Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8441
Peer-review started: February 18, 2021
First decision: April 6, 2021
Revised: April 7, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: October 6, 2021
Processing time: 222 Days and 6.6 Hours
The World Health Organization (WHO) on March 11, 2020, had declared the novel coronavirus disease 2019 (COVID-19) outbreak a global pandemic. The COVID-19 infection continues to be a pandemic and is currently causing overwhelming challenges to healthcare across the nations. Cancer patients represent a unique population vulnerable to COVID-19 infection due to their advanced age, intrinsic frailty, medical comorbidities, immunosuppression, and frequent health care visits for their underlying disease. Robust analysis of COVID-19 infection among cancer patients is crucial to aid in the optimal management of these patients.
To identify contributors of worse outcomes in patients with malignancy and COVID-19 and to describe the role of critical care.
In this review, we summarized the information from seminal articles on the presentation and management of patients with COVID-19 and malignancy that were published before December 10, 2020. We searched the Pub Med and Medline database for “COVID-19” and “Cancer”, “Malignancy”. Studies published in English, including adults with malignancy and COVID-19 infection, were eligible to be included in this review. Studies on patients that provided details on malignancy, clinical presentation, management, and outcome were included. Various details of malignancy that were included are the site of cancer, histopathological type, stage, chemotherapy, and immunotherapy. Details of COVID-19 infection that were obtained are clinical presentation, the modality of testing, imaging, management, and outcome. Critical care details that were obtained were the type of the organ dysfunction and the requirement of organ support measures, requirement of noninvasive, invasive ventilation, management of vasopressor support, and outcome. Articles that did not have patient details, opinions, letters, and articles not published in English were excluded. All articles were reviewed by 2 independent clinicians. Articles were screened for the above terminologies by independent clinicians.
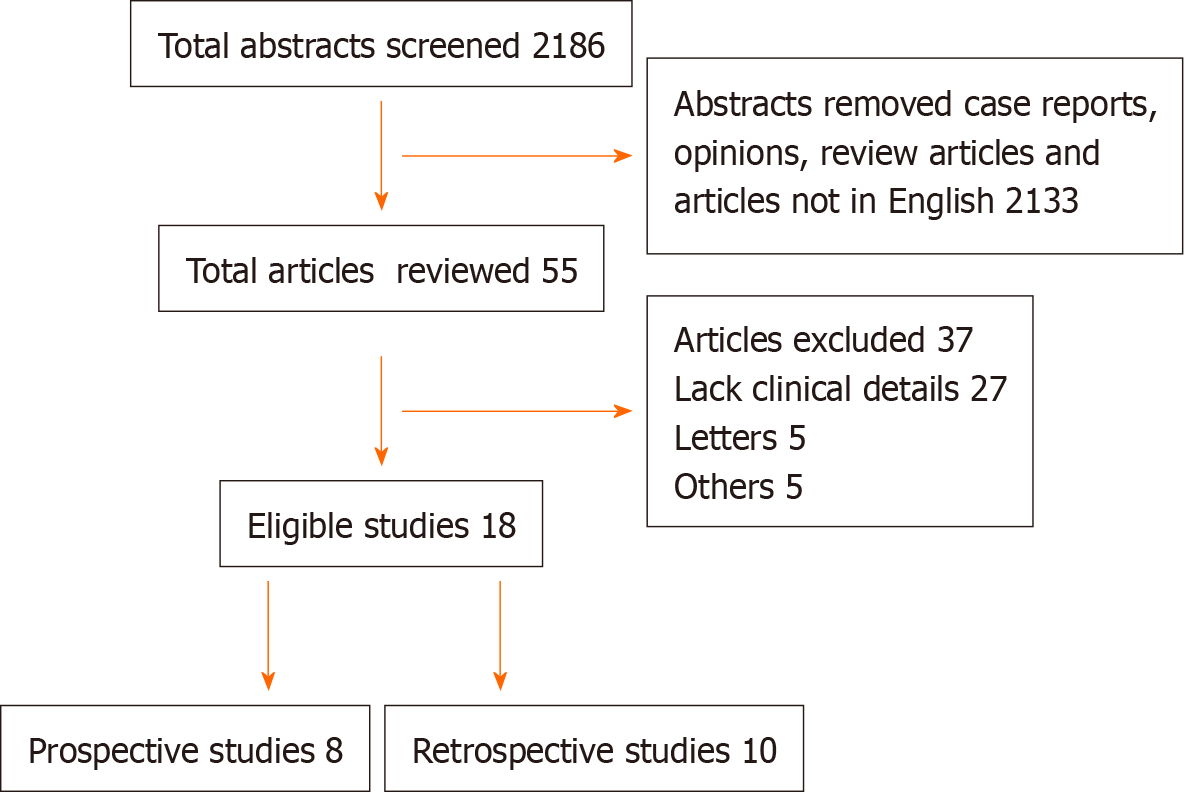
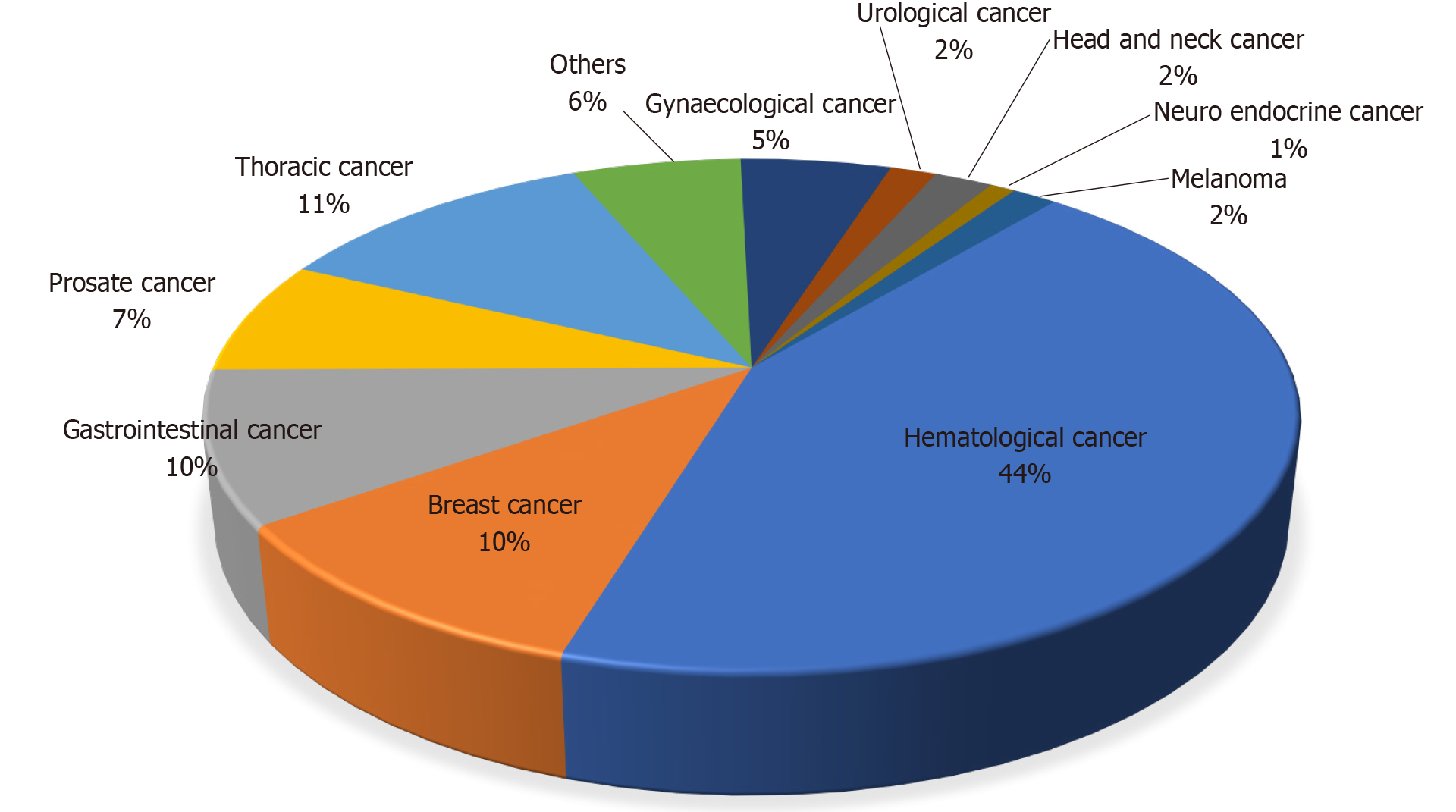
We identified two thousand one hundred eighty-six articles, among which fifty-five were studies that had included patient details pertaining to COVID-19 and cancer (Figure 1). Among these, eighteen studies were eligible and were included in this review as shown in Table 1. A total of 5199 cancer patients were reported. The mean age of patients across all the studies was 64.3 years with male predominance was noted in 12 studies. The clinical presentation and diagnosis of these patients were similar to the general population. Most commonly reported malignancies with COVID-19 infection were hematological in 44% of patients, followed by thoracic malignancy in 11% of patients. The mean number of cancer patients with COVID-19 requiring critical care was 16%. The mean mortality reported was 27.4%. Among the studies that reported the presence of organ dysfunction, respiratory failure was reported in 52% of patients, of which 11.7% required mechanical ventilation. 72% of COVID-19 cancer patients required hospitalization across all the studies. The factors which are associated with the worse outcome from COVID-19 infections among the cancer patients were male gender, age ≥ 65 years, presence of higher comorbidity burden based on Charlson comorbidity index and cumulative illness reporting scale > 6, and smoking history.
The majority of the cancer patients required intensive care due to respiratory failure and the need for mechanical ventilation. Appropriate contingency planning for these patients in terms of goals of care and judicious resource allocation in the resource-poor regions is the key. The factors associated with worse outcomes from COVID-19 infections were independent of oncological features such as tumor stage, disease status, or current provision of active anticancer therapy and it could be continued with caution.
Core Tip: Based on the analyses of 18 studies from major national and international cancer registries, it is evident that among symptomatic coronavirus disease 2019 (COVID-19) cancer patients, approximately one in six patients required intensive level of care, and one in four patients had a fatal outcome. It is crucial to identify factors associated with the worse outcome as it helps to provide prognostic enrichment while discussing the goals of care in this specific patient population. Appropriate contingency planning for these patients in terms of goals of care and judicious resource allocation in the resource-poor regions is the key. Later studies showed an absence of association between mortality from COVID-19 infection and active cytotoxic or noncytotoxic chemotherapy and it could be continued with caution.
- Citation: Ramasamy C, Mishra AK, John KJ, Lal A. Clinical considerations for critically ill COVID-19 cancer patients: A systematic review. World J Clin Cases 2021; 9(28): 8441-8452
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8441.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8441
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes a wide range of illnesses ranging from mild flu-like symptoms to severe respiratory failure leading to death. The novel coronavirus disease 2019 (COVID-19) infection continues to be a pandemic and is currently causing overwhelming challenges to healthcare across the nations. As of December 28th, more than 80 million cases have been diagnosed worldwide with 18 million cases reported from the United States of America[1]. Cancer patients represent a uniquely vulnerable population due to their advanced age, intrinsic frailty, medical comorbidities, immunosuppression, and frequent health care visits for their underlying disease. Existing data suggest that patients with hematological malignancies are more susceptible to SARS-CoV-2 infection[2-5]. The literature on COVID-19 infection in patients with cancer is limited. Robust analysis of outcomes of COVID-19 disease among cancer patients is crucial to aid in the optimal management of these patients during this continuously evolving pandemic. The impact of medical management, the role of critical care, and the multidisciplinary approach in treating patients with malignancy and COVID-19 infection are mostly unknown. In this review, we aimed to study the important epidemiological parameters and predictors of the requirement of acute critical care in patients with malignancy and COVID-19. We also aimed to study the various outcomes as reported in the literature in this subgroup of patients.
In this review, we summarized the information from seminal articles on the presentation and management of patients with COVID-19 and malignancy that were published before December 10, 2020. We searched the Pub Med and, Medline database for “COVID-19” and “Cancer”, “Malignancy”. Studies published in English, including adults with malignancy and COVID-19 infection, were eligible to be included in this review. Studies on patients that provided details on malignancy, clinical presentation, management, and outcome were included. Various details of malignancy that were included are the site of cancer, histopathological type, stage, chemotherapy, and immunotherapy. Details of COVID-19 infection that were obtained are clinical presentation, the modality of testing, imaging, management, and outcome. Critical care details that were obtained were the type of the organ dysfunction and the requirement of organ support measures, requirement of noninvasive, invasive ventilation, management of vasopressor support, and outcome. Articles that did not have patient details, opinions, letters, and articles not published in English were excluded. All articles were reviewed by 2 independent clinicians. Articles were screened for the above terminologies by independent clinicians.
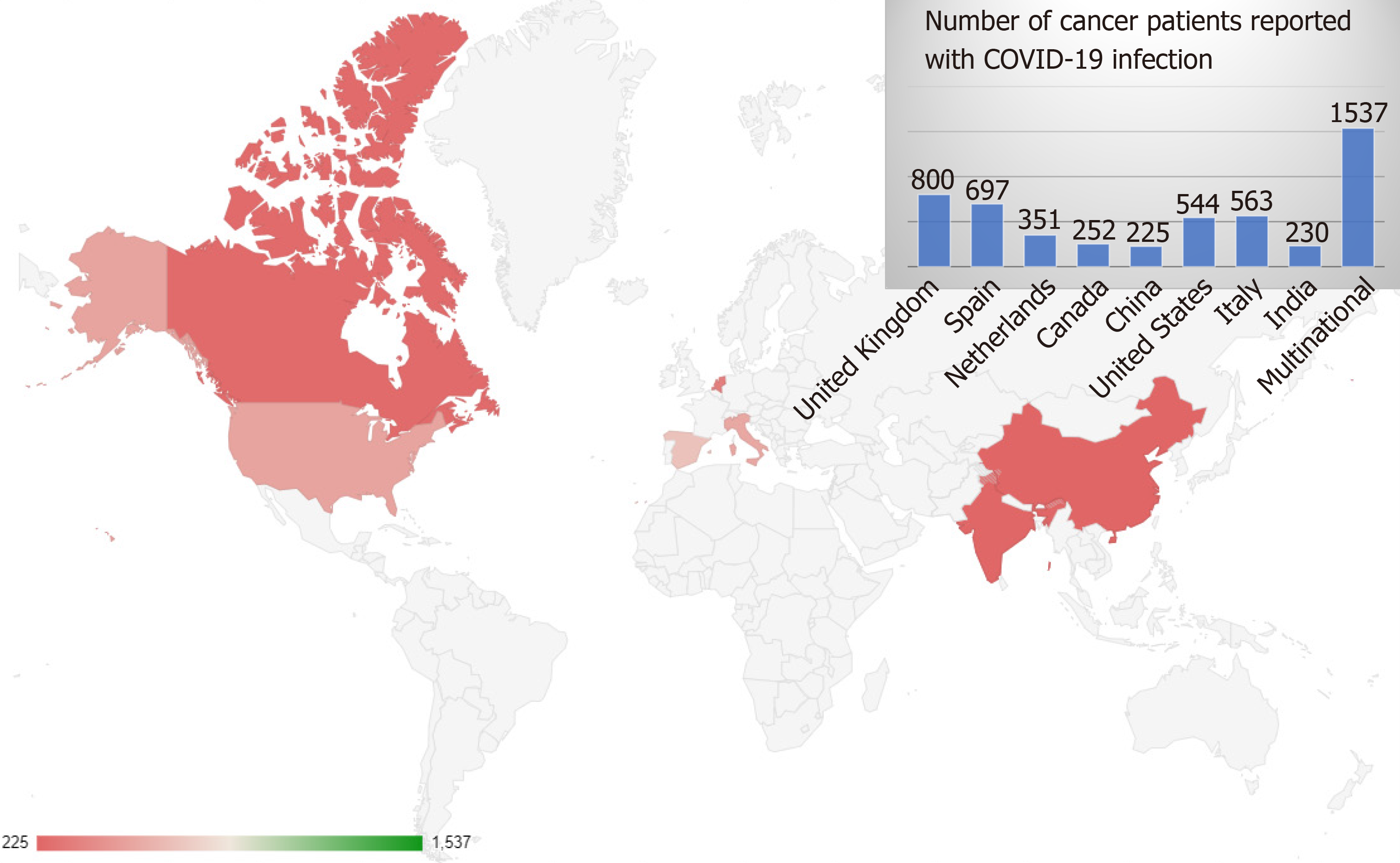
Studies from major national and international cancer registries and large single-center retrospective studies were included and interpreted. We identified two thousand one hundred eighty-six articles, among which fifty-five were studies that had included patient details on COVID-19 and cancer (Figure 1). Among these, eighteen studies were eligible and were included in this review as shown in Table 1. Eight of these studies were prospective in nature. These studies included patients from around eleven countries, from all over the world and five of these studies were multinational as shown in Figure 2. A total of five thousand one hundred ninety-nine patients were reported in the above studies. The mean age of patients across all the studies was 64.3 years. Male predominance among the study participants was noted in twelve (67%) studies.
| Ref. | Country | Study type | Mean age (yr) | Gender | COVID-19 diagnosis | No. of patients | ICU admission rates1 | Mortality rates1 |
| Garassino et al[13] (TERAVOLT) | Multinational (8 countries) | Observational-cross sectional | 68 | M: 141 (70%); F: 59 (30%) | RT-PCR, or imaging | 200 | 13 (9%) | 33% |
| Passamonti et al[2] | Italy | Retrospective | 66.8 | M: 340 (63%); F: 196 (37%) | RT-PCR | 536 | 82 (15%) | 37% |
| García-Suárez et al[3] | Spain | Prospective observational cohort study | 72 | M: 413 (60%); F: 277 (40) | RT-PCR | 697 | 55 (8%) | 33% |
| Lee et al[8] | United Kingdom | Prospective- observational | 69 | M: 449 (56%); F:349 (44%) | RT -PCR | 800 | 53 (7%) | 28% |
| (UKCCMP) | ||||||||
| Mato et al[9] | United States and Europe | Retrospective cohort | 70.5 | M: 125 (63%); F: 73 (37%) | RT-PCR | 198 | 70 (35%) | 33% |
| Fürstenau et al[5] | Europe and Israel | Randomized control trial | 61 | M: 4 (57%); F:3 (43%) | RT-PCR | 7 | 2 (29%) | 29% |
| He et al[4] | China | Case control study | 35 | M: 7 (56%); F: 6 (44%) | RT-PCR or imaging | 13 | NA | 61% |
| Fattizzo et al[30] | Italy | Case series | 77 | M: 10 (63%); F: 6 (37%) | RT-PCR | 16 | 2 (12%) | 31% |
| de Joode et al[14] | Netherlands | Observational cohort study | 70 | M: 187 (53%); F: 164 (47%) | RT-PCR or imaging | 351 | NA | 32% |
| Pinato et al[24] | Europe, United Kingdom, Italy, Spain | Retrospective observational | 69.3 | M: 127 (62%); F:77 (38%) | RT-PCR | 204 | 36 (18%) | 29% |
| Kuderer et al[6] (CCC19) | United States, Canada, Spain | Retrospective | 66 | M: 468 (50%); F: 460 (50%) | RT-PCR | 928 | 132 (14%) | 13% |
| Robilotti et al[21] | United Sates | Retrospective | NA | M: 212 (50%); F: 211 (50%) | RT-PCR | 423 | NA | 12% |
| Ramaswamy et al[11] | India | Prospective observational | 42 | M: 124 (54%); F: 106 (46%) | RT-PCR | 230 | 8 (3%) | 10% |
| Nichetti et al[12] | Italy | Prospective observational | 61 | M: 4 (36%); F: 7 (64%) | RT-PCR | 11 | 1 (9%) | 55% |
| Kuderer et al[6] | Canada | Prospective observational | 73 | M: 127 (50%); F: 150 (50%) | RT-PCR | 252 | 71 (28%) | 13% |
| Lara et al[17] | United States | Retrospective | 64 | F: 121 (100%) | RT-PCR | 121 | 20 (16%) | 14% |
| Zhang et al[16] | China | Retrospective | 66 | M: 60 (56%); F: 47 (44%) | RT-PCR or imaging | 107 | NA | 22% |
| Dai et al[15] | China | Case control study | 64 | M: 57 (55%); F: 48 (46%) | RT-PCR or imaging | 105 | 20 (19%) | 11% |
The four most common presenting symptoms of COVID-19 were fever (64%), cough (61%), fatigue or malaise (43%), and dyspnea (41%) and were similar to the general population[6]. The diagnosis was uniformly established with reverse transcriptase-polymerase chain reaction and/or radiological finding suggestive of COVID-19 infection (100%)[7,8]. However, the diagnosis which was solely based on the clinical symptoms were excluded in these studies due to the high risk of bias. Most commonly reported malignancies with COVID-19 infection were hematological in 44% of patients, followed by thoracic in 11%, gastrointestinal, and breast cancer 10% each (Figure 3).
Fourteen (78%) studies reported the requirement of inpatient critical care management among patients and all of these studies reported mortality (100%). The mean number of cancer patients with COVID-19 requiring critical care was 16% with the minimum and maximum being 3% and 35% respectively. The mean mortality reported was 27.4%, with the minimum and maximum being 10% and 61.5% respectively. The description of the various patterns of organ dysfunction was not uniform. The presence of acute hypoxic respiratory failure and requirement of mechanical ventilation was reported in 9 (50%) of the studies. Renal dysfunction, shock, and cardiac injury were reported in only 4 (22%), 2 (11%), 2 (11%) of the studies. Among the studies that reported the presence of organ dysfunction, respiratory failure was reported in 52% of patients, of which 11.7% required mechanical ventilation. The presence of renal dysfunction, shock, and cardiac injury was noted in 8%, 14.5%, and 4.3% respectively. The requirement of hospitalization was reported in 9 (50%) of the studies, with around 72% of patients requiring hospitalization across all the studies. There were significant differences in the study design, data collection, and measured outcomes among the studies which made the comparison of data difficult.
In this review, we summarized the role of intensive care in patients with COVID-19 and active malignancy. We identified that only very few studies discussed the medical management of sick patients with COVID-19 in the background of active malignancy. We identified that overall, 16% of COVID-19 cancer patients were admitted to the intensive care unit [the specific reasons for intensive care unit (ICU) admission were not available due to lack of data granularity]. The commonest cause of admission to ICU was an acute hypoxemic respiratory failure which is also the cardinal presentation of a typical symptomatic COVID-19 patient[8,9]. The reported intensive care unit admission rates among COVID-19 patients with chronic lymphomatous leukemia was 35 % which was higher than 7% and 14% reported by the United Kingdom Corona
The real burden of COVID-19 among cancer patients was unknown as the studies were done mostly in symptomatic patients requiring treatment. Asymptomatic cancer patients were not routinely tested for COVID-19 due to resource limitations during the initial phase of the pandemic. This is hypothesis-generating and further incites the discussion about how frequently should cancer patients be treated in the absence of symptoms if at all[10,11].
During the initial stages of the COVID-19 outbreak, due to the high risks of infection and limited medical resources, prioritization of certain anticancer treatments over others, temporary chemotherapy discontinuation was routinely practiced in Europe[12]. Initial data showed that chemotherapy was associated with fatal outcomes from COVID-19 infections among the cancer patients but later this association was not confirmed by later studies from national registries[8,13,14]. The United Kingdom cancer registry reported that 172 patients (22% of analyzed subjects) received interruption In their anti-cancer treatment due to potential fear from immunosuppression. They found active chemotherapy has no significant impact on mortality from COVID -19 infection (27% mortality observed on active chemotherapy patients vs 29% on non-chemotherapy patients; P = 0.467) after adjusting for age, comorbidities[8]. The absence of association between mortality and active cytotoxic or noncytotoxic chemotherapy, recent surgery within 4 wk suggests curative surgical resection, adjuvant chemotherapy, and maintenance chemotherapy could be continued during the pandemic with extreme caution[15-17]. Randomized clinical trials are necessary to confirm this hypothesis.
Interestingly, it was reported that thoracic malignancy and chronic lymphomatous leukemia patients with COVID-19 treated with tyrosine kinase inhibitors were less likely to develop severe COVID-19 infection requiring hospitalization, the requirement of oxygen support, and mechanical ventilation[9,13]. However, tyrosine kinase inhibitor does not appear to impact survival[9]. It is hypothesized that tyrosine kinase inhibitors modulate the immune response by blocking pro-inflammatory and chemoattractant cytokines in the lungs thereby mitigating hyperinflammatory immune response. Randomized clinical trials of tyrosine kinase inhibitor for the treatment of COVID-19 are ongoing and will provide more definitive evidence of the effect of these drugs in COVID-19 (NCT04375397 and NCT04380688)[9,18].
Outcomes from SARS-CoV-2 are influenced by ceilings of medical care. In cancer patients, escalation beyond ward-based care requires careful case-by-case evaluation. The decision to provide organ support to acutely ill cancer patients is made even harder in the context of a global pandemic, where saturation of clinical services imposes an often difficult prioritization of critical care resources in favor of younger and less co-morbid critically ill patients[3,6,19].
Dutch Oncology COVID-19 Consortium (DOCC) encouraged early discussion of advanced care planning and treatment strategies (do not intubate) among the vulnerable populations during the ongoing pandemic. They reported more than 80% of patients who had fatal outcomes with COVID-19 had previously discussed with their care team regarding goals of care and had opted for non-heroic measures in acute decompensation from a respiratory failure point of view (do not intubate or do not resuscitate)[14]. In the Netherlands, based on DOCC registry, cancer patients with COVID-19 infections were mainly admitted to the intensive care unit solely for mechanical ventilation. They provided most of the supportive care other than the mechanical ventilation, outside the intensive care unit. Their mortality rate of 32.4% of analyzed patients was comparable to the other national registries[4,6,8,13].
The overall reported mortality among the COVID-19 cancer patients ranges from 12% to 37% which is higher than the general population. UKCCMP, a registry spanning the United Kingdom reported a mortality rate of 28% based on the analysis of 800 cancer patients which was similar to the other European studies [teravolt study group (33%), Italian hematology alliance (37%) and Spain cancer registry (33%)][2,3,8,13]. Whereas the COVID-19 and CCC19, a multi-institution registry mainly from North America reported a mortality rate of 13% based on analysis of 928 patients[6]. This was similar to two single-center retrospective analyses from large healthcare systems in New York City, one at Mount Sinai (11%), another (12%) from analysis of 423 COVID-19 cancer patients at Memorial Sloan Kettering Cancer Center[20,21].
Overall, 7% to 35% of patients were treated in the intensive care unit. The mortality rates seen were as high as 50%[22]. The most common reported reason for the requirement of intensive care unit admission was acute hypoxic respiratory failure needing non-invasive or invasive mechanical ventilation[23]. The reason for intensive care unit admission was not included in most of the analyses and lack of granularity on the data point remains to be the constraint associated with retrospective studies[24]. Information condensed in studies such as above and our extensive review helps to provide prognostic enrichment while discussing the goals of care in this specific patient population.
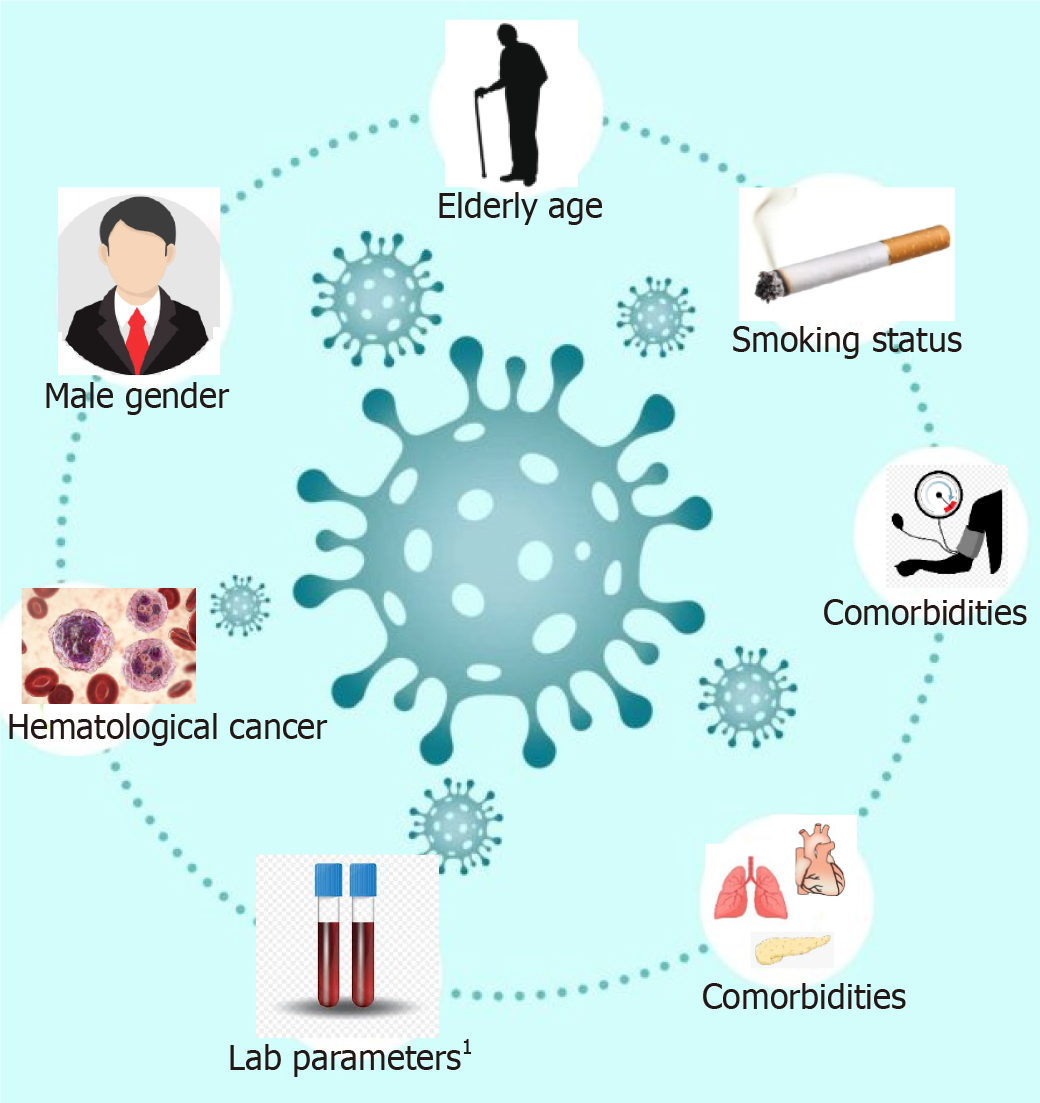
The factors which are associated with the worse outcome from COVID-19 infections among the cancer patients were male gender, age ≥ 65 years, presence of higher comorbidity burden based on Charlson comorbidity index and cumulative illness reporting scale > 6, and smoking history (Figure 4). These factors are significantly associated with the patient’s mortality independent of oncological features such as tumor stage, disease status, or current provision of active anticancer therapy[14,24]. The higher levels of inflammatory markers such as CRP, D-dimer, ferritin, and procalcitonin were associated with severe and critical COVID-19 infections[8,16].
Some of the observations reported in the studies have a biological basis in the pathogenesis of SARS-CoV-2 infection. Male gender and history of smoking are associated with the worse outcome from COVID-19 infection. In the general population, evidence regarding the history of smoking and the risk of COVID-19 infection is equivocal[25,26]. Based on the previous human and animal studies, it has been reported that angiotensin-converting enzyme2 (ACE2) receptor expression is increased among smokers which facilitates the viral entry to cells. The angiotensin-converting-enzyme 2 converts biologically active angiotensin II to angiotensin (1-9) thereby acting as a physiological counter renin-angiotensin-aldosterone system activation. The binding of the virus to the ACE2 receptor leads to its downregulation and reduced clearance of angiotensin II. It has been postulated that acute lung injury secondary to the viral infection could be due to increased levels of unbound angiotensin II and downregulation of ACE2[27].
The SARS-CoV-2 virus also depends on the proteolytic effects of transmembrane serine protease 2 (TMPRSS2)[28]. TMPRSS2 mediated cleavage of viral glycoprotein S results in the fusion of the virus with the host cell membrane. The alveolar expression of TMPRSS2 is androgen-dependent and prostate-specific[29]. The observed gender differences with a worse outcome of COVID-19 could be due to biological differences in the expression of the enzyme in addition to the difference in exposures to the virus (for example high-risk jobs, professional exposure, cigarette smoking).
Passamonti et al[2] and García-Suárez et al[3] based on the descriptive analysis reported that 50% to 62% of severe and critical COVID-19 infections among hematological cancer when compared to 26% to 43% among solid tumors vs 15% among non-cancer. In their analysis, they found that non-survivors had significantly higher baseline D-dimer levels at disease onset than survivors (0.6 mg/L vs 1.3 mg/L, P = 0.03).
Among the COVID-19 hematological malignancy patients, the lower levels of hemoglobin, lymphocyte, and platelet concentrations possibly due to the effect of hematologic therapies or attributable to COVID-19 infection itself which may predict worse outcomes[4].
Our review is timely and has certain strengths such as; it included studies with patients having COVID-19 in the background of active malignancy from major national and international cancer registries. We also attempted to identify the predictors of morbidity, worse clinical outcomes, and the role of intensive care therapy in these patients.
At the same time, our study acknowledges several limitations. There are regional and geographical differences in the threshold for testing COVID-19 infection which may add to the selection bias of not testing the asymptomatic cancer patients. Studies included in this review were retrospective and lack overall granularity of information such as details on the severity of disease, details of malignancy, and details of medical treatment, details of comorbidities, drug interactions, and outcome. Details of COVID-19 infection on the management of malignancy and vice versa were also not uniformly addressed. Details of treatment of COVID-19, duration of therapy, length of hospital stay, the long-term outcome were not uniformly available, especially at the beginning of the pandemic. Details of intensive care treatment including mode of ventilation, vasopressors of choice, renal replacement therapies, the role of sedatives and paralytics on this subgroup of populations were also not discussed. Studies with larger sample size and longer-term follow-up are needed. These limitations may also serve as hypothesis-generating questions for future studies.
As COVID-19 infection continues to affect the outcome of patients with malignancy on treatment, treating physicians need to be aware of the potential differences and contributors to outcome in patients with malignancy and COVID-19. Patients with hematological cancer and lung cancer are more vulnerable to complications of COVID-19 infections[13,14]. Superimposed bacterial infections seem to play an important role in outcomes among the hematological cancer population[4]. Limiting the exposure to novel coronavirus and prioritizing the vaccinations for these vulnerable populations should be considered. So far there is a paucity of focused data with regards to the efficacy and timing of vaccination among active cancer patients getting chemotherapy.
In this review, we identified that in the setting of active malignancy cytotoxic treatments can be continued with caution[8]. Interestingly, cancer patients treated with tyrosine kinase inhibitors were less likely to develop severe forms of COVID-19 infection. Two randomized control trials are ongoing to provide more definitive evidence. Another interesting finding was that majority of the cancer patients required intensive care due to respiratory failure and the need for mechanical ventilation. Appropriate contingency planning for these patients in terms of goals of care and judicious resource allocation in the resource-poor regions is the key.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome-coronavirus 2 has led to an unprecedented global public health crisis. Patients with cancers are particularly suspectable to morbidity and mortality from COVID-19 infection due to their medical risk factors, immune dysfunction, and frequent health care visits for their underlying disease.
To analyze the characteristics of COVID-19 infection among cancer patients which would help treating physicians in optimal management of COVID-19 cancer patients.
In this review article, authors intend to describe the role of critical care in COVID-19 cancer patients and to analyze the various factors which determine the outcome in patients with malignancy and COVID-19.
Authors searched the PubMed and, Medline database for “COVID-19” and “Cancer”, “Malignancy”. Studies published in English, including adults with malignancy and COVID-19 infection, were eligible to be included in this review. We identified two thousand one hundred eighty-six articles, among which eighteen studies were eligible and were included in this review.
A total of 5199 cancer patients were reported. Male predominance was noted in 12 studies. Most reported malignancies with COVID-19 infection were hematological in 44% of patients, followed by thoracic malignancy in 11% of patients. The mean number of cancer patients with COVID-19 requiring critical care was 16%. The mean mortality reported was 27.4%. 72% of COVID-19 cancer patients required hospitalization across all the studies. Majority of the cancer patients required intensive care due to respiratory failure and the need for mechanical ventilation. Male gender, age ≥ 65 years, presence of higher comorbidity burden and smoking history are associated with the worse outcome from COVID-19 infections among the cancer patients. These factors are significantly associated with the patient’s worse outcome independent of oncological features such as tumor stage, disease status, or current provision of active anticancer therapy.
Among symptomatic COVID-19 cancer patients, approximately one in six patients required intensive level of care, and one in four patients had a fatal outcome. It is crucial to identify factors associated with the worse outcome as it helps to provide prognostic enrichment while discussing the goals of care in this specific patient population. Appropriate contingency planning for these patients in terms of goals of care and judicious resource allocation in the resource-poor regions is the key.
In this review, we identified that in the setting of active malignancy cytotoxic treatments can be continued with caution. Cancer patients treated with tyrosine kinase inhibitors were less likely to develop severe forms of COVID-19 infection. Two randomized control trials are ongoing to provide more definitive evidence.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American College of Physicians; Society of Critical Care Medicine; American College of Chest Physicians.
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Atoum M S-Editor: Yan JP L-Editor: A P-Editor: Wang LYT
| 1. | World Health Organization. Coronavirus Disease (COVID-19) Dashboard. [cited 12 February 2021]. Available from: https://covid19.who.int. |
| 2. | Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E, Krampera M, Cairoli R, Della Porta MG, Fracchiolla N, Ladetto M, Gambacorti Passerini C, Salvini M, Marchetti M, Lemoli R, Molteni A, Busca A, Cuneo A, Romano A, Giuliani N, Galimberti S, Corso A, Morotti A, Falini B, Billio A, Gherlinzoni F, Visani G, Tisi MC, Tafuri A, Tosi P, Lanza F, Massaia M, Turrini M, Ferrara F, Gurrieri C, Vallisa D, Martelli M, Derenzini E, Guarini A, Conconi A, Cuccaro A, Cudillo L, Russo D, Ciambelli F, Scattolin AM, Luppi M, Selleri C, Ortu La Barbera E, Ferrandina C, Di Renzo N, Olivieri A, Bocchia M, Gentile M, Marchesi F, Musto P, Federici AB, Candoni A, Venditti A, Fava C, Pinto A, Galieni P, Rigacci L, Armiento D, Pane F, Oberti M, Zappasodi P, Visco C, Franchi M, Grossi PA, Bertù L, Corrao G, Pagano L, Corradini P; ITA-HEMA-COV Investigators. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737-e745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 3. | García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, Hernández-Rivas JÁ, Gil-Manso R, Kwon M, Sánchez-Godoy P, Martínez-Barranco P, Colás-Lahuerta B, Herrera P, Benito-Parra L, Alegre A, Velasco A, Matilla A, Aláez-Usón MC, Martos-Martínez R, Martínez-Chamorro C, Susana-Quiroz K, Del Campo JF, de la Fuente A, Herráez R, Pascual A, Gómez E, Pérez-Oteyza J, Ruiz E, Alonso A, González-Medina J, Martín-Buitrago LN, Canales M, González-Gascón I, Vicente-Ayuso MC, Valenciano S, Roa MG, Monteliu PE, López-Jiménez J, Escobar CE, Ortiz-Martín J, Diez-Martin JL, Martinez-Lopez J; Asociación Madrileña de Hematología y Hemoterapia (AMHH). Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 4. | He W, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y, Li L, Wang H, Chen Z, Li Q, Gale RP. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637-1645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 5. | Fürstenau M, Langerbeins P, De Silva N, Fink AM, Robrecht S, von Tresckow J, Simon F, Hohloch K, Droogendijk J, van der Klift M, van der Spek E, Illmer T, Schöttker B, Fischer K, Wendtner CM, Tausch E, Stilgenbauer S, Niemann CU, Gregor M, Kater AP, Hallek M, Eichhorst B. COVID-19 among fit patients with CLL treated with venetoclax-based combinations. Leukemia. 2020;34:2225-2229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, Del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JT, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL; COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1195] [Cited by in RCA: 1281] [Article Influence: 256.2] [Reference Citation Analysis (0)] |
| 7. | Lal A, Mishra AK, Sahu KK. CT chest findings in coronavirus disease-19 (COVID-19). J Formos Med Assoc. 2020;119:1000-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath SE, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N; UK Coronavirus Monitoring Project Team, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 837] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 9. | Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, Patel K, Osterborg A, Wojenski D, Kamdar M, Huntington SF, Davids MS, Brown JR, Antic D, Jacobs R, Ahn IE, Pu J, Isaac KM, Barr PM, Ujjani CS, Geyer MB, Berman E, Zelenetz AD, Malakhov N, Furman RR, Koropsak M, Bailey N, Hanson L, Perini GF, Ma S, Ryan CE, Wiestner A, Portell CA, Shadman M, Chong EA, Brander DM, Sundaram S, Seddon AN, Seymour E, Patel M, Martinez-Calle N, Munir T, Walewska R, Broom A, Walter H, El-Sharkawi D, Parry H, Wilson MR, Patten PEM, Hernández-Rivas JÁ, Miras F, Fernández Escalada N, Ghione P, Nabhan C, Lebowitz S, Bhavsar E, López-Jiménez J, Naya D, Garcia-Marco JA, Skånland SS, Cordoba R, Eyre TA. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Sahu KK, Mishra AK, Lal A. Comprehensive update on current outbreak of novel coronavirus infection (2019-nCoV). Ann Transl Med. 2020;8:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Ramaswamy A, Nayak L, Roy Moulik N, Sengar M, Chinnaswamy G, Jobanputra K, Shah MJ, Kapoor A, Joshi A, Kumar A, Gokarn A, Bonda A, Cheriyalinkal Parambil B, Prasad M, Bagal B, Dhamne C, Narula G, Jain H, Ghosh J, Thorat J, Bajpai J, Menon N, Khattry N, Bhargava P, Punatar S, Gulia S, Banavali S, Gupta S, Srinivas S, Rath S, Vora T, Noronha V, Patil VM, Ostwal V, Prabhash K. COVID-19 in cancer patients on active systemic therapy - Outcomes from LMIC scenario with an emphasis on need for active treatment. Cancer Med. 2020;9:8747-8753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Nichetti F, Bini M, Ambrosini M, Ottini A, Rametta A, Leporati R, Polastri D, Pircher C, Dotti K, Ferrari L, de Braud F. COVID-19 risk for patients undergoing anticancer treatment at the outpatient clinic of the National Cancer Institute of Milan: the COVINT study. ESMO Open. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, Baena J, Banna G, Berardi R, Bettini AC, Bria E, Brighenti M, Cadranel J, De Toma A, Chini C, Cortellini A, Felip E, Finocchiaro G, Garrido P, Genova C, Giusti R, Gregorc V, Grossi F, Grosso F, Intagliata S, La Verde N, Liu SV, Mazieres J, Mercadante E, Michielin O, Minuti G, Moro-Sibilot D, Pasello G, Passaro A, Scotti V, Solli P, Stroppa E, Tiseo M, Viscardi G, Voltolini L, Wu YL, Zai S, Pancaldi V, Dingemans AM, Van Meerbeeck J, Barlesi F, Wakelee H, Peters S, Horn L; TERAVOLT investigators. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 14. | de Joode K, Dumoulin DW, Tol J, Westgeest HM, Beerepoot LV, van den Berkmortel FWPJ, Mutsaers PGNJ, van Diemen NGJ, Visser OJ, Oomen-de Hoop E, Bloemendal HJ, van Laarhoven HWM, Hendriks LEL, Haanen JBAG, de Vries EGE, Dingemans AC, van der Veldt AAM; DOCC Investigators. Dutch Oncology COVID-19 consortium: Outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1142] [Cited by in RCA: 969] [Article Influence: 193.8] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Wang L, Chen Y, Wu Q, Chen G, Shen X, Wang Q, Yan Y, Yu Y, Zhong Y, Wang X, Chua MLK, Xie C. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;126:4023-4031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 17. | Lara OD, O'Cearbhaill RE, Smith MJ, Sutter ME, Knisely A, McEachron J, Gabor LR, Jee J, Fehniger JE, Lee YC, Isani SS, Wright JD, Pothuri B. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126:4294-4303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, Garcia-Marco JA, Hernández-Rivas JÁ, Mirás F, Baile M, Marquet J, Niemann CU, Reda G, Munir T, Gimeno E, Marchetti M, Quaglia FM, Varettoni M, Delgado J, Iyengar S, Janssens A, Marasca R, Ferrari A, Cuéllar-García C, Itchaki G, Špaček M, De Paoli L, Laurenti L, Levin MD, Lista E, Mauro FR, Šimkovič M, Van Der Spek E, Vandenberghe E, Trentin L, Wasik-Szczepanek E, Ruchlemer R, Bron D, De Paolis MR, Del Poeta G, Farina L, Foglietta M, Gentile M, Herishanu Y, Herold T, Jaksic O, Kater AP, Kersting S, Malerba L, Orsucci L, Popov VM, Sportoletti P, Yassin M, Pocali B, Barna G, Chiarenza A, Dos Santos G, Nikitin E, Andres M, Dimou M, Doubek M, Enrico A, Hakobyan Y, Kalashnikova O, Ortiz Pareja M, Papaioannou M, Rossi D, Shah N, Shrestha A, Stanca O, Stavroyianni N, Strugov V, Tam C, Zdrenghea M, Coscia M, Stamatopoulos K, Rossi G, Rambaldi A, Montserrat E, Foà R, Cuneo A, Ghia P. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354-2363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 19. | Mishra AK, Sahu KK, George AA, Lal A. A review of cardiac manifestations and predictors of outcome in patients with COVID - 19. Heart Lung. 2020;49:848-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, Cruz C. Do patients with cancer have a poorer prognosis of COVID-19? Ann Oncol. 2020;31:1088-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 21. | Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, Bogler Y, Caldararo M, Figueroa CJ, Glickman MS, Joanow A, Kaltsas A, Lee YJ, Lucca A, Mariano A, Morjaria S, Nawar T, Papanicolaou GA, Predmore J, Redelman-Sidi G, Schmidt E, Seo SK, Sepkowitz K, Shah MK, Wolchok JD, Hohl TM, Taur Y, Kamboj M. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 22. | Domecq JP, Lal A, Sheldrick CR, Kumar VK, Boman K, Bolesta S, Bansal V, Harhay MO, Garcia MA, Kaufman M, Danesh V, Cheruku S, Banner-Goodspeed VM, Anderson HL 3rd, Milligan PS, Denson JL, St Hill CA, Dodd KW, Martin GS, Gajic O, Walkey AJ, Kashyap R; Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group. Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2021;49:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 23. | Lal A, Mishra AK, John K, Akhtar J. Corticosteroids and rehabilitation in COVID-19 survivors. J Formos Med Assoc. 2021;120:1284-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Pinato DJ, Lee AJX, Biello F, Seguí E, Aguilar-Company J, Carbó A, Bruna R, Bower M, Rizzo G, Benafif S, Carmona C, Chopra N, Cruz CA, D'Avanzo F, Evans JS, Galazi M, Garcia-Fructuoso I, Dalla Pria A, Newsom-Davis T, Ottaviani D, Patriarca A, Reyes R, Sharkey R, Sng CCT, Wong YNS, Ferrante D, Scotti L, Avanzi GC, Bellan M, Castello LM, Marco-Hernández J, Mollà M, Pirisi M, Ruiz-Camps I, Sainaghi PP, Gaidano G, Brunet J, Tabernero J, Prat A, Gennari A. Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Usman MS, Siddiqi TJ, Khan MS, Patel UK, Shahid I, Ahmed J, Kalra A, Michos ED. Is there a smoker's paradox in COVID-19? BMJ Evid Based Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | Lowe KE, Zein J, Hatipoglu U, Attaway A. Association of Smoking and Cumulative Pack-Year Exposure With COVID-19 Outcomes in the Cleveland Clinic COVID-19 Registry. JAMA Intern Med. 2021;181:709-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med. 2020;382:2049-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1844] [Cited by in RCA: 1869] [Article Influence: 373.8] [Reference Citation Analysis (0)] |
| 28. | Mollica V, Rizzo A, Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020;16:2029-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 29. | Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020;382:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1484] [Cited by in RCA: 1565] [Article Influence: 313.0] [Reference Citation Analysis (0)] |
| 30. | Fattizzo B, Giannotta JA, Sciumè M, Cattaneo D, Bucelli C, Fracchiolla NS, Onida F, Baldini L, Barcellini W, Iurlo A. Reply to "COVID-19 in persons with haematological cancers": a focus on myeloid neoplasms and risk factors for mortality. Leukemia. 2020;34:1957-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |