Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7572
Peer-review started: March 31, 2021
First decision: May 11, 2021
Revised: May 24, 2021
Accepted: July 21, 2021
Article in press: July 21, 2021
Published online: September 6, 2021
Processing time: 152 Days and 21.9 Hours
Cardiac embolism is a common cause of ischemic stroke in young adults. Neurological complications associated with atrial myxoma most frequently include cerebral infarct due to embolus. Early complete resection of giant cardiac myxoma is the key to its treatment and prevention of stroke recurrence.
A 42-year-old, previously healthy woman was admitted to the hospital with sudden-onset inability to speak and right-sided hemiplegia. While sweeping the floor 2 h prior to hospital admission, the patient developed sudden inability to express herself or understand what others were saying, accompanied by dyskinesia of the right limb, inability to walk or hold objects, and involuntary choreiform movements of the left upper limb. The patient was diagnosed with cerebral embolism and cardiac myxoma, complicated by left middle cerebral artery occlusion. The acute stroke was treated with intravenous thrombolytic therapy and arterial embolectomy as a bridging therapy to open resection of left atrial cardiac myxoma. The patient condition improved remarkably following initial thrombolysis and embolectomy and subsequently underwent emergency open resection of the atrial cardiac myxoma. She had no recurrence during 1-year follow-up.
Strong consideration should be given to urgent intravenous thrombolysis (rt-PA, alteplase) in young adult stroke patients at the time of hospital admission. The present case demonstrated a highly successful outcome that combined throm
Core Tip: The incidence of ischemic stroke caused by cardiac mucinous tumors has been reported both nationally and internationally. Still, it is rarely reported that intravenous thrombolytic bridging artery retrieval with same-day open thoracotomy for left atrial mucinous tumors is performed in the early stage of stroke. We treated a case with cerebral embolic stroke associated with cardiac myxoma, the embolus was successfully removed, and the cardiac tumor was extirpated surgically on the day of presentation.
- Citation: Chang WS, Li N, Liu H, Yin JJ, Zhang HQ. Thrombolysis and embolectomy in treatment of acute stroke as a bridge to open-heart resection of giant cardiac myxoma: A case report. World J Clin Cases 2021; 9(25): 7572-7578
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7572.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7572
Ischemic stroke in young adults is defined as an acute ischemic cerebrovascular disease that occurs in patients aged 18-45 years[1]. Approximately 10%-14% of ischemic strokes occur in young adults[2]. Cardiac embolism is a common cause of ischemic stroke in young adults, accounting for approximately 20% to one-third of all strokes[3]. Cardiac valve and endocardial lesions are the most common, in addition to congenital heart disease, recent myocardial infarction, and cardiac tumors. Atrial myxoma represents the most common type of primary cardiac tumor in adults, accounting for as many as 83% of all primary tumors of the heart. Most cardiac myxomas (CMs) are located in the left atrium, attached to the interatrial septum. Neurological complications associated with atrial myxoma most frequently include cerebral infarct due to embolus.
A 42-year-old, previously healthy woman was admitted to the hospital with sudden-onset inability to speak and right-sided hemiplegia.
The patient suddenly was unable to express herself or understand what others were saying, accompanied by dyskinesia of the right limb, inability to walk or hold objects, and involuntary choreiform movement of the left upper limb, while sweeping the floor, 2 h prior to hospital admission. She denied nausea and vomiting, limb convulsions, incontinence, or loss of consciousness.
The patient had a free previous medical history.
The patient had no history of trauma or preceding infection.
The patient’s admission vital signs included that temperature was 36.9 °C, heart rate was 72 beats per minute, respiratory rate was 18 breaths per minute, blood pressure was 134/115 mmHg, and oxygen saturation in room air was 99%. The clinical neurological examination revealed mental clarity, depression, complete mixed aphasia, both eyes stared to the left, pupils that bilaterally were of equal size and shape, with a diameter of 3 mm, and normal light reflex. The nasolabial fold on the right side was shallow, and the tongue uncooperative. The drop test of the right limb was positive and there was no response to stimulation; the left upper arm demons
Blood analysis revealed: Neutrophils 79.5% (nL, 50%-70%), platelets 344 × 109/L (nL, 100-300 109 /L); hemoglobin 103g/L (nL, 110-150 g/L); mean hemoglobin concentration 295 g/L (nL, 320-360 g/L). The mean erythrocyte volume was 67.7 fL (nL, 82-95 fL); CRP was 78.61 mg/L (nL, 0-10 mg/L); and leukocytes, erythrocytes, coagulation, D-dimer, renal function, electrolytes as well as urine analysis were normal. Fingertip blood glucose was 7.4 mmol/L. Chest X-ray and arterial blood gas were also normal. A 12-lead electrocardiogram was normal with sinus rhythm.
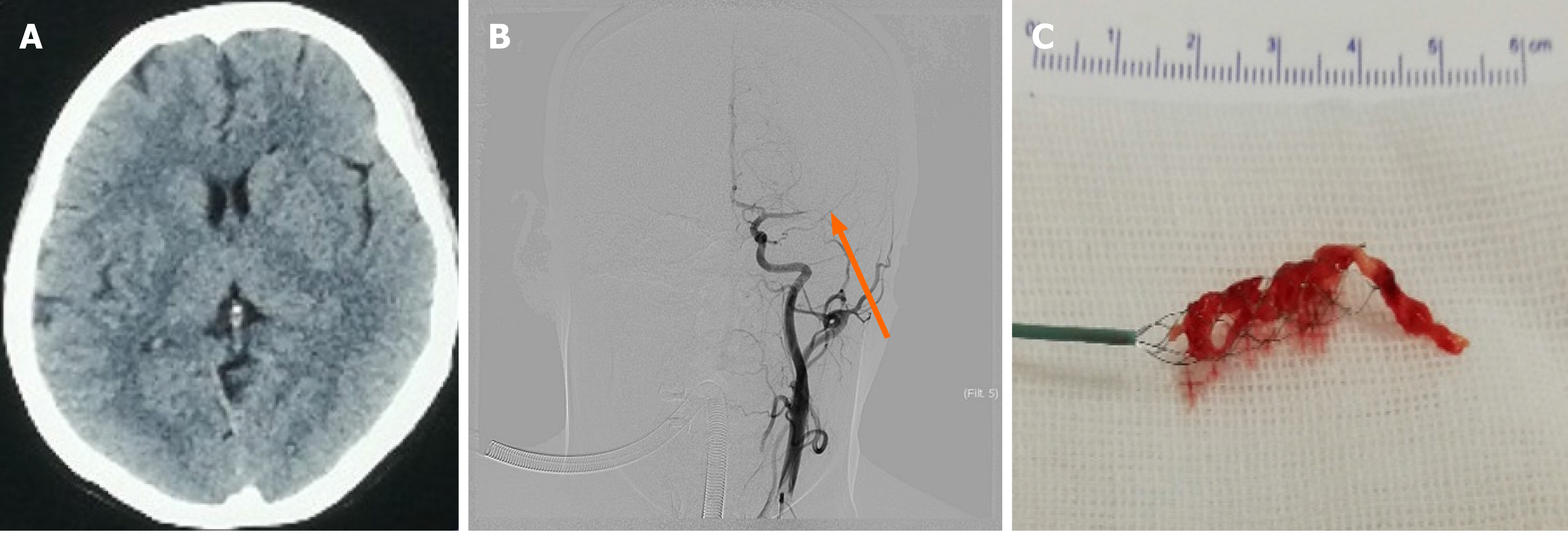
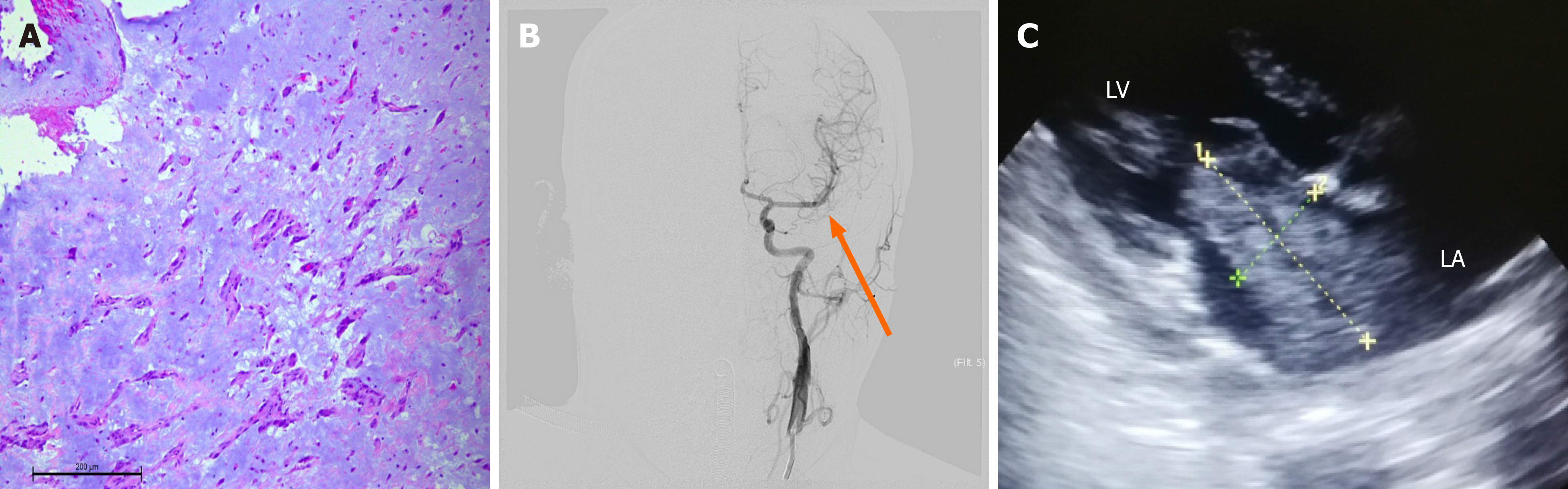
A noncontrast computed tomography (CT) scan of the brain showed no abnormality (Figure 1A). The NIHSS score was 18 points (level of consciousness question 2, level of consciousness instruction 2, gaze 2, facial paralysis 1, right upper limb 4, right lower leg 4, and aphasia 3). The patient fulfilled criteria for intravenous thrombolysis and she was successfully treated with intravenous thrombolysis using alteplase (0.9 mg/kg, 10% of the total dose was administered intravenously as a bolus, followed by an infusion of the remaining dose over 60 min) without signs of bleeding, and alteplase was initiated within 140 min of symptom onset. Cerebral angiography was conducted immediately and showed focal cerebral ischemia resulting from a distal middle cerebral artery occlusion (Figure 1B). A Solitaire FR stent and microcatheter were pulled back simultaneously during blood aspiration with a 50 cc syringe in the guiding catheter to minimize the risk of distal emboli. A red jelly-like thrombus was withdrawn out of the stent (Figure 1C) and sent for the rapid pathological examination which was suggestive of atrial myxoma (Figure 2A). A tentative diagnosis was made of cerebral arterial occlusion due to embolism from a cardiac tumor. Re-examination showed that the left middle cerebral and left anterior cerebral arteries were both patent. Successful 100% recanalization was achieved (Figure 2B). Subsequent echocardiography showed the following: A mild increase in left atrial dimension (38 mm) and an abnormal hypoechoic mass measuring 6.5 cm × 3.5 cm, with a regular shape and homogeneous internal echo, which moved with the cardiac cycle. The neoplasm remained in the mitral orifice during diastole and prolapsed into the left atrium during systole (Figure 2C).
The patient should undergo surgical treatment of the left atrial myxoma through a median sternotomy under cardiopulmonary bypass. Due to the large tumor size, the patient was at risk of cardiac arrest due to the re-shedding of mucous tissue or fragments. A preoperative conference included neurologic and cardiac anesthesiologists, a cardiac surgeon, and a neurosurgeon. There was no personal or family history. Specifically, there was no history of coronary heart disease, atrial fibrillation, or arrhythmia.
The patient’s final diagnosis was acute stroke, left middle cerebral artery occlusion, and CM.
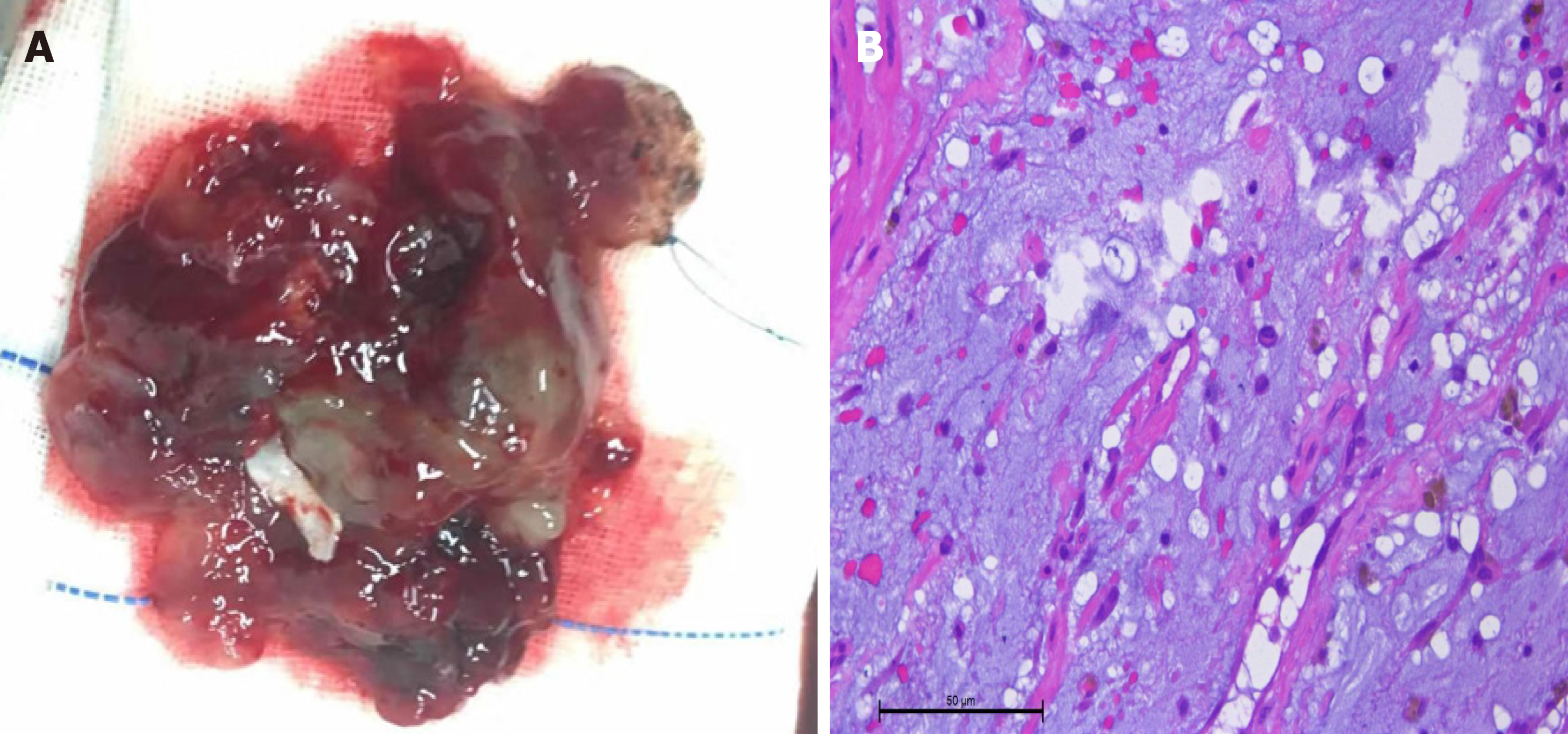
After discussion, the patient and her husband consented to surgical resection of the left atrial myxoma through a median sternotomy under cardio-pulmonary bypass. The procedure was completed without complications and pathological examination (Figure 3A) confirmed the diagnosis of myxoma (Figure 3B). Although straddling the mitral valve, the mass remained in the left atria throughout the cardiac cycle. On the second postoperative day, repeat neurological examinations showed that the patient had regained mental clarity and fluent speech, the right nasolabial sulcus was shallow, the muscle strength of the right limbs was 4/5, and there were no sensory abnor
The NIHSS score was 1, and the MRS score was 0 at the 90-d follow-up. There was no recurrence of symptoms and the patient had recovered very well without any sequelae at the 1-year follow-up. During this period, warfarin was taken orally for anticoagulation, and INR indexes were monitored.
CM account for only 0.5% of acute cerebral infarctions[4], and atrial myxoma is the most common primary cardiac tumor. Neurological complications associated with atrial myxoma most frequently include cerebral infarct due to embolus[5]. The mechanism of stroke in CM is believed to be emboli that originate from two sources: Thrombus on the surface of tumor tissue, and actual detachment of tumor tissue[6]. These frequently lead to embolic cerebral infarct, most typically in young women, with a female predominance between the third and sixth decades of life[7]. The main distribution of the infarctions is in the internal carotid artery system, although they may involve multiple vascular systems. The incidence of stroke is also associated with the position of the myxoma, and small-size myxomas cannot be ignored for the risk of stroke[8]. The young female patient had no commonly recognized high-risk factors (for example, hypertention and diabetes) predisposing to stroke, and without typical high-risk factors for cardiovascular disease and no concerning family history. The cardiac murmur detected on cardiac auscultation at the time of her hospital admission might have been a clue to cardiogenic embolism. The patient was promptly treated by thrombolysis and bridging embolectomy. Pathologically, the embolus had the form of clear mucus jelly, suggesting the presence of CM. The age and sex of the patient were consistent with those of previously reported series, with a female predominance between the third and sixth decades of life. Irrespective of the successful outcome of this patient, cerebral infarction caused by CM is a serious problem. For large vascular occlusion caused by CM, even with timely treatment utilizing rt-PA intravenous thrombolysis, success rates are still very low at approximately 10%, bleeding conversion rate is about 10.23%[5], and the curative effect is not satisfactory[9]. Mechanical arterial embolectomy can quickly open occluded blood vessels in up to 53%, but cerebral infarction due to CM has been shown to be recurrent[7]. To prevent recurrent embolic events and potentially sudden death, CM should be operated on as soon as diagnosed and safe to proceed. The longer the time interval between ischemic events and CM resection, the higher the risk of recurrent cerebral embolism[10]. Sethi[11] performed emergency peripheral vascular and cardiac surgery on a patient with acute massive cerebral infarction due to CM, and the patient performed well. Delayed surgical resection was associated with an increased risk of complications from the myxoma[4]. Shah et al[12] reported 10-, 20-, and 30-year survival rates of 77.0%, 51.8%, and 33.6% in CM. The prognosis of CM after surgical resection is good, and the recurrence rate is about 1%-4% in scattered cases[13]. Although the recurrence rate is low, warfarin anticoagulation was used for 1 year to prevent recurrence in the case.
In this case, the occurrence of a large cerebral infarct was successfully avoided after intravenous thrombolytic bridging arterial thrombectomy, and the left atrial myxoma resection was performed on the day of admission so as to reduce the risk of recurrent ischemic stroke.
The authors appreciate the patient’s cooperation sincerely. Special thanks to Ren Shuxi, PhD, from Hebei University of Technology, for his selfless help in grammar.
Manuscript source: Unsolicited manuscript
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cristina Oana M, Glumac S, Tsai ST S-Editor: Liu M L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Williams LS, Garg BP, Cohen M, Fleck JD, Biller J. Subtypes of ischemic stroke in children and young adults. Neurology. 1997;49:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Renna R, Pilato F, Profice P, Della Marca G, Broccolini A, Morosetti R, Frisullo G, Rossi E, De Stefano V, Di Lazzaro V. Risk factor and etiology analysis of ischemic stroke in young adult patients. J Stroke Cerebrovasc Dis. 2014;23:e221-e227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol. 2013;70:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Yuan SM, Humuruola G. Stroke of a cardiac myxoma origin. Rev Bras Cir Cardiovasc. 2015;30:225-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Ni B, Lu X, Gong Q, Shao Y. Simultaneous resection of left atrial myxoma and esophageal carcinoma via right thoraco-abdominal approach. J Thorac Dis. 2016;8:E531-E534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Cao GF, Bi Q, Cao L, Wang C. [The clinical characteristics of stroke in young patients with cardiac myxoma]. Zhonghua Nei Ke Za Zhi. 2017;56:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Saver JL. Dose the Merci retriever work? Stroke. 2006;37:1340-1341. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP; Multi MERCI Investigators; Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 885] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 9. | Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001;80:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 579] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Yetkin U, Celik E, Yurekli I, Gurbuz A, Our re-do extirpation in a case with recurrent giant left atrial myxoma forming mitral stenosis. J Cardiothorac Surg. 2013;170. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Sethi NK. Is it safe to proceed with thrombolytic therapy for acute ischemic stroke in a patient with cardiac myxoma? Eur Neurol. 2013;69:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Shah IK, Dearani JA, Daly RC, Suri RM, Park SJ, Joyce LD, Li Z, Schaff HV. Cardiac Myxomas: A 50-Year Experience With Resection and Analysis of Risk Factors for Recurrence. Ann Thorac Surg. 2015;100:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Barh D, Kumar A, Chatterjee S, Liloglou T. Molecular features, markers, drug targets, and prospective targeted therapeutics in cardiac myxoma. Curr Cancer Drug Targets. 2009;9:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |