Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7593
Peer-review started: May 6, 2021
First decision: June 6, 2021
Revised: June 16, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: September 6, 2021
Processing time: 116 Days and 18.9 Hours
Infliximab (IFX) is an anti-tumor necrosis factor alpha (TNF-α) agent that is widely used for the management of a variety of autoimmune and inflammatory diseases, including Crohn's disease (CD). As a result of its increasing administration, new complications have emerged. Hemorrhagic pericardial effusion, secondary to IFX therapy, is a rare but life-threatening complication.
A 27-year-old man was diagnosed with CD (Montreal A2L3B1) 6 years prior. After failing to respond to mesalazine and methylprednisolone, he took the first dose of IFX 300 mg based on his weight (60 kg, dose 5 mg/kg) on December 3, 2018. He responded well to this therapy. However, on January 21, 2019, 1 wk after the third injection, he suddenly developed dyspnea, fever, and worsening weakness and was admitted to our hospital. On admission, computed tomo
This is a case of hemorrhagic pericardial effusion following treatment with IFX. It is a rare but life-threatening complication of IFX. Early recognition helps prevent the occurrence of hemorrhagic pericardial effusion and minimize the impact on the natural evolution of the disease.
Core Tip: Pericarditis or pericardial effusion is a rare but serious complication of infliximab (IFX), especially cardiac tamponade, and most patients have rheumatoid arthritis and drug-induced lupus erythematosus. This is a case of hemorrhagic pericardial effusion following treatment with IFX. The patient was diagnosed with Crohn's disease. A high titer of anti-IFX antibodies and a strong type III immunologic reaction may be a possible cause. This suggests that clinicians need to pay attention to the occurrence of pericardial effusion in patients treated with IFX. Early recognition helps prevent the occurrence of hemorrhagic pericardial effusion and minimize its impact on the natural evolution of the disease.
- Citation: Li H, Xing H, Hu C, Sun BY, Wang S, Li WY, Qu B. Hemorrhagic pericardial effusion following treatment with infliximab: A case report and literature review . World J Clin Cases 2021; 9(25): 7593-7599
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7593.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7593
Anti-tumor necrosis factor alpha (TNF-α) agents including infliximab (IFX), adalimumab, golimumab, and certolizumab pegol are widely used in the management of a variety of autoimmune and inflammatory diseases. Crohn’s disease (CD) is characterized by chronic inflammation that mainly targets the gastrointestinal tract. Due to increased administration of anti-TNFα agents, new complications have been reported such as infections (tuberculosis, viral, bacterial and fungal infections), systemic lupus erythematosus-like reactions, arterial and venous thromboembolism, pericarditis, and pneumonitis[1-4].
Here, we present a rare case of a 27-year-old male patient with hemorrhagic pericardial effusion secondary to IFX therapy for CD. This complication disappeared following discontinuation of IFX. We also review the literature. We searched the literature using the keywords “pericarditis OR pericardial effusion OR pleuropericarditis OR cardiac tamponade AND infliximab” and found that pericarditis or pericardial effusion, is a rare but serious complication of IFX, especially cardiac tamponade. To enhance our understanding of this complication, we analyzed cases of IFX-associated pericardial complications reported in the literature.
A 27-year-old man had intermittent abdominal pain and mucosanguineous feces for 6 years. He was admitted to our hospital with complaints of dyspnea, fever, and worsening weakness.
The patient was diagnosed with CD (Montreal A2L3B1) 6 years previously. He had suffered from mucosanguineous feces, abdominal pain, and an anal fistula for 6 mo. Colonoscopy, biopsy, and multi-slice computed tomography (CT) enterography were performed. Negative blood tests were observed and no opportunistic infections were found. He was prescribed mesalazine 3 g/d for 1 year without complications but primary symptoms were only partially relieved. One year later, his symptoms of abdominal pain and loose stool were aggravated, and gastroscopy showed involvement of the upper gastrointestinal tract. He was treated with oral methylprednisolone 48 mg/d for 10 d. The dose was reduced by 4 mg every 2 wk without any improvement in symptoms. He continued to take mesalazine 2 g/d. In December 2018, reexamination with colonoscopy and biopsy showed stenosis of the sigmoid colon accompanied by numerous new ulcers. He was examined at another hospital and from then to the present, no extraintestinal manifestations occurred. The patient was advised by the doctor to undergo IFX therapy and he stopped taking mesalazine after excluding tuberculosis and other viral or bacterial infections. On December 3, 2018, he took the first dose of IFX 300 mg based on his weight (60 kg, dose 5 mg/kg). After 2 and 6 wk, he received the second and third IFX injection and a maintenance dose was planned every 8 wk thereafter. He responded well to this therapy, his symptoms disappeared and he had formed stools, and colonoscopy was planned after four doses of IFX. However, on January 21, 2019, 1 wk after the third injection, he suddenly developed dyspnea, fever, and worsening weakness and was admitted to our hospital.
The patient had no previous medical history, and no history of chest trauma or cardiac procedure.
The patient had no family history
He was febrile, tachycardiac (120 bpm), and normotensive. Our clinical considerations were heart disease or pulmonary embolism.
An electrocardiogram on admission was negative for acute changes and his troponin I and creatine kinase-MB levels were normal. There were no abnormalities in his white cell count or platelet count, and the coagulation index was normal. Viral titers including Epstein-Barr virus, cytomegalovirus, coxsackie B virus, human immunodeficiency virus and bacterial serologies were negative. A T-spot test was also negative. Blood, stool, and urine cultures were all negative. Laboratory examinations were remarkable for an elevation in C-reactive protein (CRP) to 65.24 mg/L (reference < 5.00 mg/L) and an elevated erythrocyte sedimentation rate of 48 mm/h. The procalcitonin level was normal.
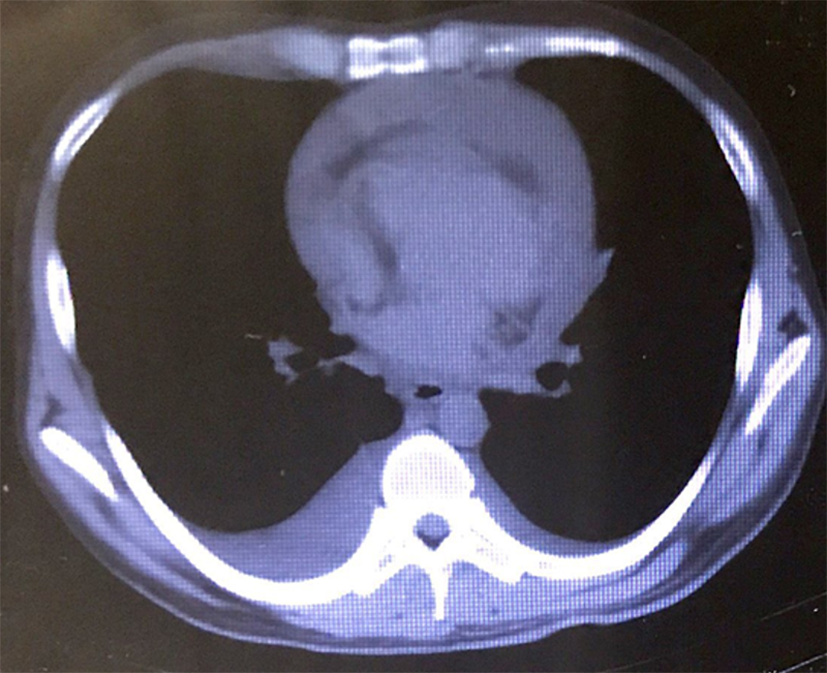
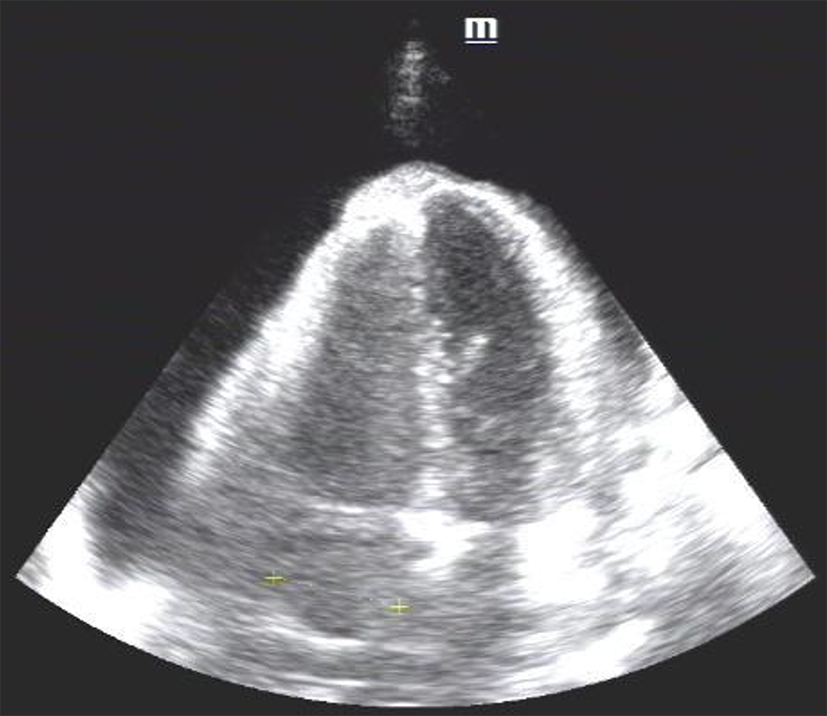
CT scan of the chest revealed a large pericardial effusion and a small right-side pleural effusion (Figure 1), and excluded the diagnosis of pulmonary embolus. An echocardiogram showed a large pericardial effusion and normal left ventricular function (Figure 2). Then successful ultrasound-guided pericardiocentesis was performed and 600 mL hemorrhagic fluid was drained.
The final diagnosis of the presented case was hemorrhagic pericardial effusion secondary to IFX therapy. Although we have not seen similar symptoms in previously treated patients, the IFX instruction manual for injection does mention pericardial effusion as a rare adverse reaction (≥ 1/10000, < 1/1000), and similar cases have been previously reported. Due to the absence of documented infection or other identifiable causes of hemorrhagic pericardial effusion, the specific time frame of IFX infusion, and no recurrence in the absence of IFX therapy, a diagnosis of hemorrhagic pericardial effusion secondary to IFX therapy was made.
The patient discontinued IFX and received supportive treatment for 3 d.
Along with a decrease in pericardial effusion, the patient’s fever and shortness of breath also gradually resolved. The patient was discharged on the 7th d with a plan to stop IFX therapy in the future. The patient was followed every 3 to 6 mo till April 2021. The total follow-up time is 26 mo. At present, his symptoms have disappeared, the fecal test and occult blood are negative, and CRP is less than 10 mg/L. Colonoscopy showed scars and a few pseudopolyps, and echocardiography showed no pericardial effusion.
Inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) are common primary diseases treated with IFX[5-8]. Pericardial effusion following IFX treatment is a rare complication. Seventeen reported cases of pericardial complications during IFX therapy, including our patient, are summarized in Table 1. Of nine reported cases with IBD, hemorrhagic pericardial effusion occurred in two patients[9], including our patient. All reported cases of cardiac tamponade due to IFX occurred in IBD patients[1-3,9]. Elderly patients with RA seem to suffer from more severe complications. It seems that these patients tend to have a high titer of anti-IFX antibodies and a strong type III immunologic reaction, and failure of IFX therapy for extra-articular manifestations may be responsible. The duration of IFX treatment varies from 1 wk to 6 years, with an average of 72.9 wk. For clinical presentation, fatigue, dyspnea, and chest pain are common in these cases. Besides, heart failure, fever, and swelling are also potential warning signs.
| Cases | Age (yr) | Gender | Primary disease | Pericardial complications | Clinical presentation | Duration of IFX Treatment | Follow-up treatment | Outcome |
| 1 | 68 | Female | RA | Transudate, constrictive | Right heart failure: leg edema and anorexia | 40 d | Infliximab discontinuation, pericardiocentesis | Pericardial effusion not increased |
| 2 | 57 | Female | RA | Hemorrhagic | Shortness of breath, right heart failure | 14 mo | Pericardiocentesis | Recurrent disease, pericardiectomy |
| 3 | 70 | Female | RA | Infective pericarditis | Cardiac failure, fatigue, dyspnea | 6 yr | Stop etanercept, pericardiocentesis and pericardial drain, antibiotics | White cell count normalized, CRP fallen |
| 4 | 75 | Female | RA | - | Dyspnea | 4 yr | Steroid | Readmitted with recurrent symptoms, pericardiocentesis |
| 5 | 60 | Female | RA | - | Cardiovascular collapse | 4 mo | Pericardiocentesis, steroid | Clinical symptoms and pericardial effusion improved |
| 6 | 45 | Male | RA | Hemorrhagic, DILE | Fever, cough, polyarthralgia, chest pain | 22 wk | Infliximab discontinuation, steroid | Clinical symptoms and pericardial effusion improved |
| 7 | 57 | Male | RA | Purulent | Collapse | 3 wk | Pericardiocentesis, antibiotics, infliximab discontinuation | Fenestration for residual pericardial effusion |
| 8 | 40 | Male | RA | - | Chest pain, chest discomfort | 15 mo | Antibiotics, steroid | Recurrent disease, pericardiectomy |
| 9 | 51 | Female | UC | DILE | Chest pain, shortness of breath, fatigue | 1 wk | Infliximab discontinuation, steroid | Clinical symptoms improved |
| 10 | 48 | Female | UC | Cardiac tamponade | Chest pain, dyspnea | 1 mo | Infliximab discontinuation, pericardiocentesis, diuresis | No recurrence or persistent cardiac dysfunction |
| 11 | 59 | Male | UC | Non-infectious | Left-sided chest pain | 1 mo | Infliximab discontinuation, NSAIDS, adalimumab | No recurrence of pericarditis |
| 12 | 60 | Male | UC | Hemorrhagic, cardiac tamponade | Shortness of breath, diffuse joint swelling and aches, general malaise | 1 wk (infliximab was reintroduced) | Infliximab discontinuation, antibiotics | Progressive shortness of breath and recumbent chest pain, pericardiocentesis, steroid and pericardial drain were performed, pericardial effusion decreased |
| 13 | 41 | Male | UC | DILE, cardiac tamponade | Pleuritic chest pain, dyspnea | 19 mo | Infliximab discontinuation, steroid | No residual pericardial effusion |
| 14 | 30 | Female | CD | Fibrinous, cardiac tamponade | Pleuritic chest pain, dyspnea | 12 mo | Infliximab discontinuation, pericardial window, mediastinal chest tube, steroid | No recurrence of symptoms of pericarditis |
| 15 | 57 | Male | CD | Purulent, cardiac tamponade | Chest pain, fever, general malaise | 8 yr | Infliximab discontinuation, pericardial window, antibiotics | Asymptomatic |
| 16 | 39 | Male | CD | DILE | Pleuritic chest pain, nausea, weakness | 8 mo | Steroid | Severe arthralgia when used infliximab one more time; no recurrence of lupus-like symptoms after infliximab discontinuation |
| 171 | 27 | Male | CD | Hemorrhagic | Dyspnea, fever and worsening weakness | 7 wk | Infliximab discontinuation, pericardiocentesis | Clinical symptoms and pericardial effusion improved |
Three cases had infections with RA or CD[2,10]. Given that IFX has an immuno-suppressive effect, this may play a role in an increased risk of infection. Drug-induced lupus erythematosus (DILE) occurred in 4 reported cases of RA, ulcerative colitis, or CD[3,4,11,12]. However, in 5 cases, pericardial fluid was not drained to identify its properties[13-15]. Patients between 50 and 60 years of age had the highest incidence of complications, and those with RA had the greatest number of complication types.
IFX is a monoclonal anti-TNFα antibody, which is widely used in IBD and other immune disorders. Patients with pericardial effusion during anti-TNFα therapy are rare, and mostly have RA and DILE[1,3,16,17]. In our patient, he had neither RA nor DILE. In this case, the association between the onset of symptoms after initiating IFX therapy and resolution of symptoms after discontinuing IFX was evident, and no other medication was administered at the same time. In addition, there was no evidence of infection. All these factors suggest that IFX infusion was the most likely etiology of hemorrhagic pericardial effusion in this patient. In 17 reported cases, most underwent performed IFX discontinuation and pericardiocentesis, including ours, and all of their clinical symptoms and pericardial effusion improved. On the other hand, one reported case did not stop IFX, and only steroid was given to control the DILE condition; severe arthralgia occurred when IFX was used one more time. Therefore, IFX discontinuation and pericardiocentesis would be effective treatments. Meanwhile, steroid, adalimumab and antibiotics for infection could also be appropriate options.
The exact mechanism of IFX-induced hemorrhagic pericardial effusion has not been clearly identified. IFX is a mouse/human chimera and monoclonal IgG1 antibody, 35% mouse-derived and 65% human-derived, and can join the variable regions of mouse antibodies to the constant regions of human IgG1. As a result of this partly murine composition and strong antigenicity, IFX might trigger an immunogenic reaction and antibody production. A high titer of anti-IFX antibodies and a strong type III immunologic reaction may be a possible cause of pericardial effusion. On the other hand, high titer of anti-IFX antibodies may decrease the drug concentration and effect, due to rapid clearance by newly produced antibodies. Therefore, we speculate that the high titers of anti-IFX antibodies in this patient, led to an autoimmune reaction to anti-TNFα agents, and the process mimicked infection. As anti-TNFα antibodies could not be used as a treatment option, further control and management of CD was challenging. With pseudopolyps and scars, he is now in the remission stage, and an intense surveillance and endoscopic examination has been scheduled.
With the increasing use of anti-TNFα agents for CD, many novel complications are being reported. Beside of biological agents, other categories being considered for the treatment CD include thalidomide, immunosuppressants, steroids, and enteral nutrition. Due to the heterogenetic mouse antigen, IFX is not always the best choice for patients, and other types of anti-TNFα agents with homologous proteins or biologicals with different targets could be considered.
Physicians should increase their awareness of this rare but life-threatening complication of IFX. Early recognition helps prevent the occurrence of hemorrhagic pericardial effusion and minimize the impact on the natural evolution of the disease.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta R, Iwashima S S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Li X
| 1. | Naseer M, Kulairi Z, Kam M. Cardiac Tamponade as a Presenting Manifestation of Infliximab-Induced Lupus in Patient Treated for Crohn's Disease. ACG Case Rep J. 2017;4:e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Harnett DT, Chandra-Sekhar HB, Hamilton SF. Drug-induced lupus erythematosus presenting with cardiac tamponade: a case report and literature review. Can J Cardiol. 2014;30:247.e11-247.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Burke JP, Kelleher B, Ramadan S, Quinlan M, Sugrue D, O'Donovan MA. Pericarditis as a complication of infliximab therapy in Crohn's disease. Inflamm Bowel Dis. 2008;14:428-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Nakamura Y, Izumi C, Nakagawa Y, Hatta K. A case of effusive-constrictive pericarditis accompanying rheumatoid arthritis: The possibility of adverse effect of TNF-inhibitor therapy. J Cardiol Cases. 2013;7:e8-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Taylor GK, Elliott L, Sosin MD, Soo SS. Complication of etanercept treatment for rheumatoid arthritis--purulent pericarditis caused by a commensal organism. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Soh MC, Hart HH, Corkill M. Pericardial effusions with tamponade and visceral constriction in patients with rheumatoid arthritis on tumour necrosis factor (TNF)-inhibitor therapy. Int J Rheum Dis. 2009;12:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Devasahayam J, Pillai U, Lacasse A. A rare case of pericarditis, complication of infliximab treatment for Crohn's disease. J Crohns Colitis. 2012;6:730-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Lather HD, Kahlenberg JM. Hemorrhagic Pericardial Effusion with Tamponade: A Rare Adverse Effect of Infliximab-Case Report and Literature Review. Case Rep Rheumatol. 2016;2016:2576496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Harney S, O'Shea FD, FitzGerald O. Peptostreptococcal pericarditis complicating anti-tumour necrosis factor alpha treatment in rheumatoid arthritis. Ann Rheum Dis. 2002;61:653-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Benucci M, Li Gobbi F, Fossi F, Manfredi M, Del Rosso A. Drug-induced lupus after treatment with infliximab in rheumatoid arthritis. J Clin Rheumatol. 2005;11:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Mirza M, Mirza M, Murugesan V, Olano A. Pericardial Effusion due to Infliximab Therapy for Ulcerative Colitis. Case Rep Gastrointest Med. 2018;2018:4324592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Ambrose NL, O'Connell PG. Anti-TNF alpha therapy does not always protect rheumatoid arthritis patients against developing pericarditis. Clin Exp Rheumatol. 2007;25:660. [PubMed] |
| 14. | O’Morain N, Kumar L, O’Carroll-Lolait C, Alakkari A, Ryan B. Infliximab Induced Cardiac Tamponade. Ir Med J. 2019;3:902. [PubMed] |
| 15. | Edwards MH, Leak AM. Pericardial effusions on anti-TNF therapy for rheumatoid arthritis--a drug side effect or uncontrolled systemic disease? Rheumatology (Oxford). 2009;48:316-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Hall SJ, Hickling P. Failure of etanercept to control extra-articular manifestations of rheumatoid arthritis. J Clin Rheumatol. 2007;13:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |