Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7542
Peer-review started: February 28, 2021
First decision: April 14, 2021
Revised: May 28, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: September 6, 2021
Processing time: 183 Days and 19.8 Hours
Congenital biliary atresia is a type of obstruction of the bile ducts inside and outside the liver, which can lead to cholestatic liver cirrhosis and eventually liver failure. The preduodenal portal vein (PD-PV) is a rare developmental malforma
A 1-mo-and-4-d-old child was admitted to the hospital in January because of yellowish skin. After surgical consultation, surgical intervention was recom
Diagnoses: (1) Congenital biliary atresia; (2) PD-PV; and (3) Congenital cardio
Core Tip: Congenital biliary atresia is a type of obstruction of the bile ducts inside and outside the liver, which can lead to cholestatic liver cirrhosis and eventually liver failure. The preduodenal portal vein is a rare developmental malformation of the portal vein. A 1-mo-and-4-d-old child was admitted to the hospital in January because of yellowish skin. After surgical consultation, surgical intervention was recommended. The child underwent laparoscopic exploration under general anesthesia. During the operation, the portal vein was located at the anterior edge of the duodenum.
- Citation: Xiang XL, Cai P, Zhao JG, Zhao HW, Jiang YL, Zhu ML, Wang Q, Zhang RY, Zhu ZW, Chen JL, Gu ZC, Zhu J. Neonatal biliary atresia combined with preduodenal portal vein: A case report. World J Clin Cases 2021; 9(25): 7542-7550
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7542
Congenital biliary atresia accounts for half of the cases of long-term neonatal ob
A female infant, G1P1, had a gestational age of 37 wk and 4 d. On the fourth day after birth, the baby developed yellowing of the facial skin, which progressively aggra
On the fourth day after birth, the baby developed yellowing of the facial skin, which progressively aggravated. The skin over the trunk region also turned yellowish, and it progressed further. Admission diagnoses were: (1) Neonatal hepatitis syndrome; (2) Abnormal liver function; and (3) Congenital cardiovascular malformations. The pa
She was admitted to the Neonatology Department with the diagnosis of “newborn jaundice” in the outpatient clinic. The meconium passed by the child was resolved within 24 h after birth, and it turned yellow within 2-3 d. Bowel movements occurred 1-2 times a day, and the color of stool was pale yellow, without clay colored stool. Urine was normal.
A female infant, G1P1, had a gestational age of 37 wk and 4 d.
Upon admission, clinical examination showed body temperature: 37 ºC, pulse: 140 beats/min, respiratory rate: 40 beats/min, weight: 3760 g, clearly conscious, good reaction, crying loudly, steady breathing, moderate yellowing of the skin on the face, trunk, and limbs, the sclera was yellowish, and the skull was not deformed. There were no special features in the face. The fontanelle measured about 2.0 cm × 2.0 cm, and it was flat. The nose did not move, the lips were not cyanosed, the neck was soft, the breath sounds of both lungs were thick, and no dry or wet rales were heard. The heart rhythm was uniform, the heart sound was medium, no murmur was heard, and the abdomen was soft. The liver was located 2 cm below the ribs, and it did not touch the spleen. Bowel sounds were normal, 3-4 sounds per minute. The umbilical cord had fallen off, the umbilicus was dry, and the umbilical chakra was not red. The muscle tension of the limbs was normal, and the foraging and sucking reflexes could be elicited.
Outpatient examination of liver function revealed: γ-glutamyl transpeptidase: 114.5 U/L, total protein: 58.5 g/L, albumin: 58.5 g/L, prealbumin: 104 mg/L, globulin: 15.6 g/L, albumin-globulin ratio: 2.75, high-sensitivity C-reactive protein: 0.32 mg/L, glutamic-pyruvic transaminase: 91.3 U/L, glutamic oxaloacetic transaminase: 166.4 U/L, indirect bilirubin: 100.02 μmol/L, direct bilirubin: 129.88 μmol /L, total bilirubin: 229.9 μmol/L; and a normal TORCH test.
Color Doppler ultrasound showed no obvious abnormal echo in the liver, gallbladder, pancreas, and kidneys. Heart Doppler ultrasound revealed interruption of the inferior vena cava and continuation of the odd vein, persistence of the left superior vena cava, and a patent foramen ovale.
After surgical consultation, surgical intervention was recommended.
(1) Congenital biliary atresia; (2) PD-PV; and (3) Congenital cardiovascular malformations.
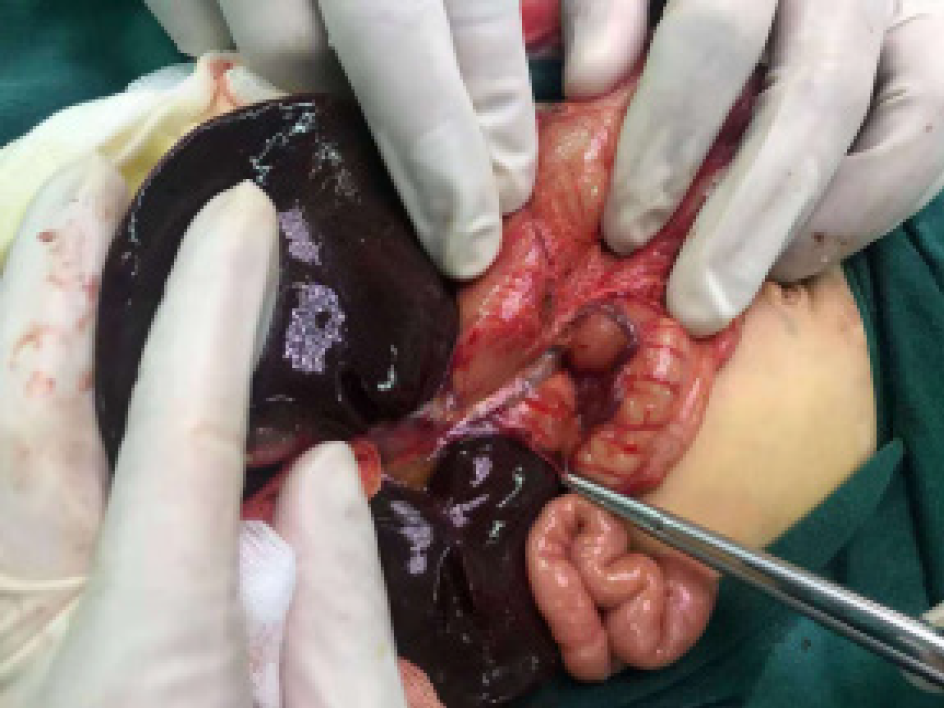
The child underwent laparoscopic exploration under general anesthesia. During the operation, the PV was located at the anterior edge of the duodenum (Figure 1). In
The child’s symptoms were gradually relieved, and then she was discharged. During follow-up, the child’s condition gradually improved, but deterioration of the child’s condition could not be ruled out, which would require liver transplantation or other treatments. Finally, the child was lost to follow-up due to change in contact infor
The relationship between neonatal biliary atresia and PD-PV should be considered in depth. In this study, the baby developed yellowing of the facial skin, which pro
Biliary atresia of the bile duct in the first part of the duodenum before the PV can occur individually, as found in such cases, or it can occur in combination. It is ca
Since 1921, Knight first described PD-PV[6], and so far, less than 100 cases have been reported in the literature. Each report often presents the case of only 1 child, and most of the reports have presented cases of only 5 children, which indicates that the de
PD-PV originates from the persistent primordial yolk vein or is related to abnormal rotation of the midgut. For example, abnormal intestinal rotation and duodenum and stomach reversal may result in PD-PV[7]. Three quarters of children with PD-PV have some concomitant malformations, such as cardiovascular malformations, gastroin
PD-PV is considered to be an external cause of congenital duodenal obstruction. Researchers have been studying how a low-pressure blood vessel can cause thick-walled duodenal obstruction[7]. In most cases, duodenal obstruction is caused by other related deformities, and PD-PV is just an accompanying deformity. In the study by Vilakazi et al[8], in only 10 cases duodenal obstruction in children was found to be caused by PD-PV alone. PD-PV may cause complete or partial duodenal obstruction. Characteristically, vomiting can occur within a few hours after birth, and feeding cannot be tolerated. Partial duodenal obstruction presents with repeated episodes of vomiting and growth retardation. Snavely and Breakell[10] reported that PD-PV cau
Preoperative diagnosis of PD-PV is very rare. This disease entity may not be dis
The prenatal diagnosis of congenital duodenal obstruction is based on obvious polyhydramnios and the double bubble sign displayed by B-ultrasound. The PD-PV is a cause of prenatally diagnosed duodenal obstruction, established by B-ultrasound, which has not been found in the literature presented in domestic and foreign reports[13].
Abdominal color Doppler ultrasound and computed tomography can be used in cases with a clear diagnosis of duodenal obstruction before surgery. If the vascular structure is found in the front of the pancreas, it has an important diagnostic value. PD-PV is a rare cause of duodenal obstruction. It is not necessary to diagnose PD-PV before surgery because all children with duodenal obstruction require laparotomy or laparoscopic exploratory surgery. However, it is very important to identify PD-PV during the operation because PD-PV occasionally does not cause obstruction, but it may only be discovered accidentally during the operation, which may result in intraoperative complications, especially in children with intestinal rotation or ab
Duodenal obstruction has the potential to progress to a surgical emergency. How
The genetic origin of PD-PV is still unclear. Although it is very rarely found in clinical practice, it is a likely cause of fetal or infantile duodenal obstruction and may cause a potential risk to surgery; thus, it should receive the attention of clinicians.
Kasai radical resection opened a new era of “uncorrectable” biliary atresia treatment. To date, Kasai radical resection is still the preferred surgical method for biliary atresia, and liver transplantation is a treatment method in case of failure of advanced Kasai radical resection[15,16]. Kasai radical surgery emphasizes early diagnosis and treat
The key to Kasai radical operation is to completely remove the hepatic hilar fibrous mass. The operation is best performed under a surgical magnifying glass, so that the side of the cut section reaches the liver parenchyma at the entry of the PV and the longitudinal level reaches the posterior wall of the PV. The depth of removal of the hilar fibrous mass is the key to this operation. Very superficial excision may not ensure reaching the appropriate small intrahepatic bile duct, and very deep excision may cause damage to the liver parenchyma and affect the healing of the surgical anasto
Various modified surgical approaches: After the classic portojejunostomy described by Kasai, although many modified procedures have been proposed to reduce the possibility of complicated cholangitis, the results are not ideal. The most commonly used modifications include external drainage and intussusception type anti-reflux valve placement. However, neither the “ventilation” nor the “valve” method has much effect on reducing the incidence of retrograde cholangitis. In the early 1990s, some scholars proposed that intussusception anti-reflux valve can reduce the occurrence of reflux cholangitis after biliary atresia. However, more recent studies have shown that targeting the regurgitant valve may be effective for anti-reflux but less effective at preventing cholangitis. A possible explanation for this dichotomy is that cystic dilatation of the intrahepatic bile duct accompanied by cholestasis has become a potential target for bacterial colonization. Therefore, although the regurgitant valve works, infection cannot be avoided.
Views on the application of laparoscopy: With the widespread application of lapa
Drug treatment after biliary atresia surgery: Effective drug treatment is extremely important for improving the prognosis after portoenterostomy. Although surgery can prolong the lifespan of children, it cannot reverse liver damage and progressive cirrhosis. Ultimately, 75%-80% of children need liver transplantation for long-term survival[18,19]. In recent years, it has been recognized that the immune-mediated da
Postoperative hormone therapy: Corticosteroids, the main component of adjuvant therapy, can significantly improve the quality of life after surgery and increase the survival. Due to the inflammatory nature of cholangitis itself and the abnormal immune mechanism, it may be related to the onset of biliary atresia. Theoretically, the application of drugs, such as steroids, after hepatoenteric anastomosis should be very effective in reducing immune-mediated liver damage, improving bile drainage, and reducing the incidence of reflux cholangitis. Since Gad et al[20] reported that short-term shock therapy with glucocorticoids can increase bile flow, many treatment institutions have adopted short-term shock therapy for 1 to 2 wk after surgery. Dillon et al[21] proposed that compared with the non-hormonal group, oral high-dose ste
Long-term application of choleretic drugs after surgery: In addition to hormones, choleretic drugs also include dehydrocholic acid, glucagon, dinoprostone, and ursodeoxycholic acid. Among them, ursodeoxycholic acid has been studied in depth. It can significantly improve the deficiency of essential fatty acids and reduce the level of bilirubin. It is currently used as a routine drug and has provided good effects. No adverse reactions have been reported. It is clinically recommended to take ursodeoxycholic acid 10 mg/(kg/d) orally. Ursodeoxycholic acid is started after the operation and usually continued for 1 to 2 years. There are also reports of oral administration throughout life.
The application of prophylactic antibiotics after surgery: In the early 1980s, the second-generation cephalosporins (cephalosporin and cefuroxime) were combined with aminoglycosides (gentamicin and amikacin). After the 1990s, third-generation cephalosporins became dominant, and they were occasionally combined with amino
With the development of liver transplantation, the prognosis of biliary atresia has greatly improved. According to current reports on liver transplantation at home and abroad, biliary atresia is the most common indication. The average survival time of children with biliary atresia without surgery is 12 mo. After Kasai surgery, more than half of the children have repeated postoperative infections, and the survival rate is only 30% to 60%. Since Strong et al[27] reported the success of the first liver trans
Kasai surgery and liver transplantation complement each other; children whose age is less than 90 d should undergo Kasai surgery first. If there is no bile flow or only temporary bile drainage after the operation, and the histological examination of the hilar region of the liver shows that the biliary tract has a small caliber and a small number of ducts, these children do not need to undergo the Kasai operation because repeated operations increase the difficulty of future liver transplantation. If the child is older than 90 d and there is no obvious chronic liver disease, then the hepatic hilar region can be dissected first to determine whether there are residual liver ducts. If there are open residual liver ducts, then the Kasai operation can be performed; otherwise liver transplantation should be performed. If the child has any obvious liver disease, such as liver cirrhosis and portal hypertension, then liver transplantation should be performed. Even if the bile drainage is satisfactory after the Kasai operation and the jaundice has gradually reduced, close follow-up should be performed over a long time. If liver disease occurs, liver transplantation should be performed as soon as possible.
In short, Kasai surgery is the first choice for treatment of biliary atresia, which may allow the child to achieve healing or buy precious time for liver transplantation. Postoperative comprehensive drug treatment plays an important role in improving the efficacy, and the success of liver transplantation significantly improves prognosis. However, it is very important to deepen our understanding of the etiology of biliary atresia, strive to improve the level of early diagnosis, and continuously improve the technique of portoenterostomy and perioperative management.
The authors thank all the medical workers who helped us (Children’s Hospital of Soochow University).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kitamura K, Saad K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Petersen C, Ure BM. What's new in biliary atresia? Eur J Pediatr Surg. 2003;13:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, Tanaka K; Japanese Biliary Atresia Registry. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Kouwenberg M, Kapusta L, van der Staak FH, Severijnen RS. Preduodenal portal vein and malrotation: what causes the obstruction? Eur J Pediatr Surg. 2008;18:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Singal AK, Ramu C, Paul S, Matthai J. Preduodenal portal vein in association with midgut malrotation and duodenal web-triple anomaly? J Pediatr Surg. 2009;44:e5-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Goel P, Bajpai M, Sharma K, Naranje P. Previously Undescribed Anomalies of Hepatic Artery and Portal Venous Anatomy in a Case of Extrahepatic Biliary Atresia and its Implications. J Indian Assoc Pediatr Surg. 2019;24:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Knight HO. An anomalous portal vein with its surgical dangers. Ann Surg. 1921;74:697-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Shimadera S, Iwai N, Deguchi E, Kimura O, Fumino S, Yokoyama T. The inv mouse as an experimental model of biliary atresia. J Pediatr Surg. 2007;42:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Vilakazi M, Ismail F, Swanepoel HM, Muller EW, Lockhat ZI. Duodenal obstruction due to a preduodenal portal vein. Afr J Paediatr Surg. 2014;11:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Tsuda Y, Nishimura K, Kawakami S, Kimura I, Nakano Y, Konishi J. Preduodenal portal vein and anomalous continuation of inferior vena cava: CT findings. J Comput Assist Tomogr. 1991;15:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Snavely JG, Breakell ES. Fatal hemorrhage from esophageal varices, due to malformations and congenital stenoses in portal venous system. Am J Med. 1954;16:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Bansal R, Dhillon KS, Kaushal G. Preduodenal portal vein: A recipe for disaster during laparoscopic cholecystectomy. J Minim Access Surg. 2019;15:63-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Walsh G, Williams MP. Congenital anomalies of the portal venous system--CT appearances with embryological considerations. Clin Radiol. 1995;50:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Choi SO, Park WH. Preduodenal portal vein: a cause of prenatally diagnosed duodenal obstruction. J Pediatr Surg. 1995;30:1521-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Zhan J, Feng J, Chen Y, Liu J, Wang B. Incidence of biliary atresia associated congenital malformations: A retrospective multicenter study in China. Asian J Surg. 2017;40:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Wang W, Zheng S, Shen C, Xiao XM. Study on the relationship between neonatal cytomegalovirus infection and biliary atresia liver fibrosis. Zhonghua Xiaoer Waike Zazhi. 2005;26:464-466. [DOI] [Full Text] |
| 16. | Wang W, Zheng S. Research on the relationship between biliary atresia and viral infection and immune system response. Guoji Erkexue Zazhi. 2006;33:270-272. [DOI] [Full Text] |
| 17. | Shen C, Zheng S, Wang W, Xiao XM. Study on the effect of operating age on the prognosis of biliary atresia after Kasai surgery. Linchuang Xiaoerwaike Zazhi. 2007;10-12. [DOI] [Full Text] |
| 18. | McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 19. | Shteyer E, Ramm GA, Xu C, White FV, Shepherd RW. Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr. 2006;42:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Gad EH, Kamel Y, Salem TA, Ali MA, Sallam AN. Short- and long-term outcomes after Kasai operation for type III biliary atresia: Twenty years of experience in a single tertiary Egyptian center-A retrospective cohort study. Ann Med Surg (Lond). 2021;62:302-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Dillon PW, Owings E, Cilley R, Field D, Curnow A, Georgeson K. Immunosuppression as adjuvant therapy for biliary atresia. J Pediatr Surg. 2001;36:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Meyers RL, Book LS, O'Gorman MA, Jackson WD, Black RE, Johnson DG, Matlak ME. High-dose steroids, ursodeoxycholic acid, and chronic intravenous antibiotics improve bile flow after Kasai procedure in infants with biliary atresia. J Pediatr Surg. 2003;38:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Wang W, Zheng S, Shen C, Xiao XM. Efficacy and safety of high-dose steroids after biliary atresia. Zhonghua Xiaoer Waike Zazhi. 2006;27:460-463. [DOI] [Full Text] |
| 24. | Zheng S, Luo Y, Wang W, Xiao XM. Histopathological analysis of intrahepatic and extrahepatic biliary system in biliary atresia. Zhongguo Xunzheng Erke Zazhi. 2007;2:253-258. |
| 25. | Zheng S, Luo Y. Modern concept of diagnosis and treatment of cholangitis after biliary atresia. Linchuang Xiaoerwaike Zazhi. 2006;113-116. |
| 26. | Bu LN, Chen HL, Chang CJ, Ni YH, Hsu HY, Lai HS, Hsu WM, Chang MH. Prophylactic oral antibiotics in prevention of recurrent cholangitis after the Kasai portoenterostomy. J Pediatr Surg. 2003;38:590-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Strong RW. Liver transplantation: current status and future prospects. J R Coll Surg Edinb. 2001;46:1-8. [PubMed] |