Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7579
Peer-review started: March 31, 2021
First decision: June 7, 2021
Revised: June 7, 2021
Accepted: July 29, 2021
Article in press: July 29, 2021
Published online: September 6, 2021
Processing time: 152 Days and 18.3 Hours
Breast adenoid cystic carcinoma (AdCC) is a rare invasive carcinoma composed of epithelial and myoepithelial cells. Microglandular adenosis (MGA) is a rare benign proliferative lesion consisting of small, uniform, and round glands formed by a single layer of epithelial cells and basement membrane without a myoepi
A 59-year-old woman was diagnosed with a newly developed density on a routine mammogram. The density was similar to or slightly lower than that of the breast parenchyma. Sonography showed an irregular mass with a slightly higher echo than that of fat. Magnetic resonance imaging showed an irregular mass with a similar T1 signal intensity and a slightly higher T2 signal intensity compared to muscles or the breast parenchyma. The lesion showed heterogeneous internal enhancement with an initially slow and delayed persistent enhancing pattern. Microscopically, the tumor was composed of invasive AdCC, in situ AdCC, and MGA. AdCC is composed of basaloid and ductal epithelial cells forming cribriform or solid sheets, or haphazardly scattered small cribriform or tubular glands. MGA showed small glands with a single epithelial lining and retained lumen. S-100 staining was strongly positive in MGA area. The patient underwent breast-conserving surgery with sentinel lymph node biopsy.
Breast AdCC arising in MGA showed unique imaging findings that was different from usual invasive cancer.
Core Tip: Many pathological or clinical studies have been reported for adenoid cystic carcinoma (AdCC) arising in microglandular adenosis (MGA), but few reports have been reported of radiological findings. In our case, it was characterized by iso- or slight hypo-density in the mammogram and slightly higher echo than that of fat in the ultrasound examination with higher T2 signal intensity and a persistent enhancing pattern in breast magnetic resonance imaging. Although AdCC shows a favorable prognosis and MGA has long been considered a benign entity, there is a risk of MGA becoming malignant and a complete resection should be performed.
- Citation: An JK, Woo JJ, Kim EK, Kwak HY. Breast adenoid cystic carcinoma arising in microglandular adenosis: A case report and review of literature. World J Clin Cases 2021; 9(25): 7579-7587
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7579.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7579
Adenoid cystic carcinoma (AdCC), a rare breast cancer, accounts for 0.1%-3.5% of all breast tumors. It is known to have a good prognosis[1-5]. AdCC is composed of two main neoplastic cells: (1) Altered myoepithelial cells grouped in nests or outlining characteristic cribriform space; and (2) Epithelial cells lining small ductule-like true lumina[6,7]. AdCC belongs to ‘rare and salivary gland-type tumors’ among epithelial tumors of the breast in World Health Organization (WHO) classification[2]. Recently, it has been reported that microRNA expression profile of breast AdCC differs from that of salivary gland AdCC[7]. It helps us understand different clinical behaviors of tumors depending on the original site.
Microglandular adenosis (MGA) is a rare benign proliferative lesion consisting of small, uniform, and round glands formed by a single layer of epithelial cells[8,9]. These glands are surrounded by basement membranes without myoepithelial cell layers. Absence of the myoepithelial layer is a characteristic feature that distinguishes MGA from ductal and lobular histology of the breast. Infiltrative proliferation of these glands and absence of the myoepithelial layer may mimic invasive breast carcinoma[8]. Although MGA is a benign entity, it may progress to atypical MGA and carcinoma arising in MGA[10-14]. Various invasive carcinomas arising in MGA have been reported. However, AdCC and its imaging findings have been rarely reported[12,15]. In this study, we report a case of AdCC arising in MGA.
A 59-year-old woman visited our hospital for a routine breast checkup. Since she first had a breast examination at our hospital in 2017, she has been undergoing regular checkups every year. Last year, her breast examination showed negative findings. However, a mammography (MG) performed this year revealed a new density.
She is currently on medication for high blood pressure and diabetes, and has no other breast symptoms.
She had no history of breast surgery.
She had no family history of cancer, including breast cancer.
The breast lesion was not clearly palpable on physical examination.
No specific laboratory examinations were performed for breast lesion in this patient.
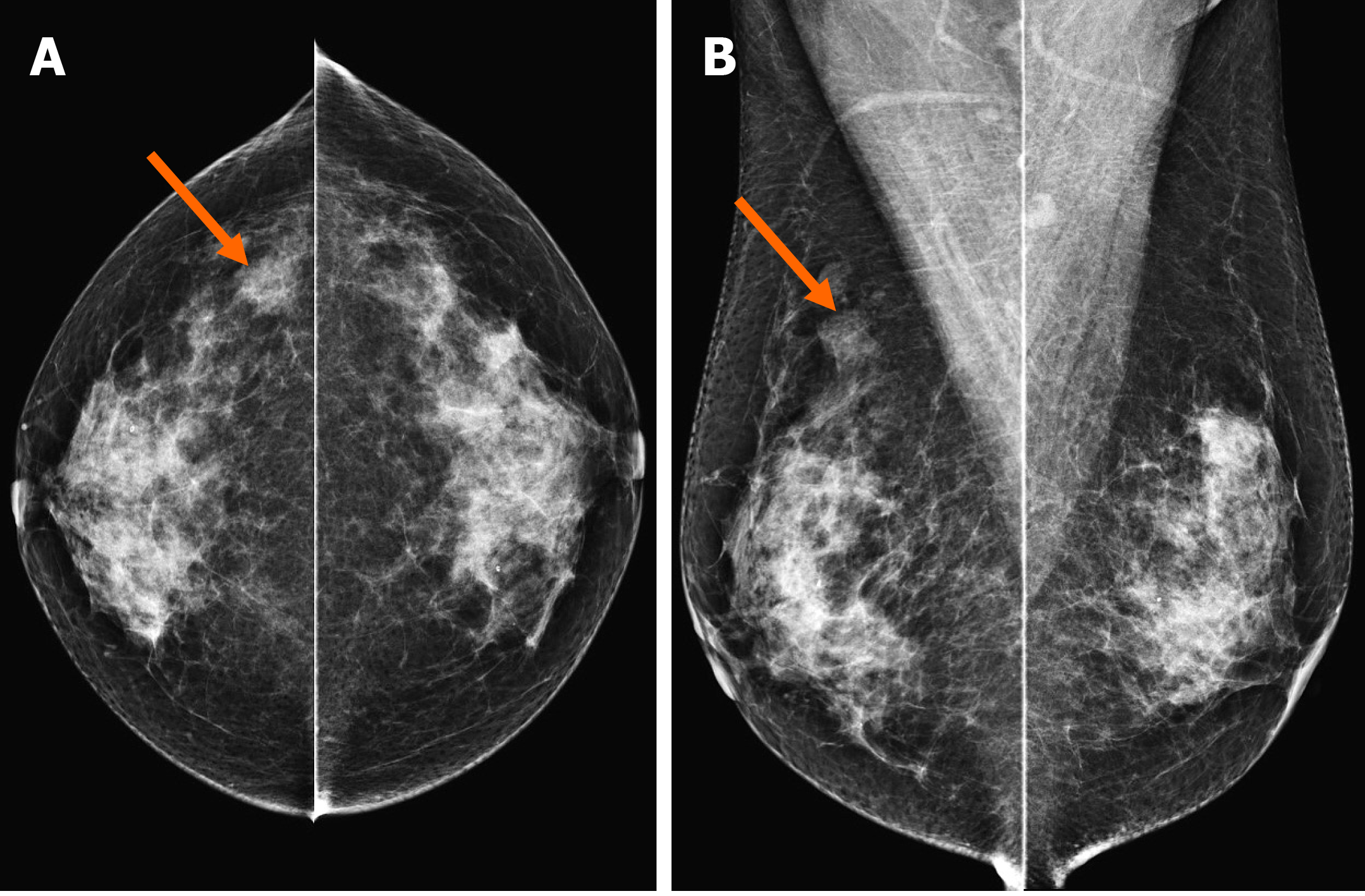
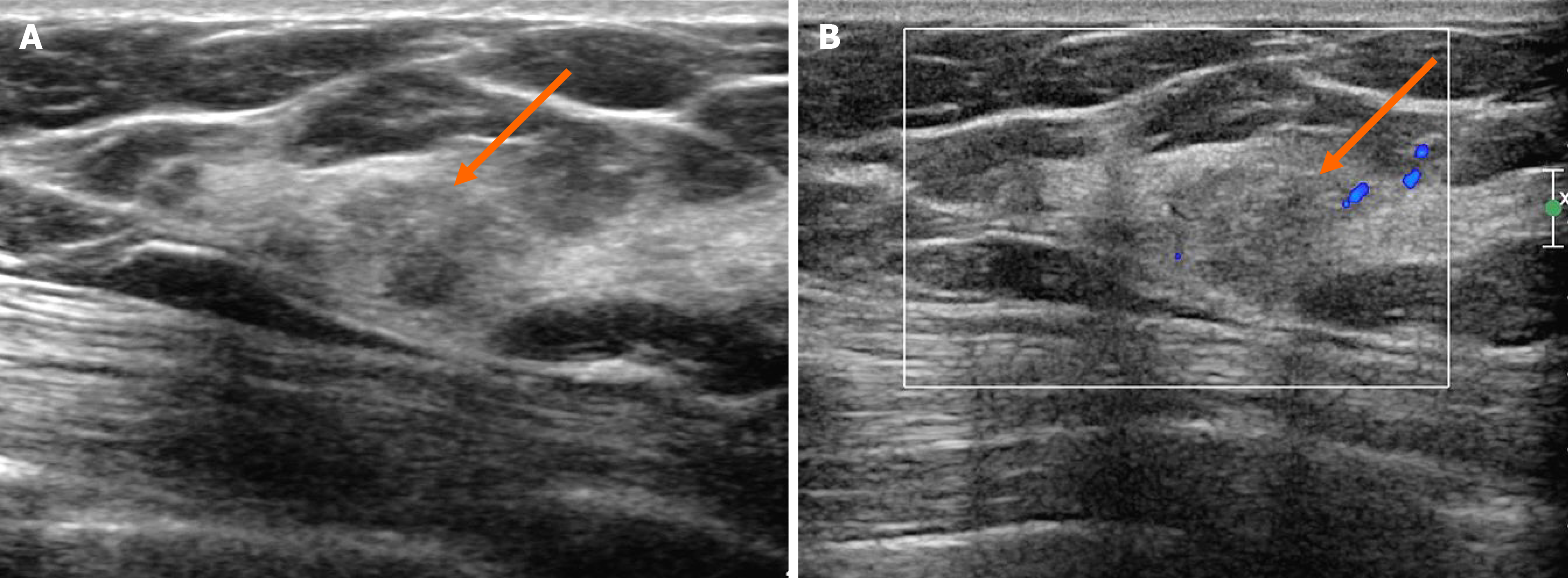
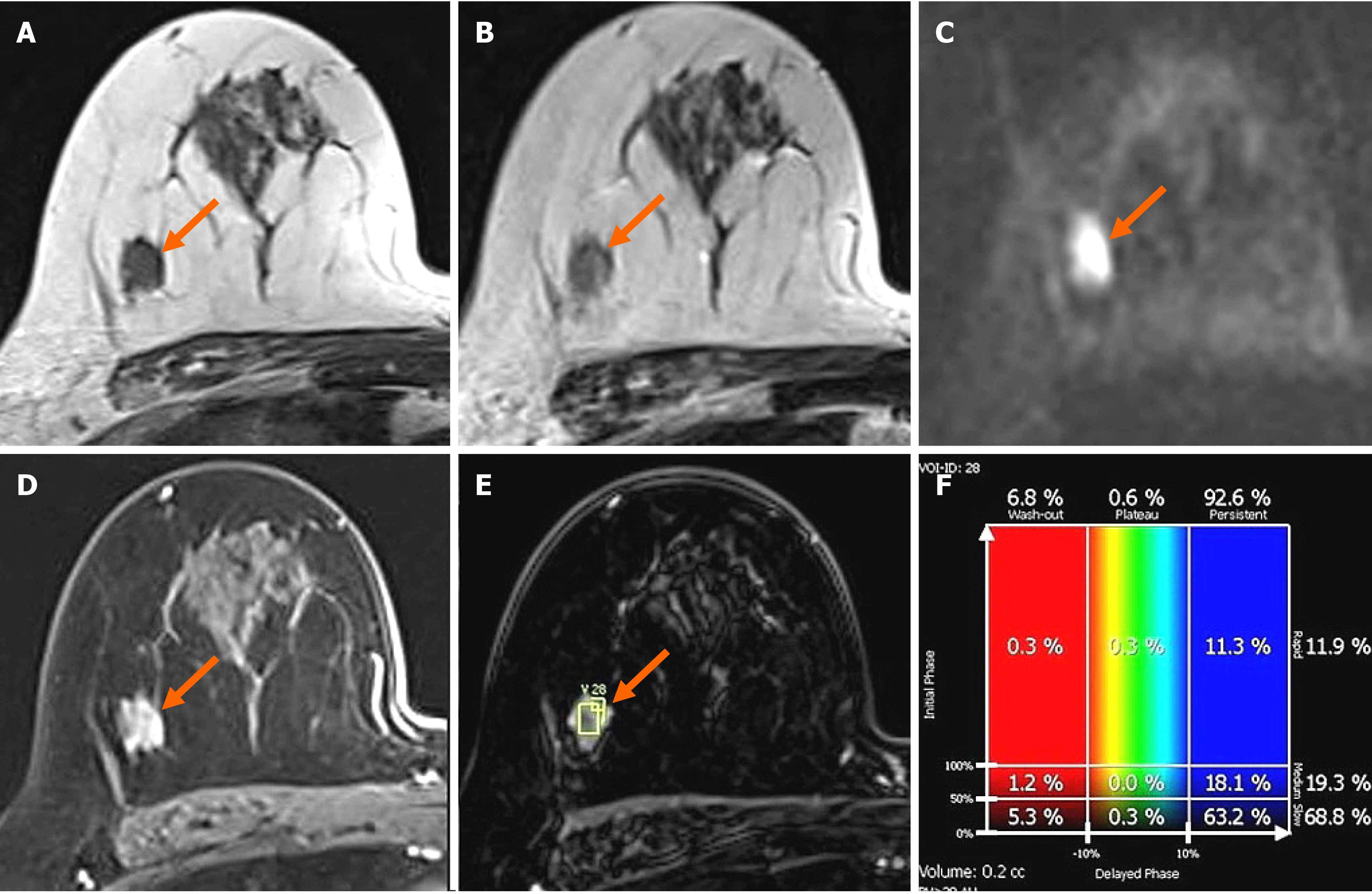
In the MG, focal asymmetry was noted in the right upper outer peripheral breast (Figure 1). The density was similar to or slightly lower than that of the breast parenchyma. It was not accompanied by distortions or microcalcifications. In the ultrasound (US) examination, the lesion was shown as a 1.5 cm, irregularly shaped mass with an angular margin (Figure 2). The echo was slightly higher than that of fat. There was no increased vascularity in the color Doppler study. In breast magnetic resonance imaging (MRI), the lesion was shown as a 1.3 cm mass with an irregular shape and margin located in the right upper outer peripheral breast, about 6 cm from the right nipple. The mass showed a similar T1 signal intensity and a slightly higher T2 signal intensity compared to muscles or the breast parenchyma (Figure 3). The lesion exhibited reduced diffusivity with hyperintensity in diffusion-weighted images and hypointensity in the apparent diffusion coefficient map compared to normal breast tissues. In the contrast enhancement study, the lesion showed heterogeneous internal enhancement with an initial slow and delayed persistent enhancing pattern. There was no axillary lymph node metastasis.
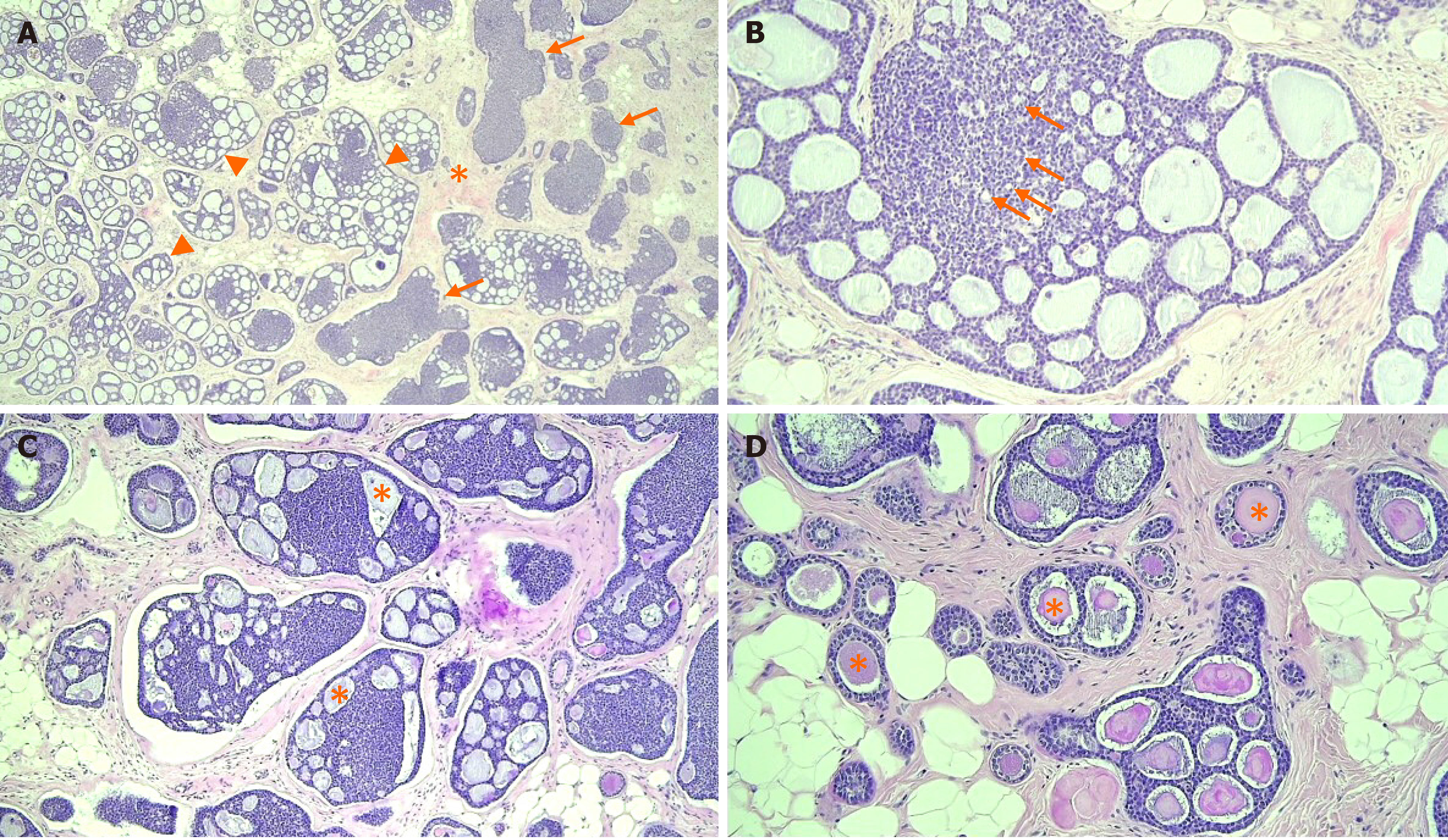
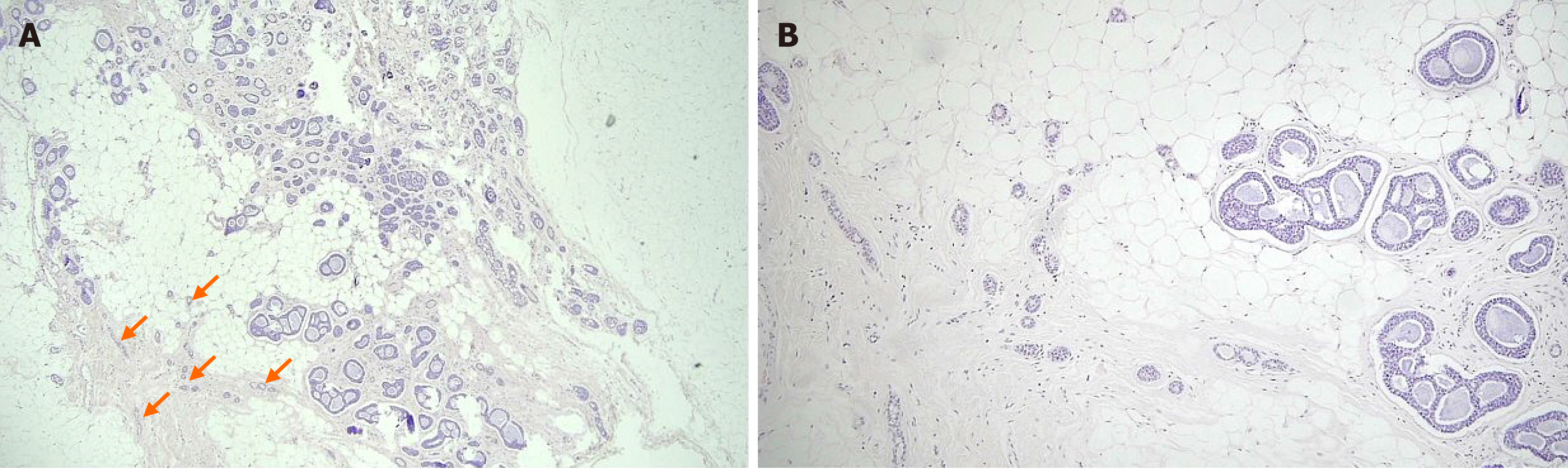
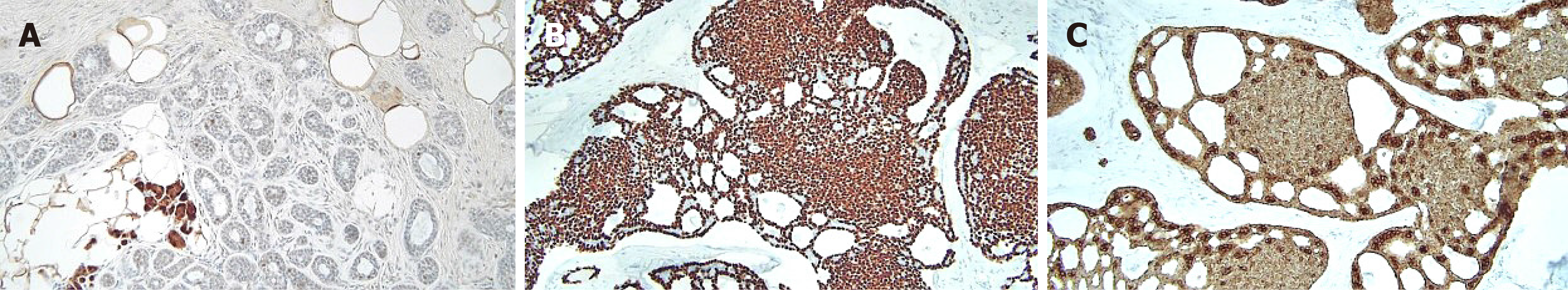
The patient underwent breast-conserving surgery and sentinel lymph node biopsy. Grossly, an ill-defined whitish tumor of roughly 1.6 cm × 1.4 cm was observed. Microscopically, invasive cancer showed diffuse infiltrating nests of cribriform or solid sheets composed of two types of cells: basaloid and ductal epithelial cells (Figure 4A and B). The cribriform space was filled with bluish or pinkish mucoid materials that were Periodic acid Schiff-positive (Figure 4C and D). Surrounding breast tissue also showed extensive intraductal proliferation of tumor cells forming small cribriform or tubules. Small round glands lined by benign-looking uniform cells were noted at the periphery of the intraductal carcinoma, suggesting in situ AdCC arising in the background of MGA (Figure 5). Benign small glands of MGA were p63 negative but S-100 positive. However, in situ AdCC was p63 positive but S-100 negative, similar to that of invasive element (Figure 6A). Immunohistochemical staining revealed myoepithelial differentiation with strong p63 positivity (Figure 6B) and epithelial differentiation with cytokeratin (CK) (Figure 6C), CD117, and epithelial membrane antigen (EMA). Triple markers of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor2 (HER2) were negative. The Ki-67 proliferation index was low (less than 17%). There was no lymphovascular or perineural invasion. There was no tumor metastasis in sentinel lymph nodes.
The lesion was confirmed as adenoid cystic carcinoma arising in microglandular adenosis.
After the breast-conserving surgery and sentinel lymph node biopsy, adjuvant radiation therapy was performed at the site of excision.
The patient had the first follow-up six months after surgery. There were no specific abnormalities other than postoperative changes.
Breast AdCC is classified into three subtypes: classic AdCC, solid-basaloid AdCC (SB-AdCC), and AdCC with high-grade transformation[2]. Classic AdCC shows a central cribriform area and a peripheral area with a tubular architecture. Both areas have the same cellular compositions of epithelial and myoepithelial cells. The glandular space is lined by epithelial-type cells that produce mucins. The pseudolumina is filled with stromal matrix, including basal membranes. Classic AdCC lacks nuclear atypia and necrosis. It shows a low mitotic count. An in situ component is rarely seen in classic AdCC. In addition to classic features of AdCC, SB-AdCC is characterized by solid nests composed of basaloid cells with marked nuclear atypia, high mitotic count, and necrosis[16]. Breast AdCC associated with high grade carcinoma is rare. It is classified into AdCC with high-grade transformation[2]. Classic AdCC shows favorable behavior. SB-AdCC and AdCC with high-grade transformation show worse prognosis than classic AdCC[2]. In immunohistochemistry, epithelial cells are positive for CK7, CK8, and EMA. In some cases, they are positive for CK5/6[17]. Myoepithelial cells show positivity for CK14, CK5/6, P63[18], and myoepithelial markers such as heavy-chain myosin, calponin, S100, and CD10. CD117 is usually strongly positive in the luminal component of AdCC. The tubular component of classic AdCC, unlike MGA, is composed of two cell types: epithelial cells and myoepithelial cells. Tubules contain mucins and basement membrane materials.
MGA has the following histologic findings: A single layer of cuboidal epithelium, clear to eosinophilic cytoplasm, rare mitotic nuclei figures, and well-preserved gland lumen and basement membranes[8,13,14,19]. Atypical MGA has features of architectural complexity, cellular expansion in the lumina, prominent nucleoli, and vesicular nuclei. In situ carcinoma from MGA shows frequent mitotic figures, obvious cytologic atypia, and obliteration of the gland lumen. Invasive carcinoma arising in MGA usually forms a microscopic solid tumor mass with infiltrative growth, desmoplastic reaction, severe cytologic atypia, and increased mitosis[13-15]. MGA, atypical MGA, and carcinoma from MGA have common immunohistochemical profiles of ER, PR, HER2, and CK5/6 negativity but CK8/18, S-100, and epithelial growth factor receptor positivity[11,13,15]. Khalifeh et al[11] have reported that Ki 67 and P53 expression could be reliable markers to distinguish MGA from atypical MGA or carcinoma from MGA because their positivity tends to increase as the lesion progresses to malignancy.
Acs et al[15] have reported 17 cases of AdCC coexisting with MGA. They suggested that AdCC might develop in a background of MGA or from MGA. They described morphological spectra of lesions with MGA, atypical MGA (also called AdCC in situ), and invasive AdCC. Altered myoepithelial cells appear to be the major neoplastic element in both AdCC and atypical MGA. Cells were stained similarly to what was seen in the myoepithelium of the breast with three markers: S-100 protein, SMA, and vimentin. MGA, atypical MGA, and AdCC show similar growth patterns with expansile or infiltrative patterns. They have a very limited metastatic capacity, showing a favorable prognosis. Rico et al[12] have reported that among carcinomas arising in MGA, not otherwise specified ductal carcinoma, metaplastic carcinoma, and salivary gland type carcinoma account for 63.4%, 35.2%, and 27.4%, respectively. Among salivary gland type carcinomas, AdCC was the most common carcinoma arising in MGA.
In our case, the tumor was composed of invasive AdCC, in situ AdCC, and MGA. Invasive AdCC showed cribriform or solid sheets composed of two cell types in the desmoplastic stroma, which was a differentiating point with in situ AdCC. Loss of basement membrane is also a characteristic of invasive cancer that could be distinguished from in situ cancer. Laminin or collagen IV staining could be helpful for the differentiation. In situ or atypical AdCC showed haphazardly scattered small cribriform or tubular tumor glands in fat tissue in the same pattern as MGA. Just next to the in situ components, small tubular glands with single epithelial lining and retained lumen were present, suggesting remaining MGA. In this area, S-100 staining was strongly positive, but p63 negative, compared to S-100 negative and p63 positive of in situ or invasive AdCC.
In the past, we have reported that ductal carcinoma in situ (DCIS) arising in MGA[20]. However, not many reports on imaging findings of carcinoma arising in MGA have been published since then. Choi and Bae[10] have reported multifocal invasive carcinoma associated with DCIS and invasive ductal carcinoma associated with encapsulated papillary carcinoma, both arising from MGA. The former showed a small nodular density in the MG and a suspicious, small hypoechoic lesion on US. The latter showed a lobulated, heterogeneous hypoechoic mass on US. Oh et al[21] have reported one case of invasive carcinoma arising in MGA. Mammogram showed an irregular isodense mass with a spiculated margin and irregular hypoechoic mass in US examination. Breast MRI showed an irregular mass with heterogeneous enhancement with a delayed washout kinetic curve. Adjacent segmental non-mass enhancement was confirmed as MGA and atypical MGA.
Imaging findings of AdCC of the breast are very diverse. In the MG, AdCC shows irregularly shaped, circumscribed, ill-defined, or microlobulated margins with iso- or hyper-density. It also appears as an architectural distortion or asymmetry[22-26]. In the US examination, it shows an irregular shape, circumscribed or ill-defined margins, and iso-, hypo- or complex echogenicity[22,23,25,27,28]. In MR images, it shows circumscribed round mass with intermediate high T2 SI and persistent enhancing curve[23], increased T2 SI with variable kinetic curves[22], spicules and early phase enhancement[28], and mixed cystic and solid mass with irregular shape and margin, and rapid and heterogeneous enhancement[25]. However, imaging findings of AdCC arising in MGA have not been described yet. To the best of our knowledge, this is the first report of imaging findings of AdCC arising in MGA.
In our case, in the MG, the lesion had no common invasive cancer features such as high density, distortion, spiculation, or suspicious microcalcifications, except for developing density. Therefore, it would be missed unless comparison with previous mammographic images was made. The density was similar to that of the breast parenchyma or slightly less. On US, sonographic echo of the lesion was slightly higher than that of fat. As the contrast between the hyperechoic breast tissue and the lesion was unclear, it was difficult to clearly distinguish the margin of the lesion in B-mode ultrasound, even with a harmonic image. Therefore, our sonographic diagnosis was a low-suspicion lesion with the possibility of a benign lesion, not breast cancer. In breast MRI, a slightly increased T2 signal intensity and a persistent enhancing pattern were seen, similar to other reports on MRI findings of AdCC. MGA might also show imaging findings similar to breast malignancy[29]. Thus, it is currently unclear which characteristics of MGA can affect imaging findings of AdCC arising in MGA. Further case collections and analysis are needed in the future to characterize this rare lesion.
For the treatment of primary AdCC of the breast, Treitl et al[1] have reported that radiation therapy or sentinel node excision besides a primary surgical therapy might not be warranted due to the tumor’s indolent course. Kulkarni et al[30] have reported that AdCC has characteristics of lower grade, hormone receptor negativity, and node negativity compared to an invasive ductal carcinoma. Therefore, AdCC was treated with less axillary surgery, fewer mastectomy, and less chemotherapy and hormone therapy. However, there is no treatment policy for AdCC arising in MGA due to the rarity of this type of malignancy. Although many published studies have suggested a relatively favorable prognosis for carcinomas arising in MGA, MGA may no longer be considered simply a benign proliferative lesion as it can progress to aggressive breast cancer. Resetkova et al[31] have emphasized the importance of complete excision of the mass and reported a recurrent case of high-grade in situ carcinoma within the residual MGA after primary surgery for this invasive tumor arising in atypical MGA. Therefore, appropriate treatment should be performed according to the patient's clinical status considering the general treatment strategy for invasive cancer with complete excision of MGA.
AdCC is a rare breast malignancy with a favorable prognosis. Although MGA has long been considered a benign entity, it has the risk of progression to malignancy. Therefore, in the case of AdCC arising in MGA, an appropriate treatment should be performed according to the clinical stage of the patient. In particular, complete resection should be performed so that no MGA remains. Imaging findings of AdCC occurring in MGA were characterized by iso- or slight hypo-density in the MG and slightly higher echo than that of fat in the US examination with higher T2 signal intensity and a persistent enhancing pattern in breast MRI.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vinh-Hung V S-Editor: Wu YXJ L-Editor: A P-Editor: Xing YX
| 1. | Treitl D, Radkani P, Rizer M, El Hussein S, Paramo JC, Mesko TW. Adenoid cystic carcinoma of the breast, 20 years of experience in a single center with review of literature. Breast Cancer. 2018;25:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, Sapino A, Sasano H, Schnitt S, Sotiriou C, van Diest P, White VA, Lokuhetty D, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 World Health Organization classification of tumours of the breast. Histopathol. 2020;77:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 468] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 3. | Kocaay AF, Celik SU, Hesimov I, Eker T, Percinel S, Demirer S. Adenoid Cystic Carcinoma of the Breast: A Clinical Case Report. Med Arch. 2016;70:392-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Dhouib F, Kallel M, Mnejja W, Fourati N, Siala W, Chaabane K, Boudawara T, Khanfir A, Daoud J. Adenoid cystic carcinoma of the breast. PAMJ-Clin Med. 2020;3:130. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Thomas DN, Asarian A, Xiao P. Adenoid cystic carcinoma of the breast. J Surg Case Rep. 2019;2019:rjy355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Ro JY, Silva EG, Gallager HS. Adenoid cystic carcinoma of the breast. Hum Pathol. 1987;18:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Miyai K, Schwartz MR, Divatia MK, Anton RC, Park YW, Ayala AG, Ro JY. Adenoid cystic carcinoma of breast: Recent advances. World J Clin Cases. 2014;2:732-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Rosen PP. Microglandular adenosis. A benign lesion simulating invasive mammary carcinoma. Am J Surg Pathol. 1983;7:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 74] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Clement PB, Azzopardi JG. Microglandular adenosis of the breast--a lesion simulating tubular carcinoma. Histopathology. 1983;7:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Choi JE, Bae YK. Invasive breast carcinoma arising in microglandular adenosis: two case reports. J Breast Cancer. 2013;16:432-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Khalifeh IM, Albarracin C, Diaz LK, Symmans FW, Edgerton ME, Hwang RF, Sneige N. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol. 2008;32:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Rico V, Shibahara Y, Monteiro M, Slodkowska E, Tam S, Zaki P, De Angelis C, Chow E, Jerzak KJ. Salivary gland-type mammary carcinoma arising in microglandular adenosis: A case report and clinicopathological review of the literature. Cancer Treat Res Commun. 2020;24:100178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Shin SJ, Simpson PT, Da Silva L, Jayanthan J, Reid L, Lakhani SR, Rosen PP. Molecular evidence for progression of microglandular adenosis (MGA) to invasive carcinoma. Am J Surg Pathol. 2009;33:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kravtsov O, Jorns JM. Microglandular Adenosis and Associated Invasive Carcinoma. Arch Pathol Lab Med. 2020;144:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Acs G, Simpson JF, Bleiweiss IJ, Hugh J, Reynolds C, Olson S, Page DL. Microglandular adenosis with transition into adenoid cystic carcinoma of the breast. Am J Surg Pathol. 2003;27:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Shin SJ, Rosen PP. Solid variant of mammary adenoid cystic carcinoma with basaloid features: a study of nine cases. Am J Surg Pathol. 2002;26:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Nakai T, Ichihara S, Kada A, Ito N, Moritani S, Kawasaki T, Uchiyama T, Itami H, Morita K, Takano M, Takeda M, Hatakeyama K, Ohbayashi C. The unique luminal staining pattern of cytokeratin 5/6 in adenoid cystic carcinoma of the breast may aid in differentiating it from its mimickers. Virchows Arch. 2016;469:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Mastropasqua MG, Maiorano E, Pruneri G, Orvieto E, Mazzarol G, Vento AR, Viale G. Immunoreactivity for c-kit and p63 as an adjunct in the diagnosis of adenoid cystic carcinoma of the breast. Mod Pathol. 2005;18:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Cascardi E, Marchiò C, Maiorano E. Microglandular Adenosis. In: Sapino A, Kulka J. Breast Pathology. Cham: Springer International Publishing, 2020: 289-293. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Jeong MS, Kang JH, Kim EK, Woo JJ, Kim HS, An JK. Ductal Carcinoma In Situ of the Breast Arising in Microglandular Adenosis. J Korean Soc Radiol. 2012;67:273-276. [DOI] [Full Text] |
| 21. | Oh SW, Lim HS, Baek JM, Lee JS. Invasive Carcinoma Arising in Microglandular Adenosis of the Breast: A Case Report and Literature Review. Iran J Radiol. 2017;14:e63463. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Glazebrook KN, Reynolds C, Smith RL, Gimenez EI, Boughey JC. Adenoid cystic carcinoma of the breast. AJR Am J Roentgenol. 2010;194:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Kim JG, Kim SY, Jung HY, Lee DY, Park SH. Radiologic and Pathological Correlation of Adenoid Cystic Carcinoma of the Breast: A Case Report. J Korean Soc Radiol. 2015;72:411-417. [DOI] [Full Text] |
| 24. | Sakhri S, Malek B, Jaidane O, Adouni O, Salma K, Chargui R, Rahal K. Adenoid cystic carcinoma of the breast, a rare entity: report of two cases and review of literature. EJMO. 2019;3:304-306. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Wang H, Liu F, Gu R, Li Y, Su F. Rare imaging appearance of adenoid cystic carcinoma of the breast: A case report. Mol Clin Oncol. 2017;7:473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Wang S, Ji X, Wei Y, Yu Z, Li N. Adenoid cystic carcinoma of the breast: Review of the literature and report of two cases. Oncol Lett. 2012;4:701-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Agafonoff S, Sobolewski A, Braverman TS. Adenoid cystic carcinoma of the breast - Discordant size on imaging and pathology: A case report and review of literature. Ann Med Surg (Lond). 2019;43:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Kashiwagi S, Asano Y, Ishihara S, Morisaki T, Takashima T, Tanaka S, Amano R, Ohsawa M, Hirakawa K, Ohira M. Adenoid Cystic Carcinoma of the Breast: A Case Report. Case Rep Oncol. 2019;12:698-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Ha SM, Woo OH, Cho KR, Seo BK, Kim CY. Radiologic Findings of Microglandular Adenosis of the Breast mimicking Breast Carcinoma: Report of a Case. J Korean Soc Breast Screening. 2014;11:37-40. |
| 30. | Kulkarni N, Pezzi CM, Greif JM, Suzanne Klimberg V, Bailey L, Korourian S, Zuraek M. Rare breast cancer: 933 adenoid cystic carcinomas from the National Cancer Data Base. Ann Surg Oncol. 2013;20:2236-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Resetkova E, Flanders DJ, Rosen PP. Ten-Year Follow-up of Mammary Carcinoma Arising in Microglandular Adenosis Treated with Breast Conservation. Arch Pathol Lab Med. 2003;127:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |