Published online Sep 6, 2021. doi: 10.12998/wjcc.v9.i25.7527
Peer-review started: February 18, 2021
First decision: April 18, 2021
Revised: April 24, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: September 6, 2021
Processing time: 193 Days and 17.5 Hours
The immune-mediated invasion of IgG4-positive plasma cells in the liver is found in some autoimmune hepatitis. Giant-cell hepatitis (GCH) is a very rare patho
A 68-year-old woman was hospitalized with fatigue, poor appetite, and yellow urine for 20 d. Liver function tests and immunological indexes were significantly abnormal and accompanied by elevated serum IgG4 levels. Liver pathology revealed severe inflammation of the interface between the portal tract and hepatocytes, portal area inflammation, plasma cell infiltration, formation of rosette cells, IgG4-positive plasma cells > 10/high-power field, IgG4/IgG > 40%, and multinucleated liver cell swelling. IgG4-related autoimmune hepatitis (AIH) combined with GCH was diagnosed, and methylprednisolone was administered at 40 mg/day. Two weeks later, the clinical symptoms disappeared, and the liver function and immunological indicators were significantly improved. Methylprednisolone was reduced at a rate of 4–8 mg per week to 8 mg/day for maintenance. A second liver biopsy 48 wk later indicated that liver inflammation and fibrosis were significantly improved. IgG4-positive plasma cells and GCH were not detected. A literature search was conducted to analyze articles reporting similar pathological phenomena.
AIH with simultaneous IgG4-positive plasma cell infiltration and GCH, liver inflammation, and fibrosis is possibly more severe than typical AIH but sensitive to corticosteroids.
Core Tip: A 68-year-old woman was hospitalized for liver function tests. Her immunological indices differed significantly from normal, and were accompanied by elevated serum IgG4 levels. Liver pathology revealed severe inflammation at the interface between the portal tract and hepatocytes, plasma cell infiltration, formation of rosette cells, IgG4-positive plasma cells > 10/high-power field, IgG4/IgG > 40%, and mul
- Citation: Tan YW, Wang JM, Chen L. Is simultaneous presence of IgG4-positive plasma cells and giant-cell hepatitis a coincidence in autoimmune hepatitis? A case report. World J Clin Cases 2021; 9(25): 7527-7534
- URL: https://www.wjgnet.com/2307-8960/full/v9/i25/7527.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i25.7527
IgG4-related disease (IgG4-RD) is a new autoimmune disease mediated by immunity[1]. This kind of disease is closely related to IgG4 lymphocytes, with increased serum IgG4 levels and IgG4-positive plasma cells infiltrating many organs and tissues. The most common organs involved include the pancreas and salivary gland, followed by the bile duct, kidney, liver, lung, and lacrimal glands. It has been recently reported that in the diagnosis of IgG4-autoimmune hepatitis (IgG4-AIH)[2,3], whether IgG4-AIH has independent subclass characteristics of classical AIH remains controversial.
Giant-cell hepatitis (GCH) or post-infantile GCH is an inflammatory disease of the liver characterized by the presence of large, thin-walled multinucleated (4-5 or more nuclei) liver cells. GCH is common in infants and young children but is very rare in adult liver diseases. In the past 30 years, scarcely more than 100 cases have been reported in the literature with an incidence of 0.1%–0.25%[4]. Therefore, the disease characteristics when the simultaneous presence of IgG4-positive plasma cells and GCH in liver tissue is accompanied by elevated serum IgG4 levels in patients with AIH are still unknown. We herein report a case of AIH with such characteristics and present the entire diagnosis and treatment process.
A 68-year-old woman was hospitalized with symptoms of asthenia, poor appetite, and yellow urine lasting more than 20 d.
The patient had no nausea, vomiting, epigastric pain, or other symptoms of dis
The patient had no history of hepatitis, blood transfusions, contact with schistosomiasis contaminated water, alcohol abuse, or use of liver-damaging drugs. The patient reported no hypertension, diabetes, coronary heart disease, or other chronic diseases.
The patient’s parents had no liver disease, and her siblings displayed no signs of liver disease.
Physical examination revealed the following: Blood pressure, 106/88 mmHg; heart rate, 71 beats/min; and body temperature, 36.8 °C. The skin and sclera were slightly yellow, and no liver palms or spider nevi were found. The abdomen was flat and soft, and there was no tenderness or rebound pain throughout the abdomen, no palpation under the sternum or lower right ribs, no palpation under the left ribcage, no percussion pain in the liver area, negative mobility dullness, and no edema in either lower limb.
A liver function test revealed the following: Total bilirubin (TBIL) 65.21 µmol/L; direct bilirubin 45.19 µmol/L; alanine aminotransferase (ALT) 441.2 U/L; aspartate aminotransferase (AST) 466.6 U/L; alkaline phosphatase (ALP) 330.4 U/L; γ-glutamyl transpeptidase (GGT) 489.6 U/L; lactate dehydrogenase 222.6 U/L; total cholesterol 6.43 mmol/L; albumin 31.2 g/L; globulin 38.6 g/L; and prealbumin 102.3 mg/L. A blood test panel revealed the following: White blood cells 3.61 × 109/L; red blood cells 3.38 × 1012/L; hemoglobin 89 g/L; platelets 75 × 109/L; neutrophil count 1.97 × 109/L; and neutrophil percentage 55.4%. Antinuclear antibody (85 U/L; < 10 U/L, enzyme-linked immunosorbent assay), anti-smooth muscle antibody, anti-liver/kidney microsomal antibody type 1, anti-nuclear glycoprotein antibody, anti-soluble acid nucleoprotein antibody, anti-hepatocyte cytoplasmic antigen type 1 antibody, anti-soluble liver antigen/hepatopancreatic antigen antibody, and other test results were negative. IgG, IgG4, and IgM levels were 29.4 g/L (< 17.1 g/L), 2.93 g/L (< 2.01 g/L), and 4.33 g/L (< 4 g/L), respectively. Viral hepatitis (A–E), Epstein–Barr virus (EBV), and cytomegalovirus (CMV) infections were ruled out.
Enhanced upper abdominal computed tomography, magnetic resonance imaging, and magnetic resonance cholangiopancreatography revealed splenomegaly but no lesions of the pancreas, bile duct, or other lesions.
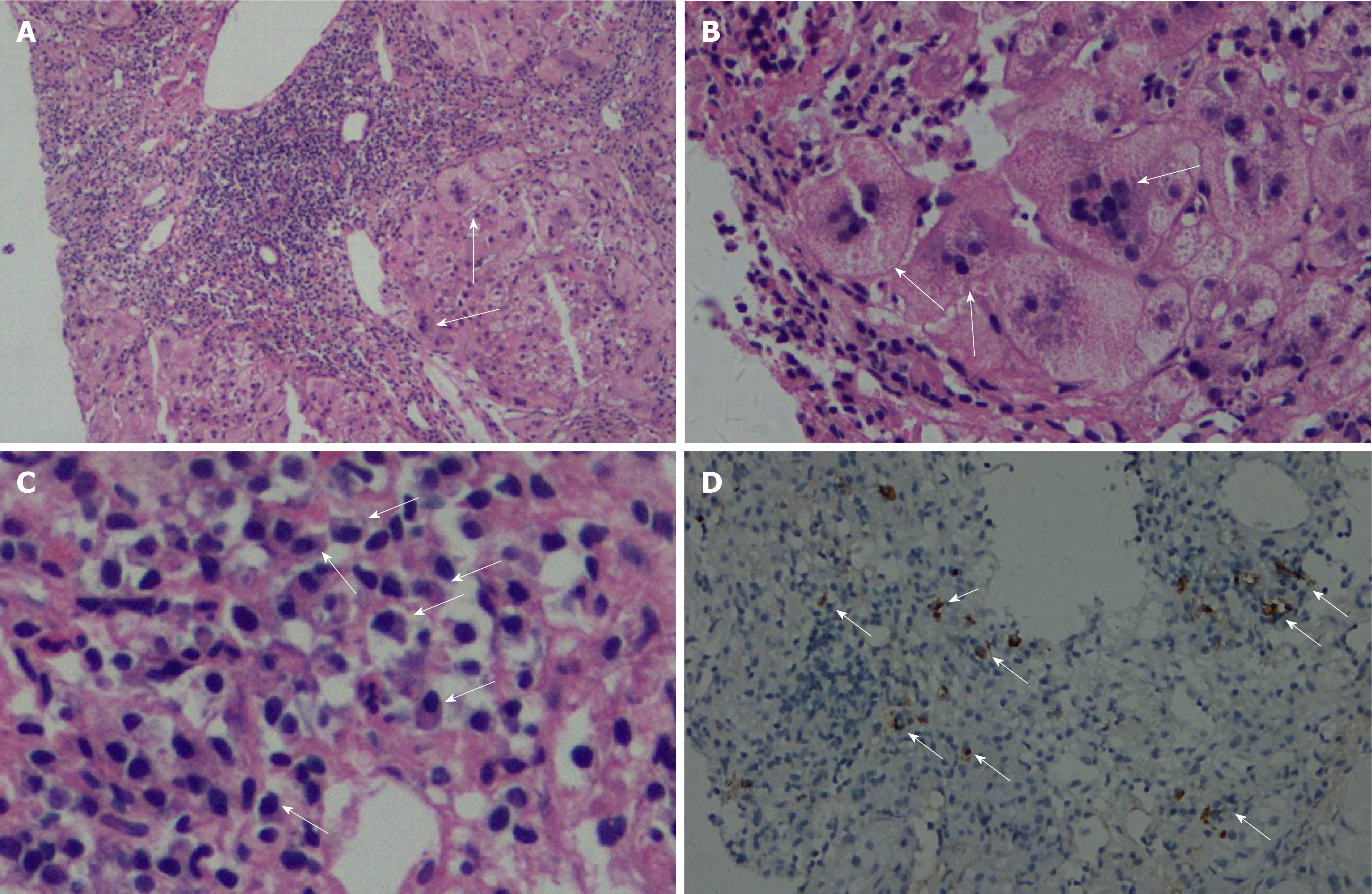
A liver biopsy was performed, and the pathology showed extensive proliferation of fibrous tissue (Ishak’s fibrosis score, 5), moderate and severe interface hepatitis, bridging necrosis and fibrosis, edema of hepatocytes as evidenced by rosette formation, multinucleated hepatocytes in each portal area (Figure 1A and B), extensive monocyte lymph infiltration, dense plasma cells in the portal area, and positive plasma cells stained with IgG4 (≥ 10/high-power field, HPF; Figure 1C and D), and IgG4/IgG > 40%. The pathological diagnosis was IgG4-related AIH and GCH.
Methylprednisolone (40 mg/d) was administered.
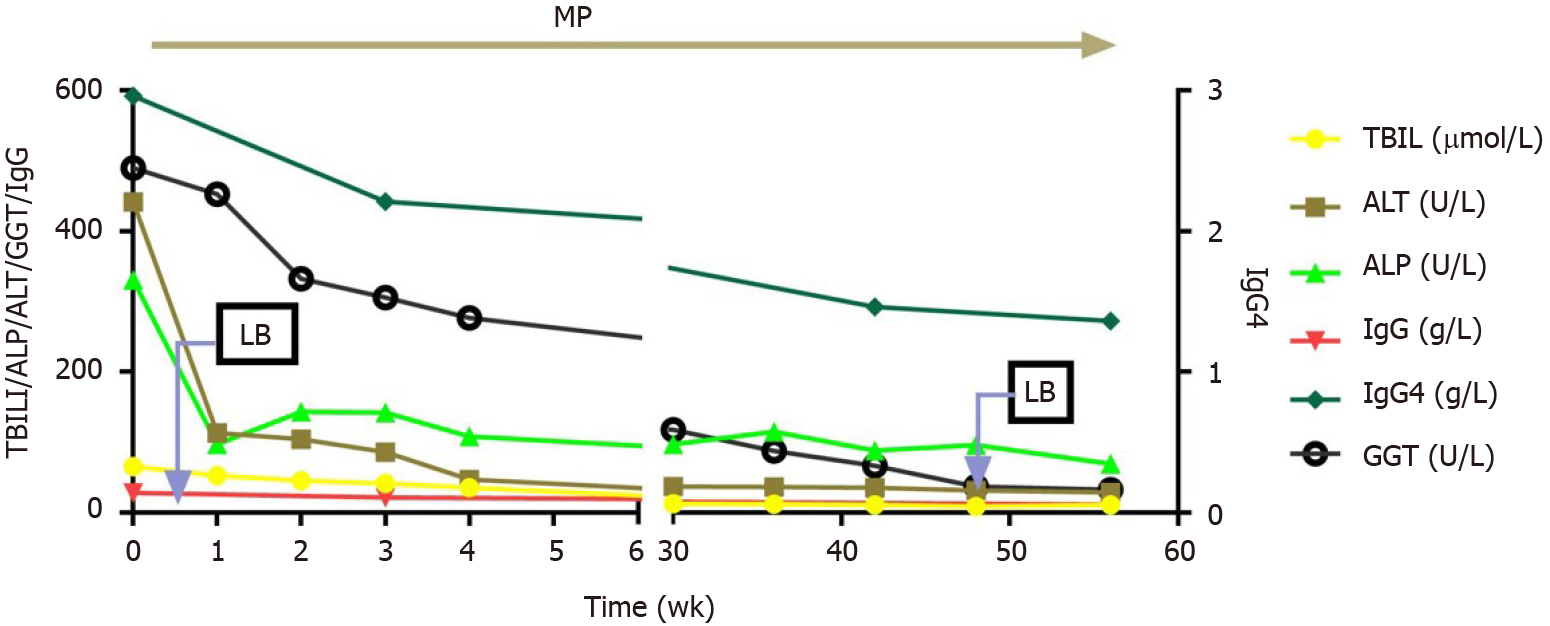
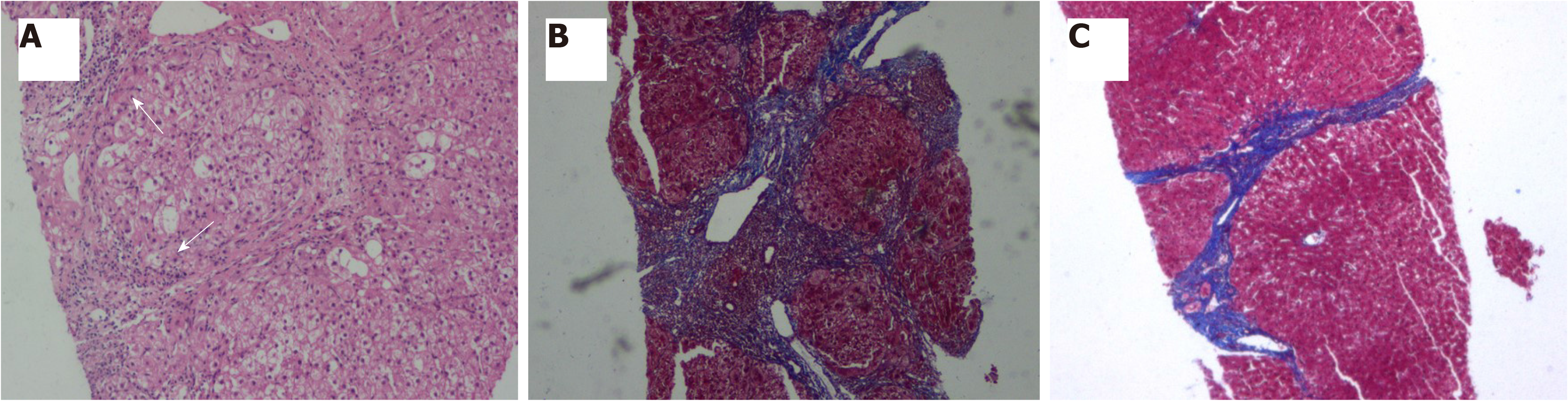
Two weeks after initial treatment, liver function moderately improved [TBIL, 45.3 µmol/L; ALT, 113 U/L; ALP, 143 U/L; GGT, 332 U/L], and immunological indices began to decline (IgG, 21.4 g/L; IgG4, 2.21 g/L). After 4 wk, methylprednisolone was reduced by 4–8 mg per week, to reach a stably maintained dose of 8 mg/d. Liver function and immunological indicators were monitored every 4–12 wk (Figure 2). A second liver biopsy was performed after 48 wk. Liver pathology indicated significant improvement in liver inflammation and necrosis, no lobular inflammation, mild inflammation in the portal area (Figure 3A), and significantly ameliorated fibrosis (from stage 5 to stage 2 according to Ishak’s fibrosis scoring system, Figure 3B and C), and scattered plasma cells. IgG4-positive immunohistochemical staining was negative, and GCH was no longer detected (Figure 3A).
Umemura et al[2] described the clinicopathological features of IgG4-related liver disease for the first time. A variety of histological manifestations was observed in 17 patients with autoimmune pancreatitis (AIP), including portal vein inflammation (35%), interfaceinterface hepatitis (24%), lobular hepatitis (29%), bile duct injury (59%), and tubular cholestasis (53%). These pathological results strongly suggest that about 20%–30% of patients with AIP have pathological manifestations similar to those of classic AIH.
Umemura et al[5] reported the first case of IgG4-AIH in 2007. Abnormal ALT, AST, and ALP levels and other abnormalities of the liver function were observed, as well as elevated serum IgG and IgG4. Typical pathological features of AIH were found in the liver biopsy histology, such as interface hepatitis, plasma cell infiltration, and formation of rosette cells. According to the diagnostic criteria of the International Autoimmune Hepatitis Group[6], our case was consistent with AIH. According to the simple AIH scoring standard introduced in 2008, we diagnosed the patient with AIH based on the following eight-point criteria: ANA+, 2 points; IgG, 2 points; typical pathological characteristics, 2 points; viral hepatitis exclusion, 2 points.
IgG4-AIH has only been reported in a few studies[7-11]. In these studies, the proportion of IgG4-AIH ranges from 3.3% to 34.6% in AIH. IgG4-AIH and AIH are not significantly different in terms of clinical characteristics, biochemistry, and immunology, including the efficacy of corticosteroids. However, the criterion for IgG4-positive plasma cells differs in the diagnosis of IgG4-AIH. Chung et al[7] and Amarapurkar et al[9] used IgG4+/HPF > 5, whereas Umemura et al[8], Canivet et al[10], and Aydemir et al[12] used IgG4+ > 10 as the diagnostic criterion. In 2016, a study summarized and analyzed published data that met the diagnostic criteria for IgG4-RD, and proposed diagnostic recommendations for IgG4-AIH[12] as follows: (1) Serum IgG4 ≥ 1.35 g/L; (2) IgG4-plasma cells/HPF in liver tissue ≥ 10 and the ratio of IgG4-positive plasma cells to plasma cells infiltrated > 40%; (3) Chronic hepatitis with banding and bridging necrosis or apparent parenchymal collapse; and (4) Incorporation of other IgG4-RD. Complying with all four items is defined as confirmed IgG4-AIH, and complying with the first three items as highly likely IgG4-AIH, whereas complying with any two items as possible IgG4-AIH. Diagnostic recommendations are still subject to clinical testing.
However, the diagnosis of IgG4-AIH is still not accepted. IgG4-AIH is not recognized as a diagnostic category in the AIH guideline specification[13]. IgG4-RD is an immune disease of the systemic system, and its pathological features include non-infectious and non-neoplastic inflammation and fibrosis[1]. Therefore, it is reasonable to believe that the liver is not an organ that can avoid injury.
However, a newly published study found that 10 (11.9%) out of 84 patients with AIH had IgG4-AIH[14]. Although the biochemical characteristics of IgG4-AIH were similar to those of classical AIH, the pathological manifestations of IgG4-AIH were more severe interface hepatitis and lobular hepatitis and more progressive fibrosis than those of AIH. After a median of 139 mo of long-term observation, the recurrence rate of IgG4-AIH was lower than that of classical AIH. Xue et al[15] observed 152 patients with AIH. They analyzed 111 patients with AIH in the low IgG4 group (< 135 mg/mL) and 41 patients in the high IgG4 group (> 135 mg/mL) and found that those in the high IgG4 group presented a higher proportion of cirrhosis than those in the low IgG4 group. In our case, severe inflammation and fibrosis were also observed during the first histological examination. After 1 year of corticosteroid treatment, the second histological examination showed that the inflammation and fibrosis of the liver improved significantly, and the liver function remained normal.
Phillips et al[16] identified GCH in the early 1990s. They found that in 10 cases of GCH, individual hepatocytes had 30 nuclei, 5 out of 10 patients died, and 5 received liver transplantation, showing a very poor prognosis of the disease. Paramyxovirus antigens were detected in the cytoplasm of two patients, suggesting that GCH may be related to a viral infection.
GCH is a rare pathological feature of the liver. A total of 36726 liver biopsies sampled across 25 years from a single center showed 50 (0.14%) cases of GCH[17]. The reasons for its appearance may include infection (hepatitis A, B, C, EBV, CMV, paramyxo-like virus, human immunodeficiency virus, HPS pulmonary syndrome, and human papillomavirus), autoimmune disease (AIH, ulcerative colitis, primary sclerosing cholangitis, primary biliary cholangitis, systemic lupus erythematosus, and rheumatoid arthritis), drugs (polyarteritis nodosa, methotrexate, mercaptopurine, amytriptyline, p-aminosalicylic acid, vinyl chloride, chropromazine, and metho
The mechanism of GCH generation is not clear, and two explanations are accepted: One is the expression of immature cells[18], in which nuclear proliferation is not accompanied by cell membrane mitosis, resulting in nuclear accumulation[19]; the other is the nuclear overreaction of liver cells under abnormal stimulation[20,21].
To search for similar articles from the PubMed/Medline database, the time set was January 1, 1960 to December 31, 2020 and the keywords set as “IgG4 or AIH and GCH”. Three cases involving the coexistence of IgG4-AIH and GCH have been reported[5,8]. Umemura et al[8] reported 70 consecutive patients with type 1 AIH from a single center between June 1985 and December 2006. GCH was found in two cases of IgG4-AIH and in 58 cases of typical AIH. In addition to elevated IgG4 in serum and IgG4-positive plasma cell expression in liver tissue, these two patients had more serious portal inflammation (> 2/3 of periportal areas), interface hepatitis (> 2/3 of periportal areas), lobular hepatitis (zonal hepatitis), plasma cells (> 20/HPF), and bridging fibrosis compared with those with classic AIH. In one case, after 5 years of treatment and follow-up, ALP and GGT levels continued to rise to 622 and 936 IU/L, respectively. The disease eventually developed into IgG4-related sclerosing cholangitis. The second case was a 42-year-old male patient with a serum IgG4 level of 642 mg/dL and a large number of IgG4-plasma cells expressed in liver tissue. After treatment with prednisone at 60 mg/d for 4 wk, the serum IgG4 level was reduced to 452 mg/dL and became normal after 7 mo. A second liver biopsy was performed and the pathology showed that although portal sclerosis was still present, portal inflammation and lobular inflammation improved markedly, and the GCH disappeared. The patient was followed for 13 years, and prednisone administration (5.0 mg/day) was maintained. Another case was reported as the first one of IgG4-AIH[5]. The authors did not emphasize the presence of GCH, but a large number of GCH hepatocytes were observed in the pathology images. The patient was sensitive to prednisone, and after 4 wk, the dose was reduced to 5 mg/day. The serum IgG4 and ALT levels were normal.
From the reported three cases of IgG4-AIH accompanied with GCH together with our case, some common features were observed: (1) The pathological lesions including inflammation, necrosis, and fibrosis are more serious than the pathological characteristics of classical AIH; (2) The disease is sensitive to corticosteroids. Except for one case that developed PSC, the other several cases had good histological improvement and biochemical response; and (3) Lastly, the more significant feature is that GCH disappears after corticosteroid therapy.
It is still difficult to explain the relationship between these two mechanisms. It can be assumed that: (1) The infection is the initiator; the evidence of not only the infection was found in GCH[16] but also the initiator of IgG4-RD. Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) are important receptors that mediate immune recognition of pathogenic microorganisms and are widely distributed on the surfaces of immune cells. Microbial antigens activating TLR and NLR on the surfaces of monocytes may promote the production of IgG4 by the B cell activating factor signaling pathway[22]; and (2) Large number of cytokines, such as IL-4, IL-5, IL-10, IL-13, and IL-21, which regulate the growth of various cells, and the transforming growth factor-β[23-26] are involved in the occurrence of IgG4-RD. Overexpression of these factors promotes the overdifferentiation of plasma cells and overproliferation of liver nuclei[27].
In conclusion, in our case and those from the literature, the simultaneous occurrence of IgG4 and GCH in AIH may reflect severe inflammation, necrosis, and fibrosis of liver injury, which may be sensitive to corticosteroid therapy, and the disappearance of GCH is evidence of pathological repair.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Komori A, Lu J, Macedo G, Masaki N, Takahashi T, Watanabe T S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Sánchez-Oro R, Alonso-Muñoz EM, Martí Romero L. Review of IgG4-related disease. Gastroenterol Hepatol. 2019;42:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Minaga K, Watanabe T, Chung H, Kudo M. Autoimmune hepatitis and IgG4-related disease. World J Gastroenterol. 2019;25:2308-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 4. | Bihari C, Rastogi A, Sarin SK. Postinfantile giant cell hepatitis: an etiological and prognostic perspective. Hepat Res Treat. 2013;2013:601290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Umemura T, Zen Y, Hamano H, Ichijo T, Kawa S, Nakanuma Y, Kiyosawa K. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1985] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 7. | Chung H, Watanabe T, Kudo M, Maenishi O, Wakatsuki Y, Chiba T. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int. 2010;30:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Umemura T, Zen Y, Hamano H, Joshita S, Ichijo T, Yoshizawa K, Kiyosawa K, Ota M, Kawa S, Nakanuma Y, Tanaka E. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. J Gastroenterol. 2011;46 Suppl 1:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Amarapurkar AD, Amarapurkar DN. Immunoglobulin IgG4 and autoimmune hepatitis. Trop Gastroenterol. 2015;36:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Canivet CM, Anty R, Patouraux S, Saint-Paul MC, Lebeaupin C, Gual P, Duclos-Vallee JC, Tran A. Immunoglobulin G4-associated autoimmune hepatitis may be found in Western countries. Dig Liver Dis. 2016;48:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Aydemir Y, Akcoren Z, Demir H, Saltik Temizel IN, Ozen H, Yuce A. Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children. J Gastroenterol Hepatol. 2019;34:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Shiokawa M, Kodama Y, Kuriyama K, Yoshimura K, Tomono T, Morita T, Kakiuchi N, Matsumori T, Mima A, Nishikawa Y, Ueda T, Tsuda M, Yamauchi Y, Minami R, Sakuma Y, Ota Y, Maruno T, Kurita A, Sawai Y, Tsuji Y, Uza N, Matsumura K, Watanabe T, Notohara K, Tsuruyama T, Seno H, Chiba T. Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016;65:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 570] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 14. | Arase Y, Matsumoto K, Anzai K, Tsuruya K, Sugiyama S, Yoshihara S, Hirose S, Uojima H, Hidaka H, Nakazawa T, Deguchi R, Kojima S, Takashimizu S, Shiraishi K, Shirai T, Kagawa T. Clinicopathological Features of Autoimmune Hepatitis with IgG4-Positive Plasma Cell Infiltration. Dig Dis. 2021;39:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Xue M, Shen Y, Fan X, Shen M, Yang L. Autoimmune Hepatitis with Elevated Serum IgG4 Levels Have a High Prevalence of Cirrhosis at Diagnosis. Can J Gastroenterol Hepatol. 2021;2021:6692511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Phillips MJ, Blendis LM, Poucell S, offterson J, Petric M, Roberts E, Levy GA, Superina RA, Greig PD, Cameron R. Syncytial giant-cell hepatitis. Sporadic hepatitis with distinctive pathological features, a severe clinical course, and paramyxoviral features. N Engl J Med. 1991;324:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 142] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Matta B, Cabello R, Rabinovitz M, Minervini M, Malik S. Post-infantile giant cell hepatitis: A single center's experience over 25 years. World J Hepatol. 2019;11:752-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (2)] |
| 18. | Perez-Atayde AR, Sirlin SM, Jonas M. Coombs-positive autoimmune hemolytic anemia and postinfantile giant cell hepatitis in children. Pediatr Pathol. 1994;14:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gorelik M, Debski R, Frangoul H. Autoimmune hemolytic anemia with giant cell hepatitis: case report and review of the literature. J Pediatr Hematol Oncol. 2004;26:837-839. [PubMed] |
| 20. | Lau JY, Koukoulis G, Mieli-Vergani G, Portmann BC, Williams R. Syncytial giant-cell hepatitis--a specific disease entity? J Hepatol. 1992;15:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Roberts E, Ford-Jones EL, Phillips MJ. Ribavirin for syncytial giant cell hepatitis. Lancet. 1993;341:640-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Watanabe T, Yamashita K, Fujikawa S, Sakurai T, Kudo M, Shiokawa M, Kodama Y, Uchida K, Okazaki K, Chiba T. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012;64:914-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 818] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 24. | Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 480] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 25. | Kiyama K, Kawabata D, Hosono Y, Kitagori K, Yukawa N, Yoshifuji H, Omura K, Fujii T, Mimori T. Serum BAFF and APRIL levels in patients with IgG4-related disease and their clinical significance. Arthritis Res Ther. 2012;14:R86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Maehara T, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Tanaka A, Shinozaki S, Kubo Y, Nakamura S. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Ann Rheum Dis. 2012;71:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | de Boer BA, Kruize YC, Rotmans PJ, Yazdanbakhsh M. Interleukin-12 suppresses immunoglobulin E production but enhances immunoglobulin G4 production by human peripheral blood mononuclear cells. Infect Immun. 1997;65:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |