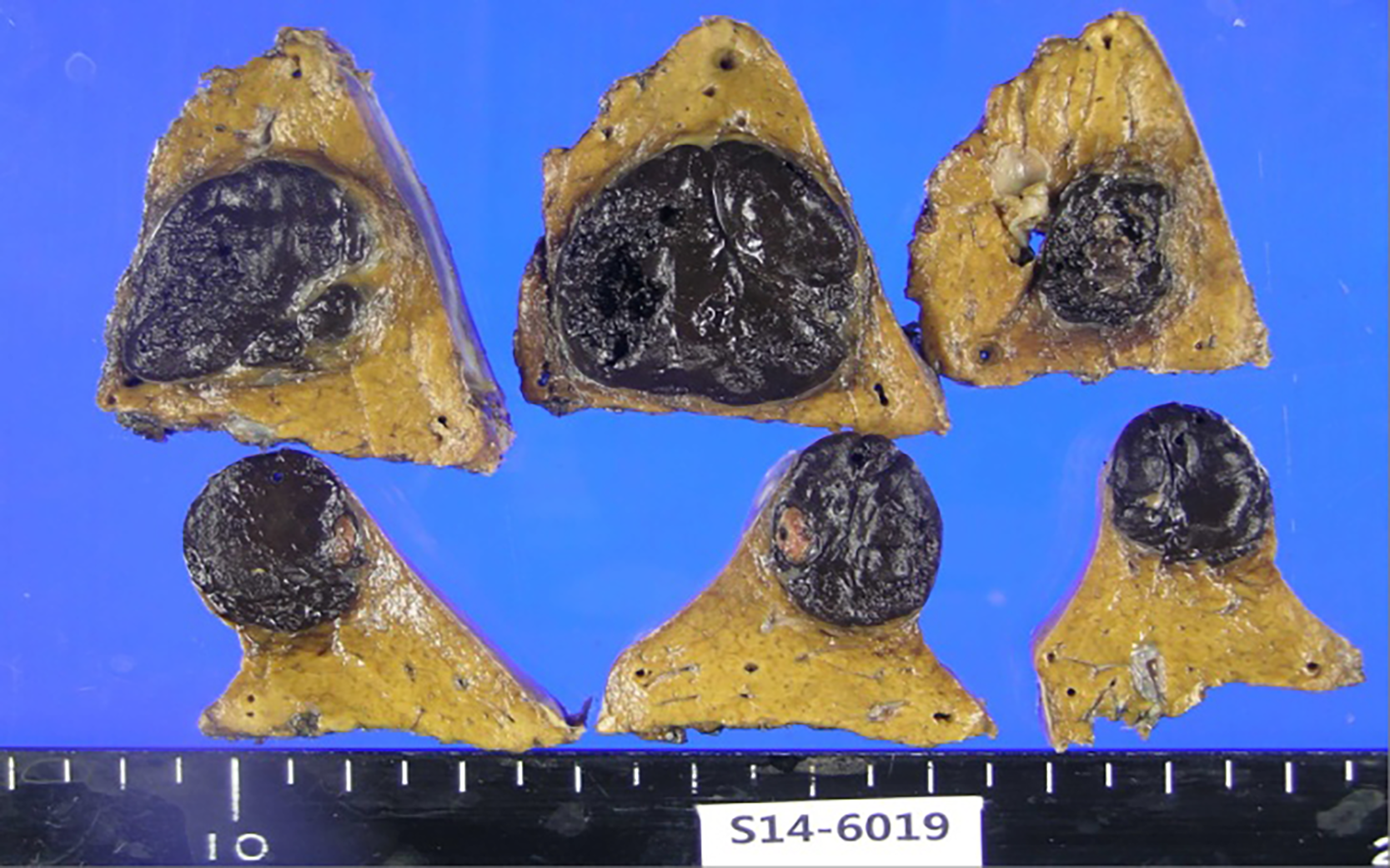
The patient underwent partial hepatectomy with the removal of segments #5 and #7 of the liver in July 2014 (Figure 4). Histological findings confirmed metastatic malignant melanoma, with clear resection margins and no lymphovascular invasion (Figure 1B).
The patient was discharged without postoperative complications. She had no signs of recurrence in the liver 3 mo later on abdominal CT and at 12 mo postoperative on PET-CT. She was lost to follow-up at 18 mo because she did not want any further follow-up care.
We report a case of surgical treatment for uveal melanoma with liver metastasis. The patient was first diagnosed with uveal melanoma in December 2012 and received systemic dacabazine chemotherapy. One year later, she underwent cervical lymph node removal and received IFN-alpha chemotherapy. In July 2014, we detected liver metastasis, and performed a partial hepatectomy. After surgery, she received dacabazine chemotherapy. Although the follow-up had been cut off after 18 mo of chemotherapy, the outcome to that point was good.
The patient had a positive prognosis because she was younger than 60 years of age, female, and had a low-severity liver metastatic burden (Eastern Cooperative Oncology Group performance status 0, normal ranges on liver function tests). Negative prognosis factors for her, however, were the short interval between uveal melanoma diagnosis and the diagnosis of multiple liver metastases. We performed a hepatectomy based on the patient’s performance status and absence of signs of distant metastasis. The results were satisfactory in that the metastatic lesion was removed with clear resection margins and no lymphovascular invasion was detected.
In a large study that enrolled 602 patients with primary uveal melanoma, Kodjikian et al[9] reported that complete surgical removal of metastases with postoperative intra-arterial chemotherapy was more effective for prolonging survival than partial removal of metastases along with postoperative intra-arterial chemotherapy or best supportive care. Other studies have demonstrated that radical resection of up to four to five liver metastases without signs of micrometastases may improve the prognosis of metastatic disease[11]. A French study of 3873 patients with uveal melanoma, including 798 who developed liver metastases, found that time to diagnosis of liver metastasis (< 24 mo after primary diagnosis), nonradical resection of liver metastases (R1–2), more than four liver metastases, and confirmed miliary metastases were all negatively associated with overall survival[12]. Close follow-up of all patients with uveal melanoma is recommended to detect possible metastases at the earliest possible time. Prompt detection of metastasis allows for surgical resection and systemic treatment aimed at complete regression.
As liver metastases are usually hypervascular, chemoembolization or intra-arterial chemotherapy are effective treatments. The reported response rate to chemoembolization is 36%, but survival benefits similar to those of systemic chemotherapy have not been reported[13]. Dacarbazine, cisplatin, fotemustine, or their combinations are frequently used in chemoembolization and intra-arterial chemotherapy[9,13,14]. In our case, the patient was given four courses of systemic single-agent dacarbazine chemotherapy and a 52-wk course of IFN-alpha.
Although we removed the metastatic lesions from the liver and there were no signs of recurrence in our patient, regular follow-up and evaluation should be performed in cases of malignancies to obtain the best possible survival outcomes.
This article reports on the successful surgical treatment of liver metastasis from uveal melanoma. Uveal melanoma itself has a poor prognosis, especially if metastasis is involved. For metastatic melanoma, liver resection may be a good option if liver metastases develop despite systemic treatment. Careful selection and implementation of an optimal treatment plan, within a timely manner, are required to achieve the best outcome for patients with uveal melanoma liver metastasis.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Buiar PG, Takemura N S-Editor: Yan JP L-Editor: A P-Editor: Wang LYT
| 1. | Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 499] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade OA, Mehta S, Forte L, Long A, Dellacava EF, Kaplan B, Shields JA. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 410] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 4. | Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6:1230-1244. [PubMed] |
| 5. | Schmidt-Pokrzywniak A, Jöckel KH, Bornfeld N, Sauerwein W, Stang A. Positive interaction between light iris color and ultraviolet radiation in relation to the risk of uveal melanoma: a case-control study. Ophthalmology. 2009;116:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Singh AD, De Potter P, Fijal BA, Shields CL, Shields JA, Elston RC. Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis. Ophthalmology. 1998;105:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001;119:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 279] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Aoyama T, Mastrangelo MJ, Berd D, Nathan FE, Shields CL, Shields JA, Rosato EL, Rosato FE, Sato T. Protracted survival after resection of metastatic uveal melanoma. Cancer. 2000;89:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Kodjikian L, Grange JD, Baldo S, Baillif S, Garweg JG, Rivoire M. Prognostic factors of liver metastases from uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2005;243:985-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, Pe'er J. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Brasiuniene B, Sokolovas V, Brasiunas V, Barakauskiene A, Strupas K. Combined treatment of uveal melanoma liver metastases. Eur J Med Res. 2011;16:71-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Mariani P, Piperno-Neumann S, Servois V, Berry MG, Dorval T, Plancher C, Couturier J, Levy-Gabriel C, Lumbroso-Le Rouic L, Desjardins L, Salmon RJ. Surgical management of liver metastases from uveal melanoma: 16 years' experience at the Institut Curie. Eur J Surg Oncol. 2009;35:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Rivoire M, Kodjikian L, Baldo S, Kaemmerlen P, Négrier S, Grange JD. Treatment of liver metastases from uveal melanoma. Ann Surg Oncol. 2005;12:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Huppert PE, Fierlbeck G, Pereira P, Schanz S, Duda SH, Wietholtz H, Rozeik C, Claussen CD. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol. 2010;74:e38-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |